Planar [Ni7] discs as double-bowl, pseudo metallacalix[6]arene host cavities†
Seán T.
Meally
a,
Georgios
Karotsis
b,
Euan K.
Brechin
b,
Giannis S.
Papaefstathiou
c,
Peter W.
Dunne
a,
Patrick
McArdle
a and
Leigh F.
Jones
*a
aSchool of Chemistry, University Road, National University of Ireland, Galway, Ireland. E-mail: leigh.jones@nuigalway.ie; Tel: +353-091-49-3462
bSchool of Chemistry, Joseph Black Building, University of Edinburgh, West Mains Road, Edinburgh, Scotland, UK
cLaboratory of Inorganic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Zografou, 15771, Greece
First published on 1st October 2009
Abstract
We report three heptanuclear [Ni7] complexes with planar disc-like cores, akin to double-bowl metallocalix[6]arenes, which form molecular H-bonded host cavities.
Polymetallic complexes of paramagnetic 1st row transition metal ions are of great current interest since they often exhibit fascinating physical properties such as spin-crossover behaviour,1 long range ordering (i.e. in 1-, 2- and 3D coordination polymers2) and single-molecule magnet (SMM) behaviour.3 NiII in particular, has shown much promise in the synthesis of both single-molecule magnets (SMMs) and spin phonon traps; the former taking advantage of its significant single-ion anisotropy and the latter its paramagnetic nature when confined within a highly symmetric cage.4–6 In addition, the use of magnetic clusters as building blocks to create supramolecular architectures (i.e. discrete polyhedra7 and 1-, 2- and 3D polymers8) using both covalent and non-covalent interactions has led to materials whose physical properties can be rather different to that of their parent paramagnetic building blocks.9
An important factor in the construction of such assemblies is the choice of ligand, since this dictates not only cluster symmetry, topology and the number of paramagnetic metal ions present, but also the inter-molecular interactions between clusters in the crystal. Our own interest in this area has recently led us to investigate the coordination chemistry of the Schiff-base ligand 2-iminomethyl-6-methoxy-phenol (HL1)† and its bromo-analogue 2-iminomethyl-4-bromo-6-methoxy-phenol (HL2) (Fig. 1)† and herein report its initial coordination and supramolecular chemistry with NiII.
![(left) Structure of the ligands HL1 and HL2 (R = H (L1), Br (L2)). (right) Molecular structures of complexes 1 (top) and 2 (bottom) viewed perpendicular and parallel to the [Ni7] plane, respectively.](/image/article/2010/CE/b914538a/b914538a-f1.gif) | ||
| Fig. 1 (left) Structure of the ligands HL1 and HL2 (R = H (L1), Br (L2)). (right) Molecular structures of complexes 1 (top) and 2 (bottom) viewed perpendicular and parallel to the [Ni7] plane, respectively. | ||
Reaction of Ni(NO3)2·6H2O and HL1 in the presence of NaOH in EtOH produces the heptanuclear complex [Ni7(µ3-OH)6(L1)6](NO3)2 (1) in 30% yield. The green hexagon shaped crystals of 1 crystallize in the trigonal space group P-3c1 (Fig. 1).‡ Heptanuclear complex 1 possesses a core comprising a hexagon of NiII ions surrounding a central NiII centre. The central NiII ion (Ni1) is located at a site with imposed ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) symmetry while the nitrogen atom (N2) of the NO3− group lies on a threefold axis. The remainder of the asymmetric unit comprises a second NiII centre (Ni2) along with one L1− unit and one hydroxy group (O1–H1) occupying general positions. Although topologically analogous [Mn7],10 [Fe7]11 and [Co7]12 complexes are known, the synthesis of 1 represents the first nickel complex to possess a planar hexagonal disc-like structure. All Ni ions are in distorted octahedral geometries with the six µ3-bridging OH− ions (O1) linking the central nickel (Ni1) to the six peripheral nickel ions (Ni2); each trigonal pyramidal OH− ion being situated alternately above and below the [Ni7] plane (Fig. 1).
