Antioxidant, anti-inflammatory and anti-browning activities of hot water extracts of oriental herbal teas
Li-Chen
Wu
*a,
Amily Fang-Ju
Jou
b,
Si-Han
Chen
b,
Chia-Ying
Tien
b,
Chih-Fu
Cheng
a,
Nien-Chu
Fan
b and
Ja-an Annie
Ho
*bc
aDepartment of Applied Chemistry, National Chi Nan University, Puli, Nantou, 545, Taiwan. E-mail: lw25@ncnu.edu.tw; Fax: +886-49-2917956
bDepartment of Chemistry, National Tsing Hua University, Hsin-chu, 300, Taiwan
cDepartment of Biochemical Science and Technology, National Taiwan University, Taipai, 106, Taiwan. E-mail: jaho@ntu.edu.tw; Fax: +886-2-3366-2271
First published on 4th October 2010
Abstract
Traditionally, antioxidants are used to scavenge reactive oxygen species (ROS), which are harmful by-products of aerobic metabolism. Inulae Flos, Horsetail, Chinese Leucas, Broomweed and Indian Wikstroemia are five herbal teas commonly consumed by Asians. Our aim was to investigate the hot water extracts of these five herbal teas for their total phenolics/flavonoid contents and antioxidant capacities. Furthermore, with inflammation and hyper-pigmentation considered as two biological processes associated with elevated cellular oxidative stress, Inulae Flos water extract was chosen for further evaluation of its inhibitory effects on the production of LPS-induced inflammatory mediators (such as, TNF-α, IL-6, IL-1β) in RAW 264.7 cells and its anti-tyrosinase activity. Our findings suggest that Inulae Flos might be an alternative source as a potential antioxidant, and a noteworthy inhibitor of production of pro-inflammatory cytokines in a dose-dependent manner. Moreover, it could also serve as a potential natural food additive to prevent browning.
Introduction
Many nutritional supplements on the market are derived from herbal extracts for their health benefits, such as antioxidation and immunomodulation.1,2,3,4 An increasing number of natural dietary supplement products are being developed in response to the increasing focus of consumers in terms of personal health. It is known that excess reactive oxygen species (ROS) tend to attack susceptible biomolecules such as nucleic acid and protein, causing oxidative imbalance of the antioxidant system. The resulting oxidative stress may lead to aging, inflammation, and other chronic diseases.5,6 It has also been reported that a wide array of diseases, ranging from coronary heart disease to cancer, are caused by inflammation.7 Undoubtedly oxidative stress underlies many human diseases, and the exploration of new, rich sources of natural antioxidants for scavenging cellular free radicals has become the top priority in developing functional foods or nutritional supplements.The consumption of herbal tea, particularly green tea, has been proven to scavenge free radicals and thus effectively reduce the risks of various chronic diseases due to its abundance of antioxidants.8–13 Antioxidants can therefore be regarded as a natural anti-inflammatory approach which helps improve health and reduce inflammation without the use of prescription medicine. Therefore, there has been an increasing interest in searching for new antioxidant nutraceuticals which have the potential health benefits to present a substitution for corticosteroids (a common anti-inflammation drug) as an approach to get rid of the root cause that leads to inflammations.
