Molecular tectonics: from 1-D interwoven racemic chains to quadruple-stranded helices†
Mei-Jin
Lin
,
Abdelaziz
Jouaiti
*,
Nathalie
Kyritsakas
and
Mir Wais
Hosseini
*
Laboratoire de Chimie de Coordination Organique (UMR 7140), Université de Strasbourg, Institut Le Bel, 4 rue Blaise Pascal, 67000, Strasbourg, France. E-mail: hosseini@chimie.u-strasbg.fr; Fax: +33 368 85 13 25; Tel: +33 368 85 13 23
First published on 27th October 2009
Abstract
The combination of two positional isomers tectons 1 and 2, based on a racemic 1,1′-spirobi(indane) scaffold bearing two pyridine units, with HgCl2 affords doubly interwoven and quadruple-stranded helical architectures, respectively.
The generation of helical assemblies is a topic of interest. These architectures, discrete or infinite in dimension, may either be purely organic or hybrid in nature engaging both organic parts and metallic centres.1 For the design of infinite helical coordination strands,2 which can be described as 1-D networks,3 one may apply the tectonic4 approach in designing specific helix inducing tectons. For example, the use of 1,1′-binaphthyl,5isomannide6 or calixarene7 based tectons has been reported. Another possibility consisting of introducing secondary interacting sites, such as oligoethylenglycol fragments, within the spacer connecting the primary coordination sites has also been explored.8 Another feature associated with the formation of helical architectures is the number of strands composing the assembly. Although single, double, and triple helices are relatively well known,2b there are very few examples of higher stranded assemblies.9
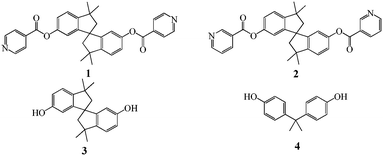
Here we report the design and synthesis of tectons 1 and 2 (Scheme 1) based on a 1,1′-spirobi(indane) scaffold bearing two pyridine units, as well as the formation of helical architectures by their combination with HgCl2.
 | ||
| Scheme 1 | ||
For the construction of helical architectures, the rigid 1,1′-spirobi(indane) is an interesting backbone.10 This unit is chiral and, due to the imposed angle between the two aromatic rings by the spiro centre, may be regarded as an analogue of binaphthol. We and others have already reported helical structures based on such a unit.5
The 1,1′-spirobi(indane) scaffold was functionalised with two pyridines as monodentate coordinating sites affording tectons 1 and 2. In both cases, the junction between the backbone and the pyridyl moiety was ensured by an ester group. Tectons 1 and 2 only differ by the position of the attachment of the pyridyl group to the spirobi(indane) moiety (position 4 for 1 and 3 for 2). For the synthesis of 1 and 2 as racemic mixtures, the starting material was bisphenol A, 4, which upon heating at 135 °C in the presence of methane sulfonic acid affords the diol3 in ca. 20% yield.10 In dry THF, the condensation of 3 with either the hydrochloride salt of isonicotinoyl chloride or nicotinoyl chloride in the presence of Et3N afforded the desired compounds 1 and 2 in 57 and 49% yields, respectively as racemic mixtures.‡
Since both 1 and 2 are neutral tectons, HgCl2 was used as the inorganic tecton for the generation of neutral helical strands.
At room temperature, a solution of tectons 1 or 2 in CHCl3 was layered with DMSO and then a solution of HgCl2 in EtOH was carefully added. Upon slow diffusion, colourless prism-type crystals, insoluble in common solvents, were obtained after several days. In both cases, crystals were analysed by X-ray diffraction on single crystals (XRD)†§ and by X-ray diffraction on powder (PXRD).