symmetry while the nitrogen atom (N2) of the NO3− group lies on a threefold axis. The remainder of the asymmetric unit comprises a second NiII centre (Ni2) along with one L1− unit and one hydroxy group (O1–H1) occupying general positions. Although topologically analogous [Mn7],10 [Fe7]11 and [Co7]12 complexes are known, the synthesis of 1 represents the first nickel complex to possess a planar hexagonal disc-like structure. All Ni ions are in distorted octahedral geometries with the six µ3-bridging OH− ions (O1) linking the central nickel (Ni1) to the six peripheral nickel ions (Ni2); each trigonal pyramidal OH− ion being situated alternately above and below the [Ni7] plane (Fig. 1).
The anionic ligands L1− (singly deprotonated at the phenolate site) bridge the peripheral NiII centres adopting a µ2-η1:η2:η1 coordination motif, lying alternately above and below the [Ni7] plane. The result is a double-bowl conformation in which the [Ni7] core is the basal plane, reminiscent of a metallocalix[6]arene concave unit (Fig. 1). Close inspection of the double-bowl conformation shows approximate bowl dimensions of (base × depth × rim diameter) 6.20 × 4.21 × 11.70 Å. In the crystal the [Ni7] units stack on top of one another resulting in a unit cell possessing four psuedo-superimposable 1D columns of [Ni7] units with each unit linked by a 120° rotation. The [Ni7] units are held into 1D columnar arrays via zig-zag shaped belts of NO3− anions (each comprising six NO3− ions) which sit above and below the individual heptanuclear complexes with C–H⋯O bonding interactions between the NO3− oxygen atoms (one unique, O4) and protons (H1A and H5) of the L1− ligands (H1A⋯O4 = 2.59 Å and H5⋯O4 = 2.44 Å). These NO3− belts thus effectively ‘zip-up’ pairs of [Ni7] moieties to form molecular cavites (each of approximate volume ∼155.9 Å3 with a [Ni7]plane-[Ni7]plane distance of 11.635 Å),13 formed by two juxtaposed pseudo metallocalix[6]arene [Ni7] bowl units. In addition they also H-bond to adjacent 1D [Ni7] columns thus completing the 3D connectivity in the unit cell (Fig. SI1). From a topological point of view, each [Ni7] is H-bonded to twelve NO3− with the latter being connecting six [Ni7] units thus creating a (6,12)-connected net with a (415)2(448.618)-alb topology (Fig. SI 2†).14,15
The H-bonded molecular cavities formed in the crystals of 1 are empty. Investigation of these enclosures as potential host cavities towards small molecule guest inclusion led to the formation of the analogous hexanuclear complex [Ni7(OH)6(L1)6](NO3)2·3MeNO2 (2), formed by dissolution of 1 in MeNO2 in ∼15% yield.† Complex 2 crystallises in the same trigonal P-3c1 space group as 1 and thus also possesses a central NiII (Ni1) with imposed ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) symmetry and a N atom (of the NO3− counter anion) lying on a threefold rotation axis (N2). Complex 2 also exhibits similar bowl dimensions of 6.20 × 4.08 × 12.04 Å while the [Ni7]plane-[Ni7]plane distance of 11.371 Å in 2 is only marginally larger than observed in 1 (11.635 Å). Indeed complex 2 differs with respect to 1 only in that the H-bonded cavities in 2 are of the required size and shape (calculated volume of ∼ 322.8 Å3) to accommodate three guest MeNO2 solvent molecules (Fig. 2). These are related crystallographically via a three fold rotation and interact within the cavity via H-bonding interactions between their