Antioxidants such as vitamin C, vitamin E, carotenoids and polyphenolics have been found for their additional bio-function as inhibitors of tyrosinase. Tyrosinase inhibitors have important applications in the food industry and the field of biomedicine. Tyrosinase inhibitors not only possess anti-browning ability in the processing of meats, vegetables and fruits,14–16 but they also exert anti-melanogenesis in mammalian cells. Tyrosinase, a multifunctional copper-containing enzyme (mono- and diphenolase activities) catalyzes melanin synthesis via the hydroxylation of tyrosine to o-diphenol and the oxidation of o-diphenol to highly reactive o-quinones,17 which then spontaneously polymerize to form compounds of high molecular weight or brown pigments, or undergo nucleophilic attack by amino acids and proteins, polyphenols, or water to form Michael type addition products18 that enhance the production of the brown color. Browning in foods, on the other hand, occurs in the presence of oxygen when the polyphenol oxidase (PPO) converts phenolic compounds into dark colored pigments. Since the fresh-cut produce (i.e., fruits and vegetables) industry is one of the fastest growing food sectors of the market, and chlorine solutions are used extensively to sanitize and extend the shelf-life of such products, concerns have been raised toward the possible formation of carcinogens from such chlorine usage. Though sodium sulfite, citric acid, cysteine, potassium sulfite, sulfur dioxide, sodium meta-bisulfite and oxyresveratrol are existing alternative commonly-used food additives that function to prevent browning in foods (i.e., fruits, vegetables and meats), substantial research efforts in exploring alternative sources of anti-browning agents are still been encouraged.14,19
Inulae Flos, Horsetail, Chinese Leucas, Broomweed, and Indian Wikstroemia, found in Asia, Europe and Pacific islands, are normally used as herbal teas for the treatment of sore throats, bronchitis, and digestive disorders by Asians. However, little information is available on the inhibitory effects of these herbal teas on antioxidation and inflammation activity.20 In this study, we quantified the hot water extracts of five commonly-used herbal teas which grew locally in Taiwan for their total phenolic content, flavonoid content, and antioxidant capacity. Moreover, studies were conducted to evaluate their inhibitory effect on the production of LPS-induced inflammatory mediators (such as IL-6, IL-1β, and TNF-α) in RAW 264.7 cells. In addition, the hot water extract of Inulae Flos was chosen to be investigated for its potential anti-tyrosinase activity.
Materials and methods
Reagents and materials
All herbs, Inulae Flos, Equisetum spp. (Horsetail), Leucas chinensis (Chinese Leucas), Scoparia dulcis L. (Broomweed), Wikstroemia indica (L.) C. A. Mey. (Indian Wikstroemia), were obtained from a local market in Kaohsiung, Taiwan. Ascorbic acid, ascorbate oxidase, aluminium chloride anhydrous (AlCl3), 2-2′-azinobis-3-ethylbenzthiazoline-6-sulfonate (ABTS), bovine serum albumin (BSA), catechin, DL-3,4-dihydroxyphenylalanine (DL-DOPA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 3-(4,5-dimethylthiazaol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethylsulfoxide (DMSO), Folin-Ciocalteau phenol reagent, ferric trichloridehexahydrate (FeCl3·6H2O), gallic acid, Griess reagent (modified), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), sodium nitrite (NaNO2), sodium acetate (CH3COONa), Tween-20, 2,4,6-tripyridyl-s-triazine (TPTZ) and 3,3′,5,5′-tetramethyl benzidine (TMB) liquid substrate system for ELISA were purchased from Sigma Chemical (St. Louis, MO, USA). Dulbecco's modified Eagles's medium (DMEM), mushroom tyrosinase and penicillin/streptomycin solution were obtained from Hyclone (Logan, Utah, USA). Fetal bovine serum (FBS) was acquired from Biological Industries (Kibbutz BeitHaemek, Israel). Raw 264.7 cell line was obtained from the Culture Collection and Research Center (CCRC, Hsinchu, Taiwan). Anhydrous sodium carbonate (Na2CO3) and sodium hydroxide (NaOH) were purchased from Merck (Darmstadt, Germany). Acetic acid (CH3COOH) and potassium persulfate (K2S2O8) were purchased from J. T. Baker (Phillipsburg, NJ, USA). Hydrochloric acid (HCl) was purchased from Riedel-de Haën (Seelze, Germany). Sulfuric acid (H2SO4) was purchased from Fluka (Buchs, Switzerland). Mouse IL-1β, and mouse IL-6 were purchased from Invitrogen (Carlsbad, CA, USA). Mouse TNF-α ELISA was purchased from Bender Medsystems (Burlingame, CA, USA).Sample preparation - extraction and isolation
The extraction of five herbal teas was initiated by soaking the dried herbs (50 g) with distilled and deionized water (D. D. water, 400 mL; resistivity not less than 18 MΩ·cm). Heating individual herbal tea under reflux at 100 °C for 3 h was then carried out. After filtration, the supernatant was collected, followed by lyophilization to obtain extract powder. All crude extracts were stored in airtight packaging at −20 °C until use.Apparatus
Lyophilization was conducted using a Thermo Electron Corporation lyophilizer (Model ModulyoD-115, Waltham, MA, USA). The absorbance (optical density) of each sample was measured using the microplate reader (Sunrise™, Tecan Trading AG, Switzerland). The structural characterization of natural flavonoids presented in the Inulae Flos herbal tea extract was conducted by Fourier Transform Mass Spectrometry (Varian 901-MS, Palo Alto, CA, USA). The HPLC system was controlled by the Agilent Technologies 1200. Separation and retention studies were carried out on a LiChroCART®125-4 RP-18 (5 μm, 250 mm × 2mm i.d.) column, which was purchased from Merck (Darmstadt, Germany). HPLC measurements were performed using the methanol–water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) mobile phase at a flow rate of 0.8 mL min−1. An isocratic elution was used for analysis of naringenin and amentoflavone. All injection volumes were 20 μL. The detection of naringenin and amentoflavone was carried out using UV-Vis spectrophotometry set at a wavelength of 283 nm and 370 respectively.