For 1·HgCl2, the structural study revealed that the crystal (Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) was composed of both enantiomers of 1 (Fig. 1a) and HgCl2. The interconnection of consecutive tectons 1 with the same chirality by HgCl2 leads to the formation of both P and M helical strands (Fig. 1b and c). For the 1,1′-spirobi(indane) backbone, an angle of 70.7° between the two phenyl groups is observed. The Hg2+ cation adopts a silghtly distorted square pyramidal geometry and its coordination sphere is composed of 2 Cl− anions (dHg–Cl = 2.34–2.35 Å, ClHgCl angle of 167.5°) and 3 N atoms (dHg–N = 2.59–2.72 Å, NHgN angles of 83.4° and 171.4°) belonging to three different tectons 1 (Fig. 1e). Among the 3 N atoms present in the coordination sphere of the Hg2+ cation, two belong to two consecutive tectons 1 of the same chiralty generating the helical strand (Fig. 1b–c) and the third one belongs to a second type of tecton 1 (angle between the two phenyl groups of the 1,1′-spirobi(indane) moiety of 78.8°) possessing the opposite handedness (Fig. 1d–f). The latter type behaves as an appended ligand and only one out of the two pyridine units are engaged in the binding of Hg2+ cation belonging to the helical strand (Fig. 1d). This is probably due to the rigidity of the backbone and to the junction between the scaffold and the pyridyl moiety in position 4. The overall structure is a 1-D interwoven architecture composed of two enantiomorphous helical strands (Fig. 1g).
) was composed of both enantiomers of 1 (Fig. 1a) and HgCl2. The interconnection of consecutive tectons 1 with the same chirality by HgCl2 leads to the formation of both P and M helical strands (Fig. 1b and c). For the 1,1′-spirobi(indane) backbone, an angle of 70.7° between the two phenyl groups is observed. The Hg2+ cation adopts a silghtly distorted square pyramidal geometry and its coordination sphere is composed of 2 Cl− anions (dHg–Cl = 2.34–2.35 Å, ClHgCl angle of 167.5°) and 3 N atoms (dHg–N = 2.59–2.72 Å, NHgN angles of 83.4° and 171.4°) belonging to three different tectons 1 (Fig. 1e). Among the 3 N atoms present in the coordination sphere of the Hg2+ cation, two belong to two consecutive tectons 1 of the same chiralty generating the helical strand (Fig. 1b–c) and the third one belongs to a second type of tecton 1 (angle between the two phenyl groups of the 1,1′-spirobi(indane) moiety of 78.8°) possessing the opposite handedness (Fig. 1d–f). The latter type behaves as an appended ligand and only one out of the two pyridine units are engaged in the binding of Hg2+ cation belonging to the helical strand (Fig. 1d). This is probably due to the rigidity of the backbone and to the junction between the scaffold and the pyridyl moiety in position 4. The overall structure is a 1-D interwoven architecture composed of two enantiomorphous helical strands (Fig. 1g).
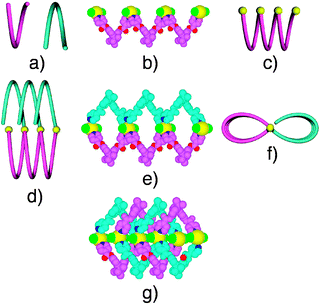 | ||
| Fig. 1 Schematic representation of the P (blue) and M (pink) helical configuration adopted by the tecton 1 (a), a portion of the helical strand generated upon bridging of consecutive tectons 1 by HgCl2 (b) and its schematic representation (c), schematic representation of helical strand bearing appended tectons 1 (d: perpendicular view and f: view along the helix axis), a portion of the structure (e) and a portion of the interwoven architecture (g). H atoms are omitted for clarity. | ||
For the second combination, i.e. tecton 2 and HgCl2, the structural analysis revealed that the crystal (trigonal, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) is composed of 2, HgCl2, 2H2O and 2CHCl3solvent molecules. The organic and inorganic tectons 2 and HgCl2 form helical strands (Fig. 2a) with opposite handedness. This is not surprising, since for 2, a racemic mixture of both enantiomers was used and consequently both P and M helical strands are found in the crystal. The metric analysis presented here concerns the strand with P helicity. As expected, except for the handedness, the same analysis holds for both strands. Within the helical strand composed of the same enantiomer, a torsion angle of 87.5° between the two phenyls is observed for the central 1,1′-spirobi(indane) scaffold. The two 3-pyridyl units connected to the backbone through ester junctions (dC–O = 1.36 Å and dC
c) is composed of 2, HgCl2, 2H2O and 2CHCl3solvent molecules. The organic and inorganic tectons 2 and HgCl2 form helical strands (Fig. 2a) with opposite handedness. This is not surprising, since for 2, a racemic mixture of both enantiomers was used and consequently both P and M helical strands are found in the crystal. The metric analysis presented here concerns the strand with P helicity. As expected, except for the handedness, the same analysis holds for both strands. Within the helical strand composed of the same enantiomer, a torsion angle of 87.5° between the two phenyls is observed for the central 1,1′-spirobi(indane) scaffold. The two 3-pyridyl units connected to the backbone through ester junctions (dC–O = 1.36 Å and dC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O = 1.20 Å) are divergently oriented towards the concave face of the scaffold and tilted by 18.7° (Fig. 2a).
