Molecular tectonics: tubular crystals with controllable channel size and orientation†
Mei-Jin
Lin
,
Abdelaziz
Jouaiti
*,
David
Pocic
,
Nathalie
Kyritsakas
,
Jean-Marc
Planeix
and
Mir Wais
Hosseini
*
Laboratoire de Chimie de Coordination Organique (UMR 7140), Université de Strasbourg, Institut Le Bel, 4 rue Blaise Pascal, 67000 Strasbourg, France. E-mail: hosseini@chimie.u-strasbg.fr
First published on 13th November 2009
Abstract
The combination of flexible neutral organic tectons based on two pyridines interconnected by a thioether or thioester type spacer with an inorganic ZnSiF6 pillar leads to the formation of 2-D coordination networks and the packing of the latter generates crystals offering controllable tubular channels with imposed orientation along the pillar axis.
Coordination networks or metal–organic frameworks (MOFs) with inner cavities have attracted considerable interest because of their ability to encapsulate and exchange guest molecules and to catalyze chemical reactions.1,2 Most of these potential applications directly depend on the type, shape and size of the cavity. Thus, it is pertinent to investigate different design principles allowing the control and modulation of the characteristics of cavities and channels at the level of construction units. In a retrosynthetic approach,3 the formation of coordination networks4 results from the self assembly of tectons capable of interacting with each other.5
Here, we report the design and generation of crystalline materials possessing channels with controllable size and orientation.
The SiF62− anion6 is an interesting unit for the design of coordination networks. Its combination with dications such as Zn, Co, Cu and organic ligands leads to the formation of pillars resulting from the bridging of metal centres through the two fluorine atoms occupying the axial positions (Fig. 1a).7,8
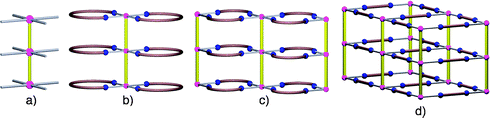 | ||
| Fig. 1 Schematic representation of the formation of a pillar (a), 1-D (b), 2-D (c) and 3-D (d) architectures resulting from the combination of ZnSiF6 with flexible or rigid organic tectons. | ||
For the design strategy, instead of looking at Zn2+ and SiF62− as two separate entities, one may consider the pillar (Fig. 1a) that they form as a construction unit (infinite tecton) offering the possibility of constructing 1-D (Fig. 1b), 2-D (Fig. 1c) or 3-D (Fig. 1d) architectures with inner cavities through the use of the coordination sphere around the zinc cations (four available sites occupy the corners of a square) regularly distributed (every ca. 7.5 Å) along the pillar. Thus, depending on the nature of the organic tecton bridging the pillars, one may control the dimensionality of the network as well as the shape, size and nature of the channels. Indeed, a flexible tecton offering the appropriate curvature should lead to the formation of 1-D tubular networks resulting from the binding of the cation by both extremities of the tecton (Fig. 1b). The use of a flexible tecton unable to bite the same Zn2+ cation should generate 2-D tubular systems for which the cyclic cavities are oriented parallel to the pillar axis (Fig. 1c) and finally, a rigid tecton should afford a 3-D cubic architecture (Fig. 1d).7,8 For the latter case, we have recently demonstrated the possibility of differentiating and modulating the size of the channels.8 In terms of design principle, controlling the nature, size, shape and orientation of the channels is important. Using the strategy presented here this can be achieved since by construction, e.g.Fig. 1c, the size and shape are imposed by the organic tecton and the orientation by the construction principles. Using a completely different approach, we have previously investigated the formation of tubular networks using calixarene or cyclophane type tectons.9
As for the organic tecton, a unit based on a flexible spacer bearing a monodentate coordinating site at each end is required. Thus, tectons 1–6 (Scheme 1) were designed and prepared. For these tectons, the coordinating site is a 4-pyridyl unit. Based on the nature of the junction between the pyridyl unit and the spacer controlling the rigidity of the building block, tectons 1–6 may be divided into two classes A (thioether) and B (thioester). We have already reported similar tectons based on oligoethyleneglycol and their use for the formation of helical strands.10
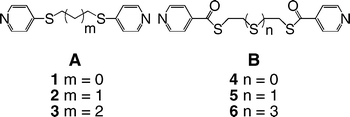 | ||
| Scheme 1 | ||
Tectons 1–3 were obtained in high yield upon reacting 4-mercaptopyridine with α,ω-alkyl dihalides (ethyl for 1, propyl for 2 and butyl for 3) in THF and in the presence of K2CO3 (see Experimental section in ESI† ).11 Tectons 4–6 were prepared by treating thioglycol (for 4), 2,2′-thiodiethanethiol (for 5) or 1,13-dimercapto-4,7,10-trithiatridecane (for 6) with the hydrochloride salt of isonicotinoyl chloride in the presence of Et3N at room temperature and in dry THF (see Experimental section in ESI† ).12
In crystallization tubes, upon slow diffusion of an EtOH solution of ZnSiF6·6H2O into a CHCl3 solution of tectons 1–6, colourless crystalline materials were obtained after several days. Whereas for compounds 2 and 6, in spite of many attempts, the solid obtained was polycrystalline, for the other four combinations, with 1 and 3–5, single crystals were obtained and analysed by X-ray diffraction techniques (Fig. 2) (See ESI crystallographic section).
