pH-Controlled change of the coordination modes of the highly symmetrical multitopic ligand and metal–oxygen arrays for constructing coordination assemblies†‡
Bo
Zheng
,
Junfeng
Bai
* and
Zhuxiu
Zhang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, P. R. China
First published on 4th September 2009
Abstract
For the first time, the effect of the pH value on the coordination assemblies of a highly symmetrical multitopic ligand has been systematically investigated, indicating that higher pH values lead to increase of the coordination ability of the ligand, the amount of hydroxide incorporated into the structures, and the degree of condensation of metal polyhedra in the resulting 3D networks.
Coordination assemblies are of high interest due to their fascinating topologies and interesting properties.1 However, the controllable synthesis of MOFs is a great challenge because many factors play an important role in their self-assembly, such as the chemical structure of the ligands chosen,2a the coordination geometry preferred by the metal,2b reaction temperature,2c the counterions,2d the solvent system,2e pH value, the metal-to-ligand ratio,2f and the method of crystallization.2g As one of the external stimuli, the pH value is especially important in the assembly of the MOFs, leading to a variation of coordination ability or configuration conversion of the ligand,3a an introduction of a hydroxide group,3b change in the coordination environment at the metal centers,3c and new topologies based on the variation of the metal-to-ligand ratio,3d and consequently, the resulting structures.3e However, systematic investigation of the pH value effect on the coordination assemblies of highly symmetrical multi-topic ligands and nuclearity variation of metal–oxygen arrays still remains rare. Furthermore, pH dependent supramolecular isomerism of coordination networks has also been less explored.3f
We are interested in constructing coordination polymers of highly symmetrical multitopic units with novel topologies and properties.4 By varying the concentration of the solution, we have isolated inorganic fullerene-like molecules, 1D or 2D coordination polymers based on the highly symmetrical [Cp*Fe(η5-P5)].4a Recently, temperature in a limited range (5–50 °C) was reported by us to control finely and reversibly the coordination modes of the ligands (from monodentate to tetradentate) and the coordination assemblies.4e Alongside our work, herein a systematic investigation of the pH value effect on the coordination assemblies based on squaric acid, a highly symmetrical multitopic ligand, has been carried out. The hydrothermal reactions of Cd(NO3)2·4H2O, squaric acid and water under similar reaction conditions but with different amounts of KOH gave the previously reported hydrated complex Cd(C4O4)(H2O)2 (1)4e,5 and three anhydrous complexes Cd3(C4O4)2(OH)2 (2), Cd2(C4O4)(OH)2 (3) and Cd2(C4O4)(OH)2 (4), respectively (Fig. 1). The results show that with increase of the pH value, the coordination mode of the squarate is changed from tetradentate in 1 to hexadentate in 2–4, and the isolated Cd(II) ions are replaced with the infinite M–O–M arrays. Interestingly, the arrays change from two types of 2D M–O–M layers in 2 and 3 to 1D denser double M–O–M chains in 4, while rearranging both edge- and corner-sharing Cd octahedra, and consequently, increasing the density of the networks.
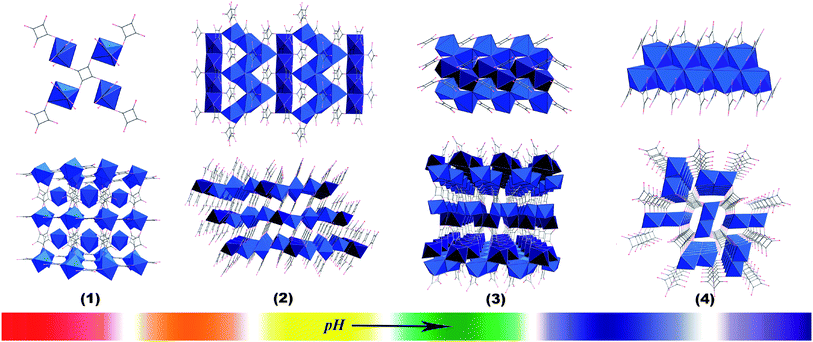 | ||
| Fig. 1 Progression of complexes 1–4 from a low pH value (far left) to a high pH value (far right), showing a trend toward higher coordination ability of the ligand and greater inorganic connectivities. Gray spheres denote carbon, while red oxygen, with CdO6 octahedra in light blue and dark blue chains. | ||
When the initial molar ratio of the reactants, (Cd2+/H2C4O4/KOH) was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, complex 1 was obtained at the lowest pH (pH = 3). Complex 1 is a 3D network structure with a typical NbO topology and was first reported by Maji et al.,5 which consists of isolated cadmium atoms that are octahedrally coordinated by four squarates at the basal positions and two water molecules at the apical positions. Each squarate binds to four different cadmium centers and functions as a µ4-(κ4 O1
6, complex 1 was obtained at the lowest pH (pH = 3). Complex 1 is a 3D network structure with a typical NbO topology and was first reported by Maji et al.,5 which consists of isolated cadmium atoms that are octahedrally coordinated by four squarates at the basal positions and two water molecules at the apical positions. Each squarate binds to four different cadmium centers and functions as a µ4-(κ4 O1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O3
O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O4) bridging ligand (Fig. S1). Complex 2 was obtained by varying the reactant ratio to 2