O atoms (O5 and O6) and the nearby µ3-OH− groups on each of the two [Ni7] units which form the cavity floors (O1⋯O5 = 3.08 Å; O1⋯O6 = 3.25 Å). As commonly observed when small molecules are located within such highly symmetrical molecular cavities,16 there is crystallographic disorder of the trigonal planar MeNO2 molecules whereby the methyl carbon atom (C10) lies on a twofold axis (see CIF for full details). When taking steric effects into account, these orientations are most likely to exist in the up-down-up anti-parallel configuration with respect to the three fold rotation symmetry they share (Fig. 2). In a similar manner to that found in 1 the NO3− ions and [Ni7] units are connected by means of C–H⋯O H-bonds (H1A⋯O4 = 2.58 Å, H2⋯O4 = 2.56 Å and H5⋯O4 = 2.43 Å) to create the alb network (Fig. SI2).†
symmetry and a N atom (of the NO3− counter anion) lying on a threefold rotation axis (N2). Complex 2 also exhibits similar bowl dimensions of 6.20 × 4.08 × 12.04 Å while the [Ni7]plane-[Ni7]plane distance of 11.371 Å in 2 is only marginally larger than observed in 1 (11.635 Å). Indeed complex 2 differs with respect to 1 only in that the H-bonded cavities in 2 are of the required size and shape (calculated volume of ∼ 322.8 Å3) to accommodate three guest MeNO2 solvent molecules (Fig. 2). These are related crystallographically via a three fold rotation and interact within the cavity via H-bonding interactions between their O atoms (O5 and O6) and the nearby µ3-OH− groups on each of the two [Ni7] units which form the cavity floors (O1⋯O5 = 3.08 Å; O1⋯O6 = 3.25 Å). As commonly observed when small molecules are located within such highly symmetrical molecular cavities,16 there is crystallographic disorder of the trigonal planar MeNO2 molecules whereby the methyl carbon atom (C10) lies on a twofold axis (see CIF for full details). When taking steric effects into account, these orientations are most likely to exist in the up-down-up anti-parallel configuration with respect to the three fold rotation symmetry they share (Fig. 2). In a similar manner to that found in 1 the NO3− ions and [Ni7] units are connected by means of C–H⋯O H-bonds (H1A⋯O4 = 2.58 Å, H2⋯O4 = 2.56 Å and H5⋯O4 = 2.43 Å) to create the alb network (Fig. SI2).†
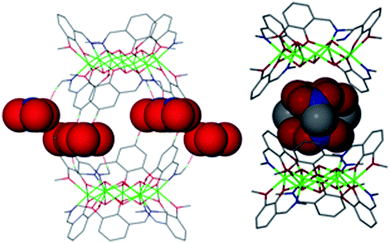 | ||
| Fig. 2 Molecular structures of 1 and 2 in the crystal highlighting the empty cavity and belt of NO3− anions in 1 (left) and the disordered guest MeNO2 molecules in 2 (right) within the host cavities. | ||
In an attempt to alter the size and shape of our molecular cavities and to probe whether we are able to control or alter its subsequent guest preferences, we decided to increase the bowl depth (cf.1 and 2) by employing the Br-analogue of HL1 in the form of the pro-ligand 2-iminomethyl-4-bromo-6-methoxy-phenol (HL2).†
This led to the formation of [Ni7(OH)6(L2)6](NO3)2·2MeCN (3) which was formed in ∼23% yield and crystallises in the monoclinic C2/c space group.‡ The NiII ion (Ni4) located at the centre of [Ni7] disk lies on an inversion centre while the remaining three metal centres (Ni1-3) and all other atoms in the asymmetric unit occupy general positions. Our hypothesis regarding changing cavity size was proved correct as the crystal structure shows the formation of a deeper bowl of dimesions 6.22 × 6.18 × 11.90 Å. Also apparent is that the individual [Ni7] units again stack into superimposable 1D columns, in this instance propagating along the b direction of the unit cell (Fig. SI3).