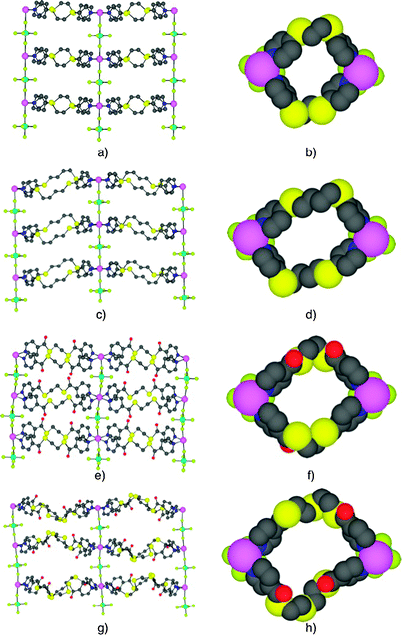 | ||
| Fig. 2 Views of portions of X-ray structures of the 2-D networks formed upon combining ZnSiF6 with 1 and 3–5 (top to bottom): perpendicular (a, c, e, g) and along (b, d, f, h) the Si–F–Zn pillar axis. H atoms and solvent molecules are not presented for sake of clarity. | ||
Furthermore, the purity of the solid phases was determined by powder X-ray diffraction (Fig. 3). This technique revealed that whereas 1·ZnSiF6, 3·ZnSiF6, 4·ZnSiF6 are the major components, i.e. rather good matching between observed and simulated peaks (Fig. 3), in the case of 5·ZnSiF6 the crystalline powder was composed of several types of crystals and the material appeared to be unstable.
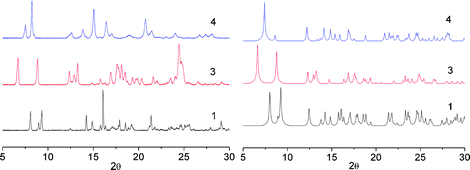 | ||
| Fig. 3 PXRD patterns obtained (left) and simulated (right) for the combination of tectons 1, 3 and 4 with ZnSiF6 showing the purity of the crystalline materials obtained. | ||
As expected from the choice of the inorganic tecton (ZnSiF6) and the design of the organic tectons 1, 3, 4 and 5, for all four cases structurally investigated, the formation of 2-D tubular coordination networks was observed (Fig. 2). In all four cases, the combination of Zn2+ with SiF62− leads to the formation of 1-D networks resulting from the bridging of Zn2+ cations by the SiF62− anions through Zn–F bonds (dZn–F in the range of 2.18–2.28 Å).
It is worth noting that the structural study on ZnSiF6·6H2O revealed that the Zn2+ cation does not form Zn–F bonds and remains coordinated to six water molecules.6 For the SiF62− moiety, the Si–F bond distance, for the F atoms located at the square base of the octahedron, is in the range of 1.65–1.68 Å which is shorter than those for the F atoms occupying the apical positions (dSi–F in the range of ca. 1.71–1.73 Å). The zinc cation also adopts the Oh geometry with its coordination sphere composed of two F and four N atoms. The two F atoms are located at the apical positions and the remaining four sites in the equatorial plane are occupied by four pyridine units belonging to four different organic tectons (dZn–N in the range of 2.08–2.10 Å). Whereas in the case of 1 (Fig. 2a) and 3 (Fig. 2c), the formation of linear pillars is observed (FZnF angle of 180°), the other two structures based on 4 (Fig. 2e) and 5 (Fig. 2g), display a puckered rod (FZnF angle of 174.5° and 171.4°, respectively). Within the 1-D networks, the distance between consecutive Zn2+ cations varies between 7.62 and 7.71 Å.