O4) bridging ligand (Fig. S1). Complex 2 was obtained by varying the reactant ratio to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 (pH = 4). X-Ray structure analysis shows that 2 crystallizes in monoclinic form with an asymmetric unit consisting of one formula, and therefore there are three crystallographically independent Cd(II) atoms. However, the coordination environments about the cadmium atoms are similar, which are all coordinated in an octahedral geometry with four oxygen atoms from four different squarate ligands in the equatorial positions and two µ3-OH groups in the apical positions. It should be noted that there exist two types of metal hydroxide-based chains in 2 (Fig. S4).† Each Cd(1)O6 octahedron shares its two pairs of 60° adjacent edges with four neighboring Cd(2) octahedra to form interesting zigzag chains, while the Cd(3) octahedra form straight linear chains in singles via sharing the trans-edges. The cadmium atoms are arranged with Cd–Cd distances of 3.5155(4) Å for the zigzag chains, and 3.5876(3) Å for the straight chain. Such two types of chains running parallel are further linked to each other by sharing hydroxides to form a novel layer parallel to the bc plane. The squarate ligands adopt two different coordination modes, which can be classified as µ6-(κ6 O1
7.5 (pH = 4). X-Ray structure analysis shows that 2 crystallizes in monoclinic form with an asymmetric unit consisting of one formula, and therefore there are three crystallographically independent Cd(II) atoms. However, the coordination environments about the cadmium atoms are similar, which are all coordinated in an octahedral geometry with four oxygen atoms from four different squarate ligands in the equatorial positions and two µ3-OH groups in the apical positions. It should be noted that there exist two types of metal hydroxide-based chains in 2 (Fig. S4).† Each Cd(1)O6 octahedron shares its two pairs of 60° adjacent edges with four neighboring Cd(2) octahedra to form interesting zigzag chains, while the Cd(3) octahedra form straight linear chains in singles via sharing the trans-edges. The cadmium atoms are arranged with Cd–Cd distances of 3.5155(4) Å for the zigzag chains, and 3.5876(3) Å for the straight chain. Such two types of chains running parallel are further linked to each other by sharing hydroxides to form a novel layer parallel to the bc plane. The squarate ligands adopt two different coordination modes, which can be classified as µ6-(κ6 O1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O1
O1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O3
O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O4) and µ6-(κ6 O1
O4) and µ6-(κ6 O1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O3
O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O4
O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O4) to bridge the layers to form the 3D structure. To the best of our knowledge, it is unprecedented that the two different µ6-hexadentate coordination modes adopted by the squarate ligand are observed within the same structure, simultaneously.6
O4) to bridge the layers to form the 3D structure. To the best of our knowledge, it is unprecedented that the two different µ6-hexadentate coordination modes adopted by the squarate ligand are observed within the same structure, simultaneously.6
By providing more base corresponding to the ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 and 2
8.5 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, two polymorphic complexes 3 and 4 could be isolated (pH = 4.5 and 6), which crystallize in the orthorhombic Pbca and the monoclinic P21/c. Although there is the same fixed stoichiometry for all components in 3 and 4, in which all squarate ligands adopt the same µ6-(κ6 O1
10, two polymorphic complexes 3 and 4 could be isolated (pH = 4.5 and 6), which crystallize in the orthorhombic Pbca and the monoclinic P21/c. Although there is the same fixed stoichiometry for all components in 3 and 4, in which all squarate ligands adopt the same µ6-(κ6 O1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O3
O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O4
O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O4) bridging coordination mode, the variation of metal oxide connectivity of edge- and corner-sharing Cd(II) octahedra leads to the resulting supramolecular isomerism. The cadmium(II) atom in 3 is coordinated in an octahedral geometry with three oxygen atoms of three different squarate ligands and three µ3-OH group (Fig. S5).† Each Cd atoms is connected through cis-edge-sharing CdO6 octahedron connection to form another different zigzag chain compared with those observed in complex 2 with Cd–Cd distances of 3.545(1) Å. These chains are further linked with each other via the corner-sharing µ3-OH to generate the novel 2D cadmium hydroxide layers parallell to the ab plane. Each squarate ligand acts in the µ6-(κ6 O1
