Reductive dissolution of Fe3O4 facilitated by the Au domain of an Fe3O4/Au hybrid nanocrystal: formation of a nanorattle structure composed of a hollow porous silica nanoshell and entrapped Au nanocrystal†
Kyung Min
Yeo
,
Jongmin
Shin
and
In Su
Lee
*
Department of Applied Chemistry, College of Applied Science, Kyung Hee University, Gyeonggi-do 449-701, Korea. E-mail: insulee97@khu.ac.kr; Fax: +82-31-202-7337; Tel: +82-31-201-3823
First published on 20th October 2009
Abstract
The Fe3O4 grain of a Fe3O4/Au hybrid nanocrystal encapsulated in a silica nanosphere was rapidly and exclusively dissolved through a reductive process facilitated by the attached Au grain, resulting in the formation of a nanorattle structure which has utility as a nanoreactor to template the growth of nanocrystals inside the cavity.
Recently, several hybrid nanocrystals, such as Au/Fe3O4, Ag/Fe3O4, CdS/FePt, and γ-Fe2O3/metal sulfides, have been synthesized by integrating chemically different species through heterojunction, and have attracted much attention due to their novel properties and unique applicability which cannot be achieved with single component nanocrystals.1 In this study, we observed the spontaneous generation of Fe3O4/Au hybrid nanocrystals during the encapsulation reaction of Fe3O4 and Au3+ complexes with silica nanospheres. Additionally, in the course of treating the hybrid nanocrystals with NaBH4, it was revealed that the Fe3O4 grains are rapidly and exclusively removed from the hybrid nanocrystals through the reductive process, facilitated by the attached Au grain. This is a unique and interesting phenomenon which had not been expected with any single component nanocrystals and has never been reported, to the best of our knowledge. Along with providing fundamental knowledge, this finding also offers a simple and novel method to fabricate “rattle type” nanostructures, which have received attention recently as catalytic and biosensing materials.2 The selective dissolution of Fe3O4 accompanied by silica etching left a Au nanocrystal inside the cavity of a hollow and porous silica nanoshell, which enabled the stabilization of the active metal core, even under severe conditions, while providing the reacting molecules with a pathway for diffusion.3 Based on the resulting nanostructure, it was envisioned that the nucleation and growth of the metal species could be guided, occurring only inside the hollow shell by the Au core, thus the nanorattle could act as a nanoreactor to spatially-confine the synthesis of nanoparticles. Herein, we report the synthesis of Fe3O4/Au hybrid nanocrystals in silica nanospheres, the reductive dissolution of their Fe3O4 grain facilitated by the attached Au, and the formation of the nanorattle structure with a porous and hollow silica nanoshell capturing a Au nanocrystal inside the cavity. We also report the successful employment of the resulting nanorattles to template Ag nanocrystal synthesis, which demonstrates their utility as a platform intermediate for a wide variety of metal core–silica shell nanoparticles (Scheme 1).
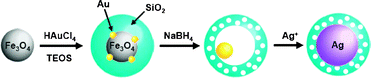 | ||
| Scheme 1 The syntheses of Fe3O4/Au@SiO2, Au@h-SiO2, and Ag@SiO2. | ||
The encapsulation of Fe3O4 nanocrystals and Au complexes within silica shells was conducted in a microemulsion system containing a HAuCl4 precursor in water droplets and 8 (±1) nm sized Fe3O4 nanocrystals in the external cyclohexane phase.4 The formation of a silica shell around the Fe3O4 nanocrystals was initiated by the successive addition of an NH4OH aqueous solution and tetraethyl orthosilicate (TEOS) and proceeded for 12 h. Transmission electron microscopy (TEM) and X-ray photoelectron spectrometry (XPS) analyses of the resulting solids revealed the reduction of Au3+ complexes during the reaction and the growth of several tiny Au nanocrystals of 1–2 nm diameter around the Fe3O4 nanocrystals, generating an Fe3O4/Au hybrid nanocrystal in a silica nanosphere of 29 (±1) nm diameter (Fe3O4/Au@SiO2) (Fig. 1, ESI†). The formation of the hybrid nanocrystal can be understood by the reduction of AuCl4− by polyoxyethylenenonylphenyl ether (Igepal CO-520, containing 50 mol% hydrophilic groups) and the preferential nucleation of Au at the Fe3O4 surface.5 A control reaction carried out in the absence of the Fe3O4 nanocrystal allowed the formation of a Au nanocrystal with an average size of 3.5 (±0.4) nm within a silica nanosphere (Au@SiO2) (ESI†).