O = 1.20 Å) are divergently oriented towards the concave face of the scaffold and tilted by 18.7° (Fig. 2a).
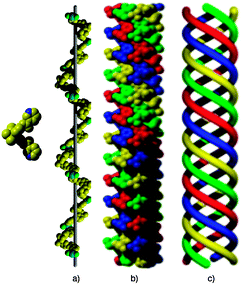 | ||
| Fig. 2 Portions of the structure of 2·HgCl2 showing the chiral tecton 2, the helical strand generated upon interconnection consecutive tectons 2 by HgCl2 (a), the quadruple-stranded helical architecture (b) and its schematic representation (c). Although both P and M helices are present in the crystal, arbitrary only the P handedness is presented. For clarity, different strands are differentiated by colour. H atoms and solvent molecules are omitted. | ||
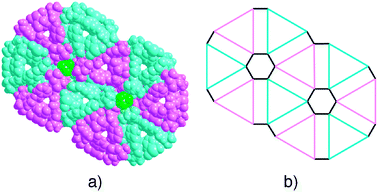
The Hg2+ cation adopts a distorted tetrahedral geometry and its coordination sphere is composed of two Cl− anions (dHg–Cl = 2.34 Å and ClHgCl angle of 154.5°) and the two N atoms (dHg–N = 2.45 Å, NHgN angle of 89.5° and ClHgN angle varying between 97.6° and 100.4°). Owing to the nearly perpendicular disposition of the spiro-annulated rings along the helical axis, the minimum translation unit (one tecton and one HgCl2) generates a helical rotation angle of 120° and thus the helical pitch of the helix, composed of three translation units, is ca. 43.47 Å (Fig. 2a). Probably, as a result of the long pitch, four strands of the same handedness form a quadruple stranded helical arrangement (Fig. 2b–c) with weak interstrand hydrogen-bonds between nicotinoyl units of different strands (distance between O and C atoms of ca. 3.14 Å). The quadruple stranded helices are not cylindrical but triangular in shape and are packed in a hexagonal fashion with alternated P and M chirality without any specific interactions between them (Fig. 3a–b).
 | ||
| Fig. 3 The cross section of the packing of 2·HgCl2 trigonal quadruple-stranded helices (a) and its schematic representation (b). For clarity, the P (blue) and M (pink) quadruple stranded helices are differentiated by colour. The Cl atoms of CHCl3 located at the centre of the hexagons are coloured in green. | ||
A view of the cross section parallel to the helical axis of the hexagonal packing of the triangular quadruple helical strands with opposite handedness (3P and 3M in alternate disposition), shows that each triangular column of a given chirality is in contact with three columns of the opposite chirality (Fig. 3b).
The packing of quadruple helical strands generates two types of channels. The inner triangular channels, with a side length of 8.8 Å, are empty, whereas the hexagonal channels with a side length of ca. 6.2 Å are found to be filled with two chloroform and two water molecules per mercury cation without any specific interaction with the helical walls.
The thermal stability of the crystalline materials was investigated by TGA which revealed that whereas 1·HgCl2 is stable up to ca. 250 °C, 2·HgCl2 starts to decompose at ca. 200 °C (Fig. 4).