O4) bridging coordination mode, the variation of metal oxide connectivity of edge- and corner-sharing Cd(II) octahedra leads to the resulting supramolecular isomerism. The cadmium(II) atom in 3 is coordinated in an octahedral geometry with three oxygen atoms of three different squarate ligands and three µ3-OH group (Fig. S5).† Each Cd atoms is connected through cis-edge-sharing CdO6 octahedron connection to form another different zigzag chain compared with those observed in complex 2 with Cd–Cd distances of 3.545(1) Å. These chains are further linked with each other via the corner-sharing µ3-OH to generate the novel 2D cadmium hydroxide layers parallell to the ab plane. Each squarate ligand acts in the µ6-(κ6 O1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O3
O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O4
O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O4) mode, by which the adjacent layers are pillared to construct a 3D coordination network along the c-axis (Fig. S6).† The asymmetric unit of 4 consists of one formula unit, which is isostructural with the reported [M2(C4O4)(OH)2] with M = Fe(II), Mn(II).6a,6b Each Cd(II) is coordinated in a slightly distorted octahedral geometry with three oxygen atoms from three different squarate ligands and three µ3-OH group (Fig. S7).† Each Cd atom is connected to four neighboring Cd atoms via sharing two pair of 60° adjacent edges, leading to the formation of 1D denser double cadmium hydroxide chains. Interestingly, the Cd⋯Cd separations are 3.4138(9) Å in the single chains and 3.3722(9) Å between the chains. The double-chain units are arranged in parallel with the a-axis and connected by squarate bridges to give a 3D framework (Fig. S8).†
O4) mode, by which the adjacent layers are pillared to construct a 3D coordination network along the c-axis (Fig. S6).† The asymmetric unit of 4 consists of one formula unit, which is isostructural with the reported [M2(C4O4)(OH)2] with M = Fe(II), Mn(II).6a,6b Each Cd(II) is coordinated in a slightly distorted octahedral geometry with three oxygen atoms from three different squarate ligands and three µ3-OH group (Fig. S7).† Each Cd atom is connected to four neighboring Cd atoms via sharing two pair of 60° adjacent edges, leading to the formation of 1D denser double cadmium hydroxide chains. Interestingly, the Cd⋯Cd separations are 3.4138(9) Å in the single chains and 3.3722(9) Å between the chains. The double-chain units are arranged in parallel with the a-axis and connected by squarate bridges to give a 3D framework (Fig. S8).†
In our case, the pH value is the key factor influencing the formation of 3D coordination polymers based on the highly symmetric multitopic ligand. Qualitatively, this can be easily seen in the structures presented in Table 1. At the lowest pH value, the aqua ligand favors the coordinaiton environment of the isolated cadmium atom and the coordination mode of the squarate favors the tetradentate in 1. Increasing the pH values in this series leads to isolation of three fully dehydrated metal hydroxide structures, 2–4, in which all the squarate ligands act as a hexadentate ligand, while the amount of hydroxide incorporated into the structures appears to follow a clear progression about the degree of metal polyhedra condensation. Moreover, the isolation of the two polymorphic complexes 3 and 4 indicates that the formation of the compact M–O–M linkages with increasing edge-sharing connectivity is favored at a higher pH, and consequently leads to increasing density of the networks.
| Molar ratioa | pH | Complex | Coordination mode of squarate | OH−/Cd2+ | Edge-sharingb | Dimensionalityc | Cd2+/103 Å | Density/g cm−3 |
|---|---|---|---|---|---|---|---|---|
| a The number in the Molar ratio column refers to the molar ratio of the reactants, Cd(NO3)2/H2C4O4/KOH. b The number of the Edge-sharing column refers to the edges in each Cd(II) octahedron shared with neighbours. c The first number in the Dimensionality column refers to the total dimensionality, the second to the M–O–M dimensionality. | ||||||||
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
3 | Cd(C4O4)(H2O)2(1) | Tetradentate | 0 | 0 | 3(0) | 5.03 | 2.175 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
4 | Cd3(C4O4)2(OH)2(2) | Hexadentate | 0.67 | (4)(2)(2) | 3(2) | 11.14 | 3.671 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 8.5 |
4.5 | Cd2(C4O4)(OH)2(3) | Hexadentate | 1 | (2) | 3(2) | 12.60 | 3.880 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
6 | Cd2(C4O4)(OH)2(4) | Hexadentate | 1 | (4) | 3(1) | 12.59 | 3.876 |
In summary, for the first time, the effect of the pH value on the coordination assemblies of a highly symmetric multitopic ligand has been systematically investigated, which clearly indicates that with increasing the pH value, the coordination mode of the squarate is changed from tetradentate in 1 to hexadentate in 2–4 and the degree of condensation of the metal polyhedra is increased (from isolated Cd(II) ions in 1 and 2D M–O–M layers in 2 and 3 to 1D denser M–O–M chains in 4). Interestingly, 3 and 4 are unprecedented supramolecular isomers due to the edge-sharing and corner-sharing octahedra of metal oxide connectivity. Our work will further facilitate the crystal engineering of functional coordination polymers based on highly symmetrical multitopic ligands.