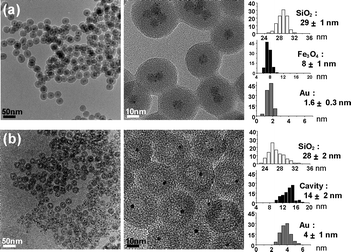 | ||
| Fig. 1 TEM images and histograms showing the size distribution of the silica nanospheres, hollow cavities, and Fe3O4 and Au grains of (a) Fe3O4/Au@SiO2 and (b) Au@h-SiO2 synthesized from 8 nm sized Fe3O4 nanocrystals. | ||
When NaBH4 (60 mM) was added to an aqueous suspension of the Fe3O4/Au@SiO2 nanospheres with stirring, the dark brown color gradually faded with the evolution of H2 gas over 30 min and a pale purple suspension was generated (Fig. 2a). The UV-Vis spectrum change showed the reduction and disappearance of the broad absorption below 500 nm, which is associated with the Fe3O4 nanocrystals, and the emergence of a surface plasmon band of the Au nanocrystals at around 520 nm (ESI†). TEM and X-ray diffraction (XRD) analyses confirmed the dissolution of Fe3O4 from the hybrid nanocrystals during the reaction (Fig. 1b, 2b). The removal of the Fe3O4 grains left voids with a 14 (±2) nm diameter in the silica nanospheres, thus leading to the formation of Au@h-SiO2 with a rattle-like nanostructure consisting of a hollow and porous silica shell with an outer diameter of 28 (±2) nm and a Au nanocrystal with an average size of 4 (±1) nm. Their nitrogen adsorption/desorption isotherm revealed the marked increase in BET surface area from 86 m2 g−1 to 371 m2 g−1 and the specification of a bimodal mesoporous system that consists of large pores with a narrow size distribution centered at 19.9 nm and small pores broadly dispersed in a 1–5 nm range (ESI†). The reduced size and the porosity of the resulting silica shell indicate the partial etching of silica during the reaction with NaBH4, which is consistent with the recent discovery reported by Yin et al.6 The larger cavity size of Au@h-SiO2, compared with the size of the removed Fe3O4 grain, can be understood by the subsequent etching of the cavity surface newly generated after Fe3O4 dissolution. When the Fe3O4 grain was etched from Fe3O4/Au@SiO2 with an aqueous HCl solution and the resulting hollow nanosphere was subsequently treated with NaBH4, as a control experiment, it was observed that the hollow cavity expanded during the NaBH4 treatment to give a hollow and porous shell, which is quite similar to that observed in Au@h-SiO2 (ESI†). The growth of a Au nanocrystal relative to that in Fe3O4/Au@SiO2 is most likely due to the coalescence or ripening of Au particles within the cavity during the etching reaction. The cavity size of Au@h-SiO2 was found to be readily controlled simply by changing the size of the sacrificial Fe3O4 nanocrystal.7 For instance, a Fe3O4/Au@SiO2 nanosphere prepared by using 5 (±1) nm sized Fe3O4 provided a Au@h-SiO2 nanorattle with an average cavity size of 10 (±1) nm (ESI†).
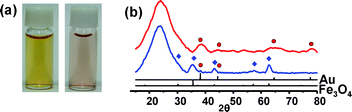 | ||
| Fig. 2 (a) Photographs and (b) XRD patterns of Fe3O4/Au@SiO2 (left photo, blue line) and Au@h-SiO2 (right photo, red line). | ||
While there are previous reports on the reductive dissolution of iron oxide occurring in several microorganisms, such a rapid and selective dissolution of the crystalline Fe3O4 phase during the abiotic reaction was an unexpected and unique finding.8 The treatment of silica nanospheres encapsulating Fe3O4 nanocrystals, Fe3O4@SiO2, prepared without the addition of HAuCl4, or their mixture with Au@SiO2, with NaBH4 did not exhibit any detectable change in the Fe3O4 crystals, while the silica nanospheres were rendered porous by etching, which proved that there was no dissolution of Fe3O4 without the attachment to Au (ESI†). Thus it can be inferred that the Au grain of the hybrid nanocrystal facilitates the electron transfer from BH4− (donor) to the adjacent Fe3O4 grain (acceptor) and catalyzes the reductive dissolution of Fe3O4. A similar electron relaying mechanism has been proposed in a number of previous reports to explain the catalytic performance of Au nanocrystals in the reduction of organic compounds by NaBH4.2b,3a,9 Very recently, Lucas et al. reported the partial dissolution of γ-Fe2O3 nanoparticles into Fe2+ ions during electrochemical reduction on a gold electrode, which also supports our proposed mechanism.10
When AgNO3 (3.0 mM) was reacted with reductive N2H4 in an aqueous suspension containing 0.3 mg ml−1 of Au@h-SiO2 nanospheres, Ag nanocrystals were grown exclusively inside the hollow shells, creating Ag@SiO2 nanospheres in which the Ag cores are well coated by porous silica shells (Fig. 3a).11 It was found that larger Ag nanocrystals were formed as the initial concentration of AgNO3 increased, which demonstrates the preferential nucleation of Ag at the Au surface inside the cavity followed by gradual growth (Fig. 3b, ESI†). The purified solids of Ag@SiO2 were readily dispersed in an aqueous suspension to generate a stable colloid (Fig. 3c and d). Control reactions with hollow and porous silica nanospheres, prepared from Fe3O4@SiO2, through etching with HCl followed by treatment with NaBH4, or Au encapsulating hollow silica nanospheres, synthesized by etching Fe3O4/Au@SiO2 with a HCl solution, gave rise to the growth of Ag with a relatively large size on the outer surface of the silica sphere (ESI†). These observations indicate that both the Au core and the porous shell are indispensable factors to spatially confine the synthesis of Ag nanocrystals inside the hollow cavities of the Au@h-SiO2 nanospheres.