30 v/v) mobile phase at a flow rate of 0.8 mL min−1. An isocratic elution was used for analysis of naringenin and amentoflavone. All injection volumes were 20 μL. The detection of naringenin and amentoflavone was carried out using UV-Vis spectrophotometry set at a wavelength of 283 nm and 370 respectively.
Determination of total phenolic content
The total phenolic content of the five oriental herbal tea extracts was determined using a modified Folin-Ciocalteu colorimetric method. In short, 0.125 mL of a known dilution of extract was mixed with 0.5 mL of Folin-Ciocalteu reagent, and allowed to react for six min. It was followed by the addition of 1.25 mL, 7% Na2CO3 solution to the mixture, and the total was brought up to 3 mL with D. D. water, and the color development was allowed to occur after 90 min. Finally the absorbance of reaction mixture was read at 760 nm. All tests were in triplicate. The measurement was compared to a calibration curve of prepared gallic acid solutions and expressed as gallic acid equivalent in milligrams.4Determination of flavonoid content
The flavonoid content was determined using a modified colorimetric method, in which 0.25 mL of the known dilution of extract was diluted with 1.25 mL of D. D. water, followed by the addition of a 75 μL 5% NaNO2 solution and allowed to stand for 5 min. Subsequently 150 μL of a 10% AlCl3 solution was added. After 6 min, 0.5 mL of 1 M NaOH solution was put in, and the mixture was diluted with another 0.0275 mL of D. D. water. Immediately after the solution was well-mixed, the absorbance of the solution at 510 nm was measured in comparison with the standard curve of prepared catechin solutions. All tests were in triplicate. The results were expressed as milligrams of catechin equivalents.1,4DPPH˙ radical-scavenging activity
The radical-scavenging activity was determined using the DPPH˙ method described previously.4 In brief, 0.5 mL of the extract dissolved in 80% ethanol was reacted with ethanolic 0.5 mM DPPH˙ solution (0.25 mL) and 100 mM acetate buffer (pH 5.5, 0.5mL). An 80% ethanol solution was used as a blank solution; the ethanolic 0.5 mM DPPH˙ solution, in the absence of sample or standard, served as the control. The decrease in absorbance of DPPH at 517 nm after 30 min of standing was measured. All tests were performed in triplicate. The antioxidant activity of the test sample is expressed as the median effective concentration for radical-scavenging activity (EC50), the amount of tested extract (mg) required for a 50% decrease in absorbance of DPPH radicals, expressed in terms of ascorbic acid equivalents. The inhibition (%) of DPPH absorbance was calculated based on the expression (Acontrol − Atest) × 100%/Acontrol, where Acontrol is the absorbance of the control (containing only DPPH˙ solution) and Atest is the absorbance of the test sample. The absorbance of DPPH˙ was plotted against the antioxidant concentrations as standard curves to calculate EC50. Results of the assay are expressed in terms of vitamin C equivalents.ABTS˙+ assay
ABTS˙+ reagent was prepared by mixing 2.45 mM potassium persulfate (K2S2O8) with 7 mM ABTS salt in 0.01 M phosphate-buffered saline (PBS, pH 7.4) and allowed to react for 16 h at room temperature in the dark. The resultant ABTS˙+ radical cation was diluted with 0.01 M PBS (pH 7.4) to give an absorbance of ca. 0.70 at 734 nm. The standards and sample extracts were diluted 100-fold with ABTS˙+ solution to a total volume of 1 mL and allowed to react for 3 more min. Control (with no standard or sample) was used as a blank and 0.99 mL of PBS were added to these controls instead. All tests were in triplicate. The inhibition (%) of ABTS˙+ absorbance was calculated based on the expression (Acontrol − Atest) × 100%/Acontrol. The absorbance of ABTS˙+ was plotted against the antioxidant concentrations as standard curves to calculate EC50. Trolox, the water-soluble α-tocopherol (vitamin E) analog, served as a standard, and the results of the assay were expressed relative to trolox in terms of TEAC (trolox equivalent antioxidant capacity).4FRASC assay