† The stacking of the [Ni7] units along b is supported by two complementary O–H⋯Br interactions which involve one µ3-OH− (H1) of a [Ni7] unit and the Br1 of a neighbouring cluster (H1⋯Br1 = 2.82 Å). More interestingly these 1D columnar stacks of [Ni7] units are linked by means of C–H⋯Br interactions via the Br atoms (Br2 and Br3 and s.e) of the bridging ligands (L2−) and –CH3 (H18B and H27B) protons of juxtaposed [Ni7] moieties (H18B⋯Br3 = 2.93 Å, H27B⋯Br2 = 2.70 Å and s.e) giving rise to a 10-connected net with a (312.428.55)-bct topology (Fig. SI4).14,15 These interactions give rise to molecular cavities which are tilted with respect to the [Ni7] planes and are interlocked in a staggered arrangement (Fig. 3). The [Ni7]plane-[Ni7]plane distance inside the cavity is 11.135 Å and represents a cavity height reduction of ∼0.5 Å cf.1 and 2. This may be attributed to the H-bonding affinity of the pendant Br-atoms (Br1) in 3, leading to a more tightly bound cavity. The approximate area of this enclosure is ∼265.9 Å3 which is larger than that within 1 (155.9) and smaller than that within 2 (322.8). As in 2, these H-bonded molecular cavities act as hosts for the encapsulation of guest solvent molecules. In this case, each cavity accommodates two MeCN molecules (large spheres in Fig. 3) which exhibit a head-to-tail conformation and are held in place through H-bonding via their N atoms (N5) with the proton (H3A) of an µ3-OH− bridging ion belonging to the nearby paramagnetic [Ni7(OH)6] core (N5⋯H3A(O3) = 2.36 Å). Efforts to encapsulate MeCN and MeNO2 solvent guests inside the cavities of 2 and 3, respectively, were unsuccessful. We may therefore hypothesise that guest molecules can only be placed within these cavities if and when they are able to orientate themselves into certain topologies comprising symmetry elements compatible with their hosts crystal lattices (Fig. 4).
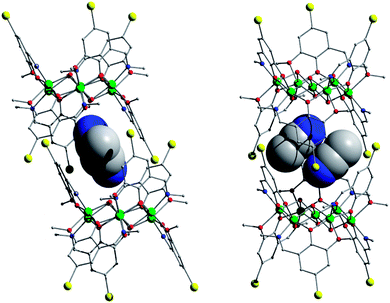 | ||
| Fig. 3 Molecular structures of 3 in the crystal showing the slightly tilted molecular cavity accommodating guest MeCN pairs (space-filled). | ||
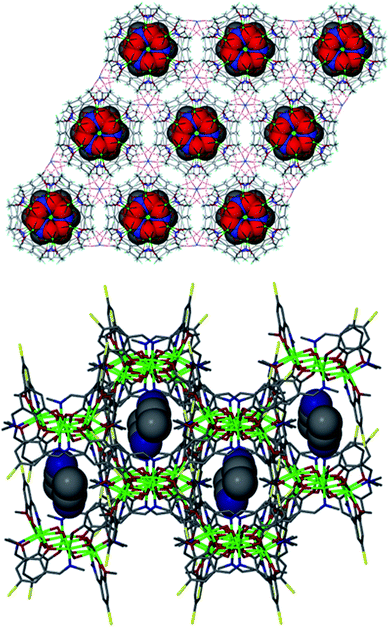 | ||
| Fig. 4 Crystal packing observed in 2 (top) and 3 (bottom) showing the molecular cavities accommodating guest MeNO2 (red spheres) and MeCN (grey/blue spheres) solvent molecules, respectively. NO3− counter anions omitted for clarity in both cases. | ||
IR spectroscopic studies on the host complexes 2 and 3 were performed to ascertain whether their guest molecules remained within their respective H-bonded cavities on drying. CHN analysis of both complexes were consistent with guest residency (ESI). The IR spectrum of 2 gave peaks at 1337 and 1555 cm−1 which are characteristic for the asymmetric and symmetric NO stretching of the guest MeNO2 molecules, respectively. Similarly a weak resonance at 2258 cm−1 (CN stretch) in the IR spectrum of 3 indicated the presence of the enclosed MeCN guest molecules. The TG trace of [Ni7(OH)6(L1)6](NO3)2·3NO2Me (2) exhibits four distinct weight loss regions, with the initial weight loss of 9.82% corresponding to the loss of the three nitromethane moieties (calculated as 10.17%) across the temperature range of 112 °C to 140 °C. The second weight loss step (of 6.30%) between 178 and 217 °C is consistent with the loss of 2 nitrates (calculated as 6.80%), while the third weight loss step, beginning at 320 °C can be attributed to the loss of two L1 ligands and upon further heating the decomposition of the remaining combustible materials occurs (SI5).†