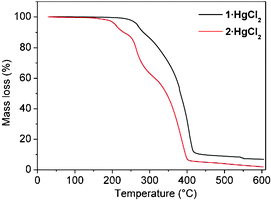 | ||
| Fig. 4 TGA traces for 1·HgCl2 and 2·HgCl2. For the latter case, the sample was dried in air for ca. 1 h. | ||
For the two crystalline materials, the purity was checked by PXRD which revealed a single set of diffraction peaks matching the simulated patterns (Fig. 5).
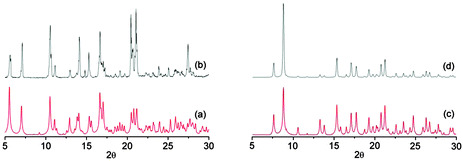 | ||
| Fig. 5 Comparison of the simulated (a and c) and recorded (b and d) PXRD patterns for 1·HgCl2 and 2·HgCl2, respectively. | ||
In conclusion, the 1,1′-spirobi(indane) backbone is a helix directing unit. The combination of racemic pyridine based tectons 1 and 2 with HgCl2, depending on the position of attachment of the coordinating sites to the scaffold, leads to the formation of either a double stranded interwoven helical arrangement or to a quadruple-stranded metallaorganic helix. The synthesis and propensity to generate helical architectures by other 1,1′-spirobi(indane) derivatives as well as combinations of 1 and 2 with other metallatectons are currently under investigation.
We thank the Université de Strasbourg, the Institut Universitaire de France, the Ministry of Education and Research, the CNRS and Marie Curie Est Actions FUMASSEC Network (Contrat N° MEST-CT-2005-020992) for financial support.
Notes and references
- (a) J.-M. Lehn, A. Rigault, J. Siegel, J. Harrowfield, B. Chevrier and D. Moras, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 2565 CAS; (b) C. Piguet, G. Bernardinelli and G. Hopfgartner, Chem. Rev., 1997, 97, 2005 CrossRef CAS; (c) C. Kaes, M. W. Hosseini, C. E. F. Rickard, B. W. Skelton and A. White, Angew. Chem., Int. Ed., 1998, 37, 920 CrossRef CAS; (d) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (e) M. Albrecht, Chem. Rev., 2001, 101, 3457 CrossRef CAS; (f) S. P. Anthony and T. P. Radhakrishnan, Chem. Commun., 2004, 1058 RSC.
- (a) E. D. Constable, in Comprehensive Supramolecular Chemistry, ed. J. Atwood, J. E. D. Davies, D. D. MacNicol and F. Vögtle, Pergamon, 1996, 9 (vol. ed. J. P. Sauvage and M. W. Hosseini), p. 213 Search PubMed; (b) L. Han and M. Hong, Inorg. Chem. Commun., 2005, 8, 406 CrossRef CAS; (c) D. Bradshaw, J. B. Claridge, E. J. Cussen, T. J. Prior and M. J. Rosseinsky, Acc. Chem. Res., 2005, 38, 273 CrossRef CAS.
- M. W. Hosseini, CrystEngComm, 2004, 6, 318 RSC.
- (a) S. Mann, Nature, 1993, 365, 499 CrossRef CAS; (b) M. Simard, D. Su and J. D. Wuest, J. Am. Chem. Soc., 1991, 113, 4696 CrossRef; (c) M. W. Hosseini, Acc. Chem. Res., 2005, 38, 313 CrossRef CAS.
- (a) Y. Cui, S. J. Lee and W. B. Lin, J. Am. Chem. Soc., 2003, 125, 6014 CrossRef CAS; (b) R. Wang, L. Xu, X. Li, Y. Li, Q. Shi, Z. Zhou, M. Hong and A. S. C. Chan, Eur. J. Inorg. Chem., 2004, 1595 CrossRef CAS; (c) C. D. Wu and W. Lin, Angew. Chem., Int. Ed., 2005, 44, 1958 CrossRef CAS; (d) A. Jouaiti, M. W. Hosseini, N. Kyritsakas, P. Grosshans and J.-M. Planeix, Chem. Commun., 2006, 3078 RSC; (e) J. Heo, Y.-M. Jeon and C. A. Mirkin, J. Am. Chem. Soc., 2007, 129, 7712 CrossRef CAS.