Working FRASC reagent was first prepared by mixing 80 μL of 300 mM acetate buffer with 10 μL of 10 mM TPTZ (2,4,6-tripyridyl-s-triazine), which was dissolved in 40 mM HCl; and 10 μL of 20 mM FeCl3·6H2O. One pair well set is used to account for the presence of total antioxidant in the sample (so-called experimental well), and another well is used to represent the background (so-called background well). 10 μL of D. D. water was added into the antioxidant wells (experiment wells), while 10 μL of 4 IU mL−1 ascorbate oxidase was added into the background wells. Ascorbic acid served as the standard and was prepared to generate 0, 2, 4, 6, 8, 10 nmol of ascorbic acid/well.21 100 μL of test samples or ascorbic acid standards were dispensed into a paired set of wells in a 96-well plate. After the addition of 100 μL of the working FRASC reagent into the wells containing either ascorbic acid standard or test samples, the measurement of absorbance at 593 nm was taken within 2–3 min. All tests were in triplicate. The ascorbic acid concentration in the sample (mM) was calculated based on the expression of (Ae − Ac) × 100/slope of the standard curve, where Ae is the absorbance of the experiment well, and Ac is the absorbance of the control well with ascorbate oxidase.Cell culture
The mouse macrophage cell line Raw 264.7 were used in all experiments, which were maintained at 37 °C and 5% CO2 in Dulbecco's modified Eagles' medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution. Cells were plated at a density of 5 × 104 cells mL−1 in a 96-well plate, and allowed to attach for 8 h, followed by the 24 h incubation with various herbal tea extract.Cell viability
A colorimetric MTT assay was utilized to measure the mitochondrial activity in viable cells based on the conversion of MTT to formazan crystals by mitochondrial enzymes. The cell culturing procedure was as described above. Aliquots (20 μL) of the MTT stock solution were pipetted into each well and the plate was continued to be incubated at 37 °C in a humidified 5% CO2 incubator. After 4 h, the medium was removed by aspiration and DMSO (100 μL) was added to each well to dissolve the formazan. Ten min later, the optical density of each well was measured spectrophotometrically at 570 nm. Results are exhibited from three replicate runs.Measurement of nitric oxide in LPS-treated RAW 264.7 cells
The cell culturing procedure was as previously described. The cells were then incubated with 1 μg mL−1 of lipopolysaccharide (LPS) to induce an inflammatory process. After the harvest of the cells, the supernatants were collected for the measurement of nitric oxide (NO) by Griess Reagent method. The nitrite concentration in the samples was determined by adding 100 μL of Griess reagent (0.4 g Griess reagent powder dissolved in 10 mL D. D. water) to 100 μL sample. The absorbance at 540 nm was then acquired thereafter to incubate for 10 min.22 The inhibitory effect was standardized with respect to the control group treated with LPS but without extracts in terms of A/A0 (%), where A is the value of A540 generated by the Griess reaction at a given concentration of a herbal tea, and A0 is that obtained from the control group treated with LPS but without extracts. Again all tests were in triplicate.Assessment of pro-inflammatory mediators in LPS-treated RAW 264.7 cells by ELISA