Initial magnetic measurements indicate weak ferromagnetic exchange between the metal centres; the data obtained for 1 and 3 is plotted in Fig. 5. The room temperature χMT value of 7.76 cm3 mol−1 (1) and 7.90 cm3 K mol−1 (3) are consistent with that expected for 7 non-interacting NiII ions with g = 2.1 (∼7.7 cm3 K mol−1). As the temperature is decreased the value of χMT increases slowly, reaching maximum values of ∼8.5 cm3 K mol−1 at 40 K for 1 and ∼10 cm3 K mol−1 at 25 K for 3, before decreasing below these temperatures to minimum values of 5.5 cm3 K mol−1 and 7.9 cm3 K mol−1, respectively at 5 K. The observed behaviour is suggestive of very weak ferromagnetic intramolecular exchange, with the low temperature (T < 40 K) decrease in χMT ascribed to relatively strong inter-molecular antiferromagnetic exchange, consistent with the packing of the [Ni7] molecules in the crystal. Indeed the maxima in χMT for both complexes are well below that expected for an isolated S = 7 spin ground state (38 cm3 K mol−1 for g = 2.00). A fit of the 1/χMversusT using only the 300–50 K data affords Weiss constants (Θ) of +18.7 K (1) and 29.0 K (3) (Fig. SI6).† The exchange interactions are likely much smaller than the single ion zfs (weak exchange limit) and thus the multiple low lying states cannot properly be described as total S states. This picture is also reflected in the magnetisation versus field data (collected in the ranges 0.5–7.0 T and 2–7 K and plotted in the inset of Fig. 5) which shows M increasing only slowly with H, rather than quickly reaching saturation as one would expect for an isolated spin ground state. This is indicative of the population of low lying levels with a smaller magnetic moment, which only become depopulated with the application of a large field, and so we cannot describe the system within the giant spin approximation.
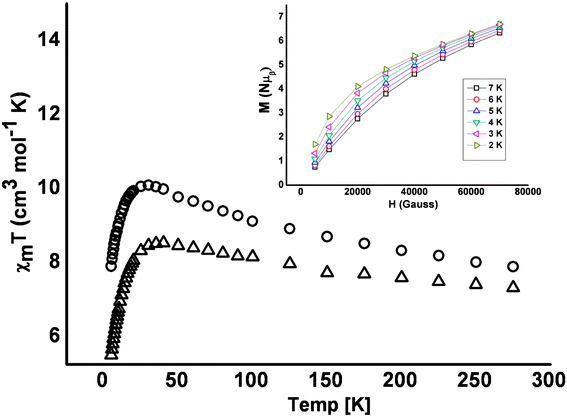 | ||
| Fig. 5 Plot of χMTvs.T for complexes 1 (△) and 3 (○) measured in the 300–5 K temperature range in an applied field of 0.1 T. (inset) Plot of magnetisation (MNµβ) vs. H (Gauss) for 1 obtained in the 7–2 K temperature range. | ||
Guest detection on 2 and 3 using 1H NMR proved inconclusive due to significant spectral broadening and therefore the diamagnetic ZnII analogues to the host/guest complexes 2 and 3 are currently being sought in order to assess their dynamic solution behaviour using NMR titration methods.16 Work on functionalising HL1 and HL2 to alter the size and/or shape of the resultant molecular cavities in order to incorporate species such as anions, cations and fluorescent molecules towards molecular sensor materials is currently underway.
Acknowledgements
We wish to thank the NUI Galway Millennium Fund (S.M./L.F.J.) and the Special Account for Research Grants of the National and Kapodistrian University of Athens (G.S.P.). We would also like to thank Dermot McGrath (NUIG) for the TGA analysis.Notes and references
- (a) K. S. Murray, Eur. J. Inorg. Chem., 2008, 3101–3121 CrossRef CAS; (b) A. B. Gaspar, M. Munoz, M. Carmen and J. A. Real, J. Mater. Chem., 2006, 16, 2522–2533 RSC; (c) A. Hauser, J. Jeftic, H. Romstedt, R. Hinek and H. Spiering, Coord. Chem. Rev., 1999, 190–192, 471–491 CrossRef CAS.