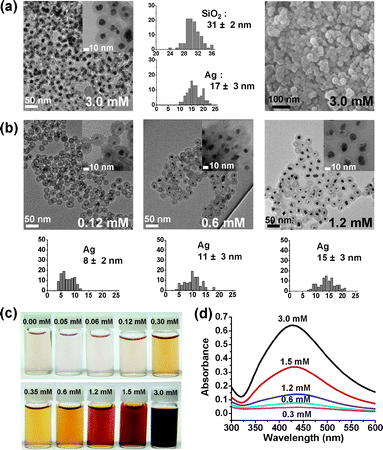 | ||
| Fig. 3 (a) TEM, HRTEM, and SEM images and histograms showing the size distribution of the silica nanospheres and Ag nanocrystals of Ag@SiO2. (b) TEM images and histograms showing the size distribution of Ag nanocrystals at various AgNO3 concentrations. (c) Photographs and (d) UV-Vis absorption spectra of aqueous suspensions containing Ag@SiO2 nanospheres (0.8 mg ml−1). | ||
In summary, we synthesized Fe3O4/Au hybrid nanocrystals encapsulated in silica nanospheres and found the rapid and selective dissolution of their Fe3O4 grains through a reductive process, facilitated by the adjacent Au, which has never been achieved with any single component nanocrystals. By employing the reductive Fe3O4 dissolution, we demonstrated a method to generate a nanorattle structure consisting of a hollow and porous silica nanoshell and a Au nanocrystal. We also demonstrated the utility of the nanorattles as nanoreactors to template the growth of nanocrystals inside the cavities. We believe that the results of this study will provide a novel approach for developing a variety of core–shell nanomaterials, which have many advantages in catalytic and biosensing applications.
This work was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2008-314-C00192).
Notes and references
- (a) W. Shi, H. Zeng, Y. Sahoo, T. Y. Ohulchanskyy, Y. Ding, Z. L. Wang and P. N. Prasad, Nano Lett., 2006, 6, 875 CrossRef CAS; (b) K.-W. Kwon and M. Shim, J. Am. Chem. Soc., 2005, 127, 10269 CrossRef CAS; (c) H. W. Gu, Z. M. Yang, J. H. Gao, C. K. Chang and B. Xu, J. Am. Chem. Soc., 2005, 127, 34 CrossRef CAS; (d) S. Kudera, L. Carbone, M. F. Casula, R. Cingolani, A. Falqui, E. Snoeck, W. J. Parak and L. Manna, Nano Lett., 2005, 5, 445 CrossRef CAS; (e) H. Yu, M. Chen, P. M. Rice, S. X. Wang, R. L. White and S. Sun, Nano Lett., 2005, 5, 379 CrossRef CAS; (f) T. Teranishi, Y. Inoue, M. Nakaya, Y. Oumi and T. Sano, J. Am. Chem. Soc., 2004, 126, 9914 CrossRef CAS; (g) T. Mokari, E. Rothenberg, I. Popov, R. Costi and U. Banin, Science, 2004, 304, 1787 CrossRef CAS.