As expected from the design of tectons 1 and 3–5, which are based on flexible spacers capable of adopting a curved conformation but not able to bite the same metal centre, the interconnection of consecutive pillars leads to the formation of 2-D tubular architectures schematically presented in Fig. 1c. Alternatively, the 2-D coordination networks may be described as catenated cationic [2+2] metallamacrocycles (two Zn2+ cations and two ligands) forming 1-D networks interconnected by SiF62− anions. The size of the metallamacrocycles ([26]MC for 1, [30]MC for 3 and 4 and [36]MC for 5, reflected by the distance between the two endocyclic Zn2+ cations (12.09 Å, 14.51 Å, 14.53 Å and 16.50 Å for 1 and 3–5 respectively)), is controlled by the nature of the tectons adopting a gauche conformation (Fig. 2b, d, f and h). As a result of the design of the system imposing the parallel arrangement of metallamacrocycles by the use of the square planar base of the octahedron around the Zn2+ cation, the channels are parallel to the pillar axis. The channels are filled by solvent molecules (CHCl3 for 1, CHCl3 and EtOH for 3, 4 and 5) without any specific interactions with the tubular cavities. Whereas for structures engaging tectons 1 and 4, the solvent molecules could be located, in the cases of 3 and 5, they were found to be disordered and could not be refined and the SQUEEZE command was used.
The potential solvent accessible volumes, calculated using the Platon software,13 are 1128, 1626, 1670 and 2181 Å3, corresponding to 30.9%, 36.6%, 36.2% and 38.7% of the total unit cell volume for 1, 3, 4 and 5 respectively.
Along the direction perpendicular to the ZnSiF6 pillars, the arrangement of tubular 2-D networks follows the close packing principle by the formation of concave–convex mosaics (Fig. 4a). In the direction of the ZnSiF6 pillars, the consecutive planes are slipped by ca. 3.82 Å which corresponds to approximately half the distance between two consecutive zinc atoms (Fig. 4b). As a consequence of the packing of the 2-D networks, in all four cases, the crystal presents only tubular channels along the pillars.
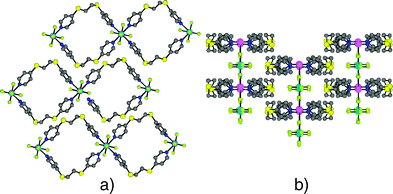 | ||
| Fig. 4 The packing of 2-D tubular planes 1·ZnSiF6 perpendicular to (a) and along (b) the ZnSiF6 pillars. H atoms are omitted for clarity. | ||
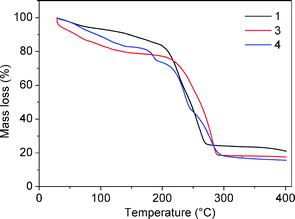
The thermal stability of the crystalline materials generated upon combining tectons 1, 3 and 4 with ZnSiF6 was investigated by taking TGA measurements in the range of 30–400 °C (under nitrogen, 2 °C per minute) (Fig. 5). This study revealed the loss of solvent molecules between ca. 30 and ca. 150 °C prior to decomposition of the samples which occurred at ca. 200 °C for 1 and 3 and at 180 °C for 4.
In conclusion, we have demonstrated that the combination of flexible bismonodentate organic tectons bearing two pyridines oriented in a divergent fashion with ZnSiF6 units forming a single stranded pillar, leads, by design, to the formation of 2-D coordination networks based on the interconnection of catenated zinc metallamacrocycles of various size by SiF62− anions. The packing of the sheets generates crystals offering only tubular channels oriented along the pillar axis. The shape as well as the size of the tubular channels may be modulated by the nature of the metallathiacrown ethers through the choice of the organic tectons. Extension to other functionalized flexible tectons and metal cations as well as the study of solvent uptake, exchange and release is currently under way.
We thank the Université de Strasbourg, the International Centre for Frontier Research in Chemistry (FRC), Strasbourg, the Institut Universitaire de France, the Ministry of Education and Research, the CNRS and Marie Curie Est Actions FUMASSEC Network (Contract No. MEST-CT-2005-020992) for financial support.
Notes and references
- B. F. Abrahams, B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1991, 113, 3606 CrossRef CAS.