The cell culturing procedure was again as described above. The cells were then incubated with 1 μg mL−1 of lipopolysaccharide (LPS) to induce the elevated amount production of pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α), Interleukin-6 (IL-6) and Interleukin-1β (IL-1β), which are three major pro-inflammatory mediators. In order to investigate the potential anti-inflammatory effects of Inulae Flos, its hot water extract was added to the LPS-treated cultured cells (5 × 104 cells/well) at final concentrations of 50, 100 and 200 μg mL−1. After 24 h of co-culturing, 100 μL of medium samples were sampled and subjected to analysis of selected pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α). Various cytokine levels in cell supernatant were determined by enzyme-linked immunosorbent assays (ELISA) according to the manufacturers' instructions (Mouse IL-1β/IL-6 CytoSet™, Invitrogen, Carlsbad, CA, USA; Mouse TNF-α module set, Bender MedSystems, Burlingame, CA, USA), in which most of the following reaction occurred at ambient temperature unless described otherwise. 100 μL of corresponding capture antibody (1–100 μg mL−1) was added to a 96-well plate and incubated at 4 °C overnight. Each well was subsequently washed with washing buffer (0.05% Tween 20 in phosphate buffered saline, PBS) once, after which 300 μL of assay buffer (0.05% Tween 20 in PBS containing 0.5% (w/v) BSA) was added and allowed to incubate for 1 h to block nonspecific binding sites. It was followed by the addition of 100 μL of standard samples into designated wells, and thereafter 50 μL of working detection antibody (biotin-antibody conjugate, 0.2 μg mL−1) was added into each well and allowed to incubate for 2 h. After the removal of unbound working detection antibody, 100 μL of streptavidin-HRP solution (0.2 μg mL−1) was added to each well and incubated for 30 min. Finally 100 μL of TMB substrate solution was dispensed to each well and incubated for 30 min in the dark. At the very last, 100 μL of stop solution (1.8 N H2SO4) was added to terminate the reaction. The absorbance at 450 nm (reference absorbance at 650 nm) was obtained by an ELISA reader. The inhibitory effect was standardized with respect to the control group treated with LPS but without extracts in terms of A/A0 (%), where A is the value of A450 generated by the ELISA assay at a given concentration of an herbal tea, and A0 is that obtained from the control group treated with LPS but without extracts. All tests were in triplicate.Enzyme inhibition assay and enzymatic kinetic analysis
This assay was performed based on the modification of procedures reported by Mason and Peterson.23 Mushroom tyrosinase was used for this assay because of its commercial availability. The inhibitory effects of the five herbal tea extracts on tyrosinase were measured by determining the oxidation rate of DL-DOPA as a substrate. The activity of tyrosinase was determined spectrophotometrically by examining dopachrome formation at 475 nm. In short, 0.8 mL of 0.625 mM DL-DOPA solution was mixed with 0.1 mL of Inulae Flos extracts separately as inhibitors (diluted with 0.05 M PBS, pH 6.5) to a final concentration of 2 mg mL−1. The tyrosinase solution (100 units, 0.1 mL) was then added to the mixture and immediate measurement of the absorbance at 475 nm was taken and continuously monitored for 6.5 min to determine the initial rate of increase in the dopachrome concentration. The inhibitory effect of Inulae Flos is expressed as the percentage required to provide 50% inhibition (IC50). To evaluate the kinetics of the potential inhibitor candidate Inulae Flos, the tyrosinase activity corresponding to the different concentrations of DL-DOPA was investigated. For various concentrations of inhibitors (0, 4, 6 mg mL−1), four replicates of the absorbance reading for varied concentrations of DL-DOPA (0.08, 0.12, 0.16, 0.2 mM) were converted to the reciprocal of the value of absorbance. The reciprocal of velocity was then plotted versus the reciprocal of concentration of the substrate. The apparent Michaelis constant (Km), the maximum velocity (Vmax) and the type of inhibition were analyzed using a Lineweaver–Burk plot.Results and discussion
Content of phenolics compounds (total phenolics and flavonoids)
The total phenolic content and flavonoid content of the five herbal tea extracts were determined. Of the five tested samples, the phenolics and flavonoid contents of Inulae Flos were the highest, with 1452.35 ± 21.33 mg gallic acid equivalents/100 g dried herb and 1220.75 ± 48.35 mg catechin equivalents/