The authors gratefully acknowledge support from the Major State Basic Research Development Programs (Nos. 2006CB806104 and 2007CB936302), the NSFC (No. 20771058), the Science Foundation of Innovative Research Team of NSFC (No. 20721002), and the Specialized Research Fund for the Doctoral Program of the Ministry of Education of China (No. 200802840011).
Notes and references
- (a) S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS; (b) C. N. R. Rao, S. Natarajan and R. Vaidhyanathan, Angew. Chem., Int. Ed., 2004, 43, 1466–1496 CrossRef CAS; (c) G. Férey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 217–225 CrossRef CAS; (d) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS; (e) M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319–330 CrossRef CAS.
- (a) Y. T. Wang, H. H. Fan, H. Z. Wang and X. M. Chen, Inorg. Chem., 2005, 44, 4148–4150 CrossRef CAS; (b) L. Carlucci, G. Ciani, D. M. Proserpio and S. Rizzato, New J. Chem., 2003, 27, 483–489 RSC; (c) P. M. Forster, A. R. Burbank, C. Livage, G. Férey and A. K. Cheetham, Chem. Commun., 2004, 368–369 RSC; M. L. Tong, S. Kitagawa, H. C. Chang and M. Ohba, Chem. Commun., 2004, 418–419 RSC; P. Mahata, M. Prabu and S. Natarajan, Inorg. Chem., 2008, 47, 8451–8463 CrossRef CAS; (d) G. H. Cui, J. R. Li, J. L. Tian, X. H. Bu and S. R. Batten, Cryst. Growth Des., 2005, 5, 1775–1780 CrossRef CAS; (e) I. S. Lee, D. MokShin and Y. K. Chung, Chem.–Eur. J., 2004, 10, 3158–3165 CrossRef CAS; (f) X. Q. Lu, J. J. Jiang, C. L. Chen, B. S. Kang and C. Y. Su, Inorg. Chem., 2005, 44, 4515–4521 CrossRef; (g) D. M. Shin, I. S. Lee, D. Cho and Y. K. Chung, Inorg. Chem., 2003, 42, 7722–7724 CrossRef CAS.
- (a) Y. J. Kim and D. Y. Jung, Chem. Commun., 2002, 908–909 RSC; W. Bi, R. Cao, D. Sun, D. Yuan, X. Li, Y. Wang, X. Li and M. Hong, Chem. Commun., 2004, 2104–2105 RSC; L. Zhang, J. Zhang, Z. J. Li, Y. Y. Qin, Q. P. Lin and Y. G. Yao, Chem.–Eur. J., 2009, 15, 989–1000 CrossRef CAS; Q. Yu, X. Q. Zhang, H. D. Bian, H. Liang and S. P. Yan, Cryst. Growth Des., 2008, 8, 1140–1146 CrossRef CAS; S. T. Wu, L. S. Long, R. B. Huang and L. S. Zheng, Cryst. Growth Des., 2007, 7, 1746–1752 CrossRef CAS; (b) L. F. Ma, L. Y. Wang, D. H. Lu, S. R. Batten and J. G. Wang, Cryst. Growth Des., 2009, 9, 1741–1749 CrossRef CAS; (c) C. T. Yang, B. Moubaraki, K. S. Murray and J. J. Vittal, Dalton Trans., 2003, 880–889 RSC; C. T. Yang and J. J. Vittal, Inorg. Chim. Acta, 2003, 344, 65–76 CrossRef CAS; (d) C. Liu, F. Luo, W. P. Liao, D. Q. Li, X. F. Wang and R. Dronskowski, Cryst. Growth Des., 2007, 7, 2282–2285 CrossRef; (e) J. Q. Sha, J. Peng, Y. Q. Lan, Z. M. Su, H. J. Pang, A. X. Tian, P. P. Zhang and M. Zhu, Inorg. Chem., 2008, 47, 5145–5153 CrossRef CAS; (f) P. K. Chen, Y. X. Che, L. Xue and J. M. Zheng, J. Solid State Chem., 2006, 179, 2656–2662 CrossRef CAS.