- (a) A. J. Blake, N. R. Champness, P. Hubberstey, W.-S. Li, M. A. Withersby and M. Schröder, Coord. Chem. Rev., 1999, 183, 117 CrossRef CAS; (b) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (c) M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O’Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS; (d) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS; (e) S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (f) G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC; (g) D. Bradshaw, J. B. Claridge, E. J. Cussen, T. J. Prior and M. J. Rosseinsky, Acc. Chem. Res., 2005, 38, 273 CrossRef CAS.
- (a) G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS; (b) M. W. Hosseini, Chem. Commun., 2005, 5825 RSC.
- (a) A. F. Wells, Three-dimensional Nets and Polyhedra, Wiley, New York, 1977 Search PubMed; (b) M. W. Hosseini, CrystEngComm, 2004, 6, 318 RSC.
- (a) M. Simard, D. Su and J. D. Wuest, J. Am. Chem. Soc., 1991, 113, 4696 CrossRef; (b) S. Mann, Nature, 1993, 365, 499 CrossRef CAS; (c) M. W. Hosseini, Acc. Chem. Res., 2005, 38, 313 CrossRef CAS.
- S. Ray, A. Zalkin and D. H. Templeton, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1973, 29, 2741 CrossRef CAS.
- (a) R. A. J. Driessen, F. B. Hulsbergen, W. J. Vermin and J. Reedijk, Inorg. Chem., 1982, 21, 3594 CrossRef CAS; (b) F. S. Keij, R. A. G. De Graaff, J. G. Haasnoot and J. Reedijk, Inorg. Chim. Acta, 1989, 156, 65 CrossRef CAS; (c) S. Subramanian and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 1995, 34, 2127 CrossRef CAS; (d) S.-I. Noro, S. Kitagawa, M. Kondo and K. Seki, Angew. Chem., Int. Ed., 2000, 39, 2082 CrossRef CAS; (e) S.-I. Noro, R. Kitaura, M. Kondo, S. Kitagawa, T. Ishii, H. Matsuzaka and M. Yamashita, J. Am. Chem. Soc., 2002, 124, 2568 CrossRef CAS; (f) M.-C. Suen and J.-C. Wang, Struct. Chem., 2006, 17, 315 CrossRef CAS; (g) M.-C. Suen, Z.-K. Chan, J.-D. Chen, J.-C. Wang and C.-H. Hung, Polyhedron, 2006, 25, 2325 CrossRef CAS.
- M. J. Lin, A. Jouaiti, N. Kyritsakas and M. W. Hosseini, CrystEngComm, 2009, 11, 189 RSC.
- (a) G. Mislin, E. Graf, M. W. Hosseini, A. De Cian, N. Kyritsakas and J. Fischer, Chem. Commun., 1998, 2545 RSC; (b) M. Loï, M. W. Hosseini, A. Jouaiti, A. De Cian and J. Fischer, Eur. J. Inorg. Chem., 1999, 1981 CrossRef CAS; (c) C. Kleina, E. Graf, M. W. Hosseini, A. De Cian and J. Fischer, Chem. Commun., 2000, 239 RSC; (d) G. Laugel, E. Graf, M. W. Hosseini, J.-M. Planeix and N. Kyritsakas, New J. Chem., 2006, 30, 1340 RSC; (e) M. N. Kozlova, S. Ferlay, S. E. Solovieva, I. S. Antipin, A. I. Konovalov, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2007, 5126 RSC.
- (a) B. Schmaltz, A. Jouaiti, M. W. Hosseini and A. De Cian, Chem. Commun., 2001, 1242 RSC; (b) A. Jouaiti, M. W. Hosseini and N. Kyritsakas, Chem. Commun., 2003, 472 RSC; (c) P. Grosshans, A. Jouaiti, V. Bulach, J.-M. Planeix, M. W. Hosseini and J.-F. Nicoud, Chem. Commun., 2003, 1336 RSC; (d) J. Bourlier, M. W. Hosseini, J.-M. Planeix and N. Kyritsakas, New J. Chem., 2007, 31, 25 RSC.
- Y.-B. Xie, J.-R. Li, C. Zhang and X.-H. Bu, Cryst. Growth Des., 2005, 5, 1743 CrossRef CAS.
- (a) W. Rosen and D. H. Busch, J. Am. Chem. Soc., 1969, 91, 4694 CrossRef CAS; (b) D. Pocic, PhD thesis, Universite Louis Paster de Strasbourg, 2005 Search PubMed.
- A. L. Spek, Acta Crystallogr., Sect. A, 1990, 46, C34.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and crystallographic data (in cif format). CCDC 743171, 743172, 743173 and 743174. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b915665h |
| This journal is © The Royal Society of Chemistry 2010 |