- (a) R. Robson, Dalton Trans., 2008, 5113 RSC; (b) S. R. Batten and K. S. Murray, Coord. Chem. Rev., 2003, 246, 103–130 CrossRef CAS; (c) C. Janiak, Dalton Trans., 2003, 2781–2804 RSC; (d) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS.
- (a) R. Sessoli, D. Gatteschi, A. Caneshi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS; (b) R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS.
- (a) J. Krzystek, J.-H. Park, M. W. Meisel, M. A. Hitchman, H. Stratemeier, L.-C. Brunel and J. Tesler, Inorg. Chem., 2002, 41, 4478 CrossRef CAS; (b) G. Rogez, J. N. Rebilly, A. L. Barra, L. Sorace, G. Blondin, N. Kirchner, M. Duran, J. van Slageren, S. Parsons, L. Ricard, A. Marvilliers and T. Mallah, Angew. Chem., Int. Ed., 2005, 44, 1876 CrossRef CAS.
- For nickel based polymetallic complexes (inc. SMMs) see: (a) M. S. El Fallah, E. Rentschler, A. Caneshi, R. Sessoli and D. Gatteschi, Inorg. Chem., 1996, 35, 3723 CrossRef; (b) T. S. Stamatatos, E. Diamantopoulou, C. P. Raptopoulou, V. Psycharis, A. Escuer and S. P. Perlepes, Inorg. Chem., 2007, 46, 2350–2352 CrossRef CAS; (c) E. Diamantopoulou, C. P. Raptopoulou, A. Terzis, V. Tangoulis and S. P. Perlepes, Polyhedron, 2002, 21, 2117 CrossRef CAS; (d) M. Murrie, H. Stoeckli-Evans and Hans U. Güdel, Angew. Chem., Int. Ed., 2001, 40, 1957 CrossRef CAS; (e) S. T. Ochsenbein, M. Murrie, E. Rusanov, H. Stoeckli-Evans, C. Sekine and Hans U. Güdel, Inorg. Chem., 2002, 41, 5133 CrossRef CAS; (f) T. C. Stamatatos, K. A. Abboud, S. P. Perlepes and G. Christou, Dalton Trans., 2007, 3861 RSC.
- S. Caretta, P. Santini, G. Amoretti, M. Affronte, A. Candini, A. Ghirra, I. S. Tidmarsh, R. H. Laye, R. Shaw and E. J. L. McInnes, Phys. Rev. Lett., 2006, 97, 207201 CrossRef CAS.
- (a) W. Wernsdorfer, N. Aliaga-Alcalde, D. N. Hendrickson and G. Christou, Nature, 2002, 416, 406 CrossRef; (b) S. Hill, R. S. Edwards, N. Aliaga-Alcalde and G. Christou, Science, 2003, 302, 1015 CrossRef CAS; (c) C. C. Stoumpos, R. Inglis, G. Karotsis, L. F. Jones, A. Collins, S. Parsons, C. J. Milios, G. S. Papaefstathiou and E. K. Brechin, Cryst. Growth Des., 2009, 9, 24–27 CrossRef CAS.
- (a) E. E. Moushi, T. C. Stamatatos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2006, 45, 7722–7725 CrossRef CAS; (b) H. Miyasaka, K. Nakata, L. Lecren, C. Coulon, Y. Nakazawa, T. Fujisaki, K. Sugiura, M. Yamashita and R. Clerac, J. Am. Chem. Soc., 2006, 128, 3770–3783 CrossRef CAS; (c) T. C. Stamatatos, K. M. Poole, D. Foguet-Albiol, K. A. Abboud, T. A. O'Brien and G. Christou, Inorg. Chem., 2008, 47, 6593–6595 CrossRef CAS; (d) L. F. Jones, A. Prescimone, M. Evangelisti and E. K. Brechin, Chem. Commun., 2009, 2023–2025 RSC.