- (a) P. Grosshans, A. Jouaiti, V. Bulach, J.-M. Planeix, M. W. Hosseini and J.-F. Nicoud, Chem. Commun., 2003, 1336 RSC; (b) P. Grosshans, A. Jouaiti, V. Bulach, J.-M. Planeix, M. W. Hosseini and J.-F. Nicoud, CrystEngComm, 2003, 5, 414 RSC; (c) P. Grosshans, A. Jouaiti, V. Bulach, J.-M. Planeix, M. W. Hosseini and J.-F. Nicoud, C. R. Chim., 2004, 7, 189 CrossRef CAS.
- W. Jaunky, M. W. Hosseini, J.-M. Planeix, A. De Cian, N. Kyritsakas and J. Fischer, Chem. Commun., 1999, 2313 RSC.
- (a) B. Schmaltz, A. Jouaiti, M. W. Hosseini and A. De Cian, Chem. Commun., 2001, 1242 RSC; (b) A. Jouaiti, M. W. Hosseini and N. Kyritsakas-Gruber, Chem. Commun., 2003, 472 RSC; (c) J. Bourlier, M. W. Hosseini, J.-M. Planeix and N. Kyritsakas, New J. Chem., 2007, 31, 25 RSC.
- (a) R. H. Wang, Y. F. Zhou, Y. Q. Sun, D. Q. Yuan, L. Han, B. Y. Lou, B. L. Wu and M. C. Hong, Cryst. Growth Des., 2005, 5, 251 CrossRef CAS; (b) X. Zhang, X. Zhou and D. Li, Cryst. Growth Des., 2006, 6, 1440 CrossRef CAS; (c) G. O. Lloyd, J. L. Atwood and L. J. Barbour, Chem. Commun., 2005, 1845 RSC; (d) P. Byrne, G. O. Lloyd, K. M. Anderson, N. Clarke and J. W. Steed, Chem. Commun., 2008, 3720 RSC; (e) D. R. Xiao, Y. G. Li, E. B. Wang, L. L. Fan, H. Y. An, Z. M. Su and L. Xu, Inorg. Chem., 2007, 46, 4158 CrossRef.
- A. M. Kendhale, R. Gonnade, P. R. Rajamohanan, H. J. Hofmann and G. J. Sanjayan, Chem. Commun., 2008, 2541 RSC.
- G. M. Sheldrick, Programs for the Refinement of Crystal Structures, University of Göttingen, Göttingen, Germany, 1996 Search PubMed.
Footnotes |
| † CCDC 743764 and 743765. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b916249f |
| ‡ General method for the synthesis of organic tectons1 and 2: Under argon and at rt, to a degassed solution of 310 (1.54 g, 5 mmol) in dry THF (20 mL), the hydrochloride salt of isonicotinoyl chloride or nicotinoyl chloride (10 mmol) was added and the mixture was stirred at rt for 15 min before Et3N (5 mL) was added and stirring was further continued for two days. After evaporation to dryness, a saturated aqueous solution of Na2CO3 (40 mL) was added to the residue and the mixture extracted with CHCl3 (3 × 30 mL). The organic solvent was removed and the residue purified by column chromatography [SiO2, CHCl3], affording the pure compounds 1 and 2, respectively, as white powders. Tecton 1 (1.48 g, 57%): 1H-NMR (300 MHz, 298 K, CDCl3): δ [ppm] = 1.27–1.41 (d, 12H, J = 12), 2.30–2.45 (dd, 4H, J = 19.5, 13.2), 6.68 (d, 2H, J = 2.4), 7.1 (dd, 2H, J = 6, 2.1), 7.24 (m, 2H), 8.0 (dd, 4H, J = 3.0, 1.5), 8.8 (dd, 2H, J = 2.7, 1.5); 13C-NMR (75 MHz, 298 K, CDCl3): δ [ppm] = 30.2, 43.3, 57.6, 59.4, 116.9, 120.4, 122.9, 123.1, 127.9, 149.9, 150.3, 150.8, 