- (a) J. Gao, G. Liang, J. S. Cheung, Y. Pan, Y. Kuang, F. Zhao, B. Zhang, X. Zhang, E. X. Wu and B. Xu, J. Am. Chem. Soc., 2008, 130, 11828 CrossRef CAS; (b) J. Lee, J. C. Park and H. Song, Adv. Mater., 2008, 20, 1523 CrossRef CAS; (c) C.-J. Jia, L.-D. Sun, F. Luo, X.-D. Han, L. J. Heyderman, Z.-G. Yan, C.-H. Yan, K. Zheng, Z. Zhang, M. Takano, N. Hayashi, M. Eltschka, M. Kälui, U. Rüdiger, T. Kasama, L. Cervera-Gontard, R. E. Dunin-Borkowski, G. Tzvetkov and J. Raabe, J. Am. Chem. Soc., 2008, 130, 16968 CrossRef CAS; (d) J. Li and H. C. Zeng, Angew. Chem., Int. Ed., 2005, 44, 4342 CrossRef CAS; (e) S. Liu, Z. Zhang and M.-Y. Han, Adv. Mater., 2005, 17, 1862 CrossRef CAS; (f) K. Kamata, Y. Lu and Y. Xia, J. Am. Chem. Soc., 2003, 125, 2384 CrossRef CAS.
- (a) J. Lee, J. C. Park, J. U. Bang and H. Song, Chem. Mater., 2008, 20, 5839 CrossRef CAS; (b) Y. H. Ng, S. Ikeda, T. Harada, S. Higashida, T. Sakata, H. Mori and M. Matsumura, Adv. Mater., 2007, 19, 597 CrossRef CAS; (c) P. M. Arnal, M. Comotti and F. Schüth, Angew. Chem., Int. Ed., 2006, 45, 8224 CrossRef CAS; (d) S. Ikeda, S. Ishino, T. Harada, N. Okamoto, T. Sakata, H. Mori, S. Kuwabata, T. Torimoto and M. Matsumura, Angew. Chem., Int. Ed., 2006, 45, 7063 CrossRef CAS; (e) Y. Yin, R. M. Rioux, C. K. Erdonmex, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS.
- (a) J. Shin, H. Kim and I. S. Lee, Chem. Commun., 2008, 5553 RSC; (b) D. C. Lee, F. V. Mikulec, J. M. Pelaez, B. Koo and B. A. Korgel, J. Phys. Chem. B, 2006, 110, 11160 CrossRef CAS; (c) D. K. Yi, S. T. Selvan, S. S. Lee, G. C. Papaefthymiou, D. Kundaliya and J. Y. Ying, J. Am. Chem. Soc., 2005, 127, 4990 CrossRef CAS.
- (a) T. Sakai and P. Alexandridis, Langmuir, 2004, 20, 8426 CrossRef CAS; (b) T. Sakai and P. Alexandridis, J. Phys. Chem. B, 2005, 109, 7766 CrossRef CAS.
- T. Zhang, J. Ge, Y. Hu, Q. Zhang, S. Aloni and Y. Yin, Angew. Chem., Int. Ed., 2008, 47, 5806 CrossRef CAS.
- (a) J. Yang, J. Lee, J. Kang, K. Lee, J.-S. Suh, H.-G. Yoon, Y.-M. Huh and S. Haam, Langmuir, 2008, 24, 3417 CrossRef CAS; (b) D. K. Yi, S. S. Lee, G. C. Papaefthymiou and J. Y. Ying, Chem. Mater., 2006, 18, 614 CrossRef CAS.
- Y.-S. Luu and J. A. Ramsay, World J. Microbiol. Biotechnol., 2003, 19, 215 CrossRef CAS.
- (a) S. Panigrahi, S. Basu, S. Praharaj, S. Pande, S. Jana, A. Pal, S. K. Ghosh and T. Pal, J. Phys. Chem. C, 2007, 111, 4596 CrossRef CAS; (b) J.-P. Deng, W.-C. Shih and C.-Y. Mou, J. Phys. Chem. C, 2007, 111, 9723 CrossRef CAS; (c) K. Hayakawa, T. Yoshimura and K. Esumi, Langmuir, 2003, 19, 5517 CrossRef CAS.
- I. T. Lucas, E. Dubois, J. Chevalet, S. Durand-Vidal and S. Joiret, Phys. Chem. Chem. Phys., 2008, 10, 3274 RSC.
- There is a recent report on the synthesis of Au@SiO2 by reducing a Au precursor in the presence of hollow silica and removing some Au aggregates which eventually formed outside the silica shell. S. Cavaliere-Jaricot, M. Darbandi and T. Nann, Chem. Commun., 2007, 2031 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and characterization, XPS of Fe3O4/Au@SiO2, UV-Vis spectra and N2 sorption isotherms of Au@h-SiO2, and TEM images of Au@h-SiO2 synthesized from 5 nm sized Fe3O4 nanocrystals, Ag@SiO2, and adducts obtained from the control reactions. See DOI: 10.1039/b915240g |
| This journal is © The Royal Society of Chemistry 2010 |