- (a) J. Bai, A. V. Virovets and M. Scheer, Science, 2003, 300, 781–783 CrossRef CAS; (b) J. Bai, A. V. Virovets and M. Scheer, Angew. Chem., Int. Ed., 2002, 41, 1737–1740 CrossRef CAS; (c) M. Scheer, A. Schindler, R. Merkle, B. P. Johnson, M. Linseis, R. Winter, C. E. Anson and A. V. Virovets, J. Am. Chem. Soc., 2007, 129, 13386–13387 CrossRef CAS; (d) B. Zheng, H. Dong, J. Bai, Y. Z. Li, S. H. Li and M. Scheer, J. Am. Chem. Soc., 2008, 130, 7778–7779 CrossRef CAS; (e) S. N. Wang, H. Xing, Y. Z. Li, J. Bai, M. Scheer, Y. Pan and X. Z. You, Chem. Commun., 2007, 2293–2295 RSC.
- T. K. Maji, G. Mostafa, S. Sain, J. S. Prasad and N. R. Chaudhuri, CrystEngComm, 2001, 3, 155–158 RSC.
- (a) D. S. Yufit, D. J. Price, J. A. K. Howard, S. O. H. Gutschke, A. K. Powell and P. T. Wood, Chem. Commun., 1999, 1561–1562 RSC; (b) J. C. Trombe, L. Sabadie and P. Millet, Solid State Sci., 2002, 4, 1209–1212 CrossRef CAS; (c) S. O. H. Gutschke, M. Molinier, A. K. Powell and P. T. Wood, Angew. Chem., Int. Ed. Engl., 1997, 36, 991–992 CrossRef CAS.
Footnotes |
† Electronic supplementary information (ESI) available![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Detailed experimental procedures, structural description, PXRD patterns, and TGA of 1–4. CCDC reference numbers 732255 and 732257 for complexes 2–4. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b917258k Detailed experimental procedures, structural description, PXRD patterns, and TGA of 1–4. CCDC reference numbers 732255 and 732257 for complexes 2–4. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b917258k |
| ‡ Crystal data for 1: C4H4O6Cd, Mr = 260.47, rhombohedral, space group R-3, a = 11.7673(9), b = 11.7673(9), c = 14.9293(16) Å, α = 90, β = 90, γ = 120°, V = 1790.3(3) Å3, Z = 9, µ = 2.726 mm−1, Dc = 2.174 Mg m−3, F(000) = 1116, T = 291(2) K, 3051 reflections collected, 746 unique with Rint = 0.0776, R1 = 0.0270, wR2 = 0.0596 (I > 2σ(I)) and GOF = 1.103. Crystal data for 2: C8H2O10Cd3, Mr = 595.33, monoclinic, space group C 2/m, a = 13.2366(13), b = 7.1753(6), c = 12.7690(12) Å, α = 90, β = 117.344(2), γ = 90°, V = 1077.25(17) Å3, Z = 4, µ = 5.925 mm−1, Dc = 3.671 Mg m−3, F(000) = 1096, T = 291(2) K, 2915 reflections collected, 1094 unique with Rint = 0.0319, R1 = 0.0221, wR2 = 0.0586 (I > 2σ(I)) and GOF = 1.063. Crystal data for 3: C2HO3Cd, Mr = 185.43, orthorhombic, space group Pbca, a = 6.8958(3), b = 5.8147(3), c = 15.8331(8) Å, α = 90, β = 90, γ = 90°, V = 634.86(5) Å3, Z = 8, µ = 6.680 mm−1, Dc = 3.880 Mg m−3, F(000) = 680, T = 291(2) K, 3062 reflections collected, 597 unique with Rint = 0.0607, R1 = 0.0200, wR2 = 0.0441 (I > 2σ(I)) and GOF = 1.075. Crystal data for 4: C4H2O6Cd2, Mr = 370.86, monoclinic, space group P21/c, a = 3.5793(3), b = 10.4878(9), c = 8.5834(7) Å, α = 90, β = 99.5510(10), γ = 90°, V = 317.75(5) Å3, Z = 2, µ = 6.673 mm−1, Dc = 3.876 Mg m−3, F(000) = 340, T = 291(2) K, 1675 reflections collected, 559 unique with Rint = 0.0607, R1 = 0.0275, wR2 = 0.0546 (I > 2σ(I)) and GOF = 0.975. |
| This journal is © The Royal Society of Chemistry 2010 |