151.7, 163.9; Tecton 2 (1.27 g, 49%): 1H-NMR (300 MHz, 298 K, CDCl3): δ [ppm] = 1.38–1.42 (d, 12H, J = 12), 2.31–2.46 (dd, 4H, J = 16.8, 13.2), 6.69 (d, 2H, J = 2.1), 7.07–7.10 (dd, 2H, J = 6, 2.1), 7.21–7.24 (d, 2H, J = 8.4), 7.40–7.44 (m, 2H), 8.37–8.41 (m, 2H), 8.81–8.83 (m, 2H), 9.33–9.34 (m, 2H); 13C-NMR (75 MHz, 298 K, CDCl3): δ [ppm] = 30.3, 43.3, 57.6, 59.4, 117.1, 120.6, 122.9, 123.4, 125.6, 137.5, 150.0, 150. 1, 151.7, 153.9, 164.0. |
§ Data were collected at 173(2) K on a Bruker APEX8 CCD Diffractometer equipped with an Oxford Cryosystem liquid N2 device, using graphite-monochromated Mo-Kα (λ = 0.71073) radiation. For both structures, diffraction data were corrected for absorption. The structures were solved using SHELXS-97 and refined by full matrix least-squares on F2 using SHELXL-97.11 The hydrogen atoms were introduced at calculated positions and not refined (riding model). Data for 1·HgCl2: C66H60Cl2HgN4O8, M = 1308.67, Triclinic, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.1808(12) Å, b = 14.0766(12) Å, c = 17.3666(15) Å, α = 67.326(2)°, β = 89.185(2)°, γ = 76.700(3)°, V = 2883.5(4) Å3, T = 173(2) K, Z = 2, Dc = 1.507 g cm−3, μ = 2.822 mm−1, 20 , a = 13.1808(12) Å, b = 14.0766(12) Å, c = 17.3666(15) Å, α = 67.326(2)°, β = 89.185(2)°, γ = 76.700(3)°, V = 2883.5(4) Å3, T = 173(2) K, Z = 2, Dc = 1.507 g cm−3, μ = 2.822 mm−1, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 466 collected reflections, 12 466 collected reflections, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 independent (Rint = 0.0379), GOF = 1.062, R1 = 0.0622, wR2 = 0.1613 for I > 2σ(I) and R1 = 0.0888, wR2 = 0.1738 for all data. 2·HgCl2: 6(C33H30Cl2HgN2O4)·2CHCl3·2H2O, M = 4983.25, Hexagonal, space group R 910 independent (Rint = 0.0379), GOF = 1.062, R1 = 0.0622, wR2 = 0.1613 for I > 2σ(I) and R1 = 0.0888, wR2 = 0.1738 for all data. 2·HgCl2: 6(C33H30Cl2HgN2O4)·2CHCl3·2H2O, M = 4983.25, Hexagonal, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, a = b = 39.9988(10) Å, c = 10.8667(3) Å, α = β = 90°, γ = 120°, V = 15056.4(7) Å3, T = 173(2) K, Z = 3, Dc = 1.649 g cm−3, μ = 4.878 mm−1, 120 c, a = b = 39.9988(10) Å, c = 10.8667(3) Å, α = β = 90°, γ = 120°, V = 15056.4(7) Å3, T = 173(2) K, Z = 3, Dc = 1.649 g cm−3, μ = 4.878 mm−1, 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 collected reflections, 3864 independent (Rint = 0.0305), GOF = 1.088, R1 = 0.0285, wR2 = 0.0750 for I > 2σ(I) and R1 = 0.0414, wR2 = 0.0881 for all data. 760 collected reflections, 3864 independent (Rint = 0.0305), GOF = 1.088, R1 = 0.0285, wR2 = 0.0750 for I > 2σ(I) and R1 = 0.0414, wR2 = 0.0881 for all data. |
| This journal is © The Royal Society of Chemistry 2010 |