- (a) C. Boskovic, R. Bircher, P. L. W. Tregenna-Piggott, H. U. Güdel, C. Paulsen, W. Wernsdorfer, A.-L. Barra, E. Khatsko, A. Neels and H. Stoeckli-Evans, J. Am. Chem. Soc., 2003, 125, 14046 CrossRef CAS; (b) J. Yoo, W. Wernsdorfer, E.-C. Yang, M. Nakano, A. L. Rheingold and D. N. Hendrickson, Inorg. Chem., 2005, 44, 3377 CrossRef CAS.
- (a) N. C. Harden, M. A. Bolcar, W. Wernsdorfer, K. A. Abboud, W. E. Streib and G. Christou, Inorg. Chem., 2003, 42, 7067 CrossRef CAS; (b) M. A. Bolcar, S. M. J. Aubin, K. Folting, D. N Hendrickson and G. Christou, Chem. Commun., 1997, 1485 RSC; (c) B. Pilawa, M. T. Kelemen, S. Wanka, A. Geisselmann and A. L. Barra, Europhys. Lett., 1998, 43, 7 CrossRef CAS.
- H. Oshio, N. Hoshino, T. Ito, M. Nakano, F. Renz and H. Gütlich, Angew. Chem., Int. Ed., 2003, 42, 223 CrossRef CAS.
- S.-H. Zhang, Y. Song, H. Liang and M.-H. Zeng, CrystEngComm, 2009, 11, 865 RSC.
- L. J. Barbour, MCAVITY: Program for calculating the molecular volume of closed capsules, University of Missouri-Columbia, Columbia, MO, USA, 2003, http://www.x-seed.net/cavity.html. The free volume within the cavities of 2 and 3 was calculated in the absence of guests Search PubMed.
- V. A. Blatov, IUCr CompComm Newsletter, 2006, 4–38 Search PubMed.
- M. O'Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782 CrossRef CAS.
- (a) N. P. Power, S. J. Dalgarno and J. L. Atwood, Angew. Chem., Int. Ed., 2007, 46, 8601–8604 CrossRef CAS; (b) M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS and references herein; (c) M. D. Pluth and K. N. Raymond, Chem. Soc. Rev., 2007, 36, 161–171 RSC; (d) A. Pastor and E. Martínez-Vivente, Coord. Chem. Rev., 2008, 252, 2314–2345 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details on the synthesis of 1–3 and ligands L1 and L2. CCDC reference numbers 741051–741053. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b914538a |
| ‡ Crystal data for 1: C54H66N8O24Ni7, M = 1622.12, trigonal, space group P-3c/1, a = b = 13.806(2), c = 23.270(5) Å, α = 90, β = 90, γ = 120°, V = 3841.2(11) Å3, T = 150(2) K, Z = 2, Dc = 1.402 g cm3, 2306 reflections collected of which 1376 were unique (Rint = 0.0802), R1 [I > 2σ(I)] = 0.1209, wR2 = 0.2446 (F2, all data). Crystal data for 2.3NO2Me: C57H75N11O30Ni7, M = 1709.25, trigonal, space group P-3c/1, a = b = 13.933(2), c = 22.742(5) Å, α = 90, β = 90, γ = 120°, V = 3823.4(11) Å3, T = 150(2) K, Z = 2, Dc = 1.485 g cm3, 2333 reflections collected of which 1835 were unique (Rint = 0.0502), R1 [I > 2σ(I)] = 0.0604, wR2 = 0.1725 (F2, all data). Crystal data for 3.2MeCN: C58H66Br6N10O24Ni7, [2(C2H3N), 2(NO3)], M = 2177.64, monoclinic, space group C2/c, a = 28.8575(14), b = 11.1352(3), c = 27.4079(13) Å, α = 90, β = 109.603(3), γ = 90.00°, V = 8296.6(7) Å3, T = 150(2) K, Z = 4, Dc = 1.743 g cm3, 7348 reflections collected of which 3779 were unique (Rint = 0.0511), R1 [I > 2σ(I)] = 0.1090, wR2 = 0.1325 (F2, all data). CCDC 722288–722291. |
| This journal is © The Royal Society of Chemistry 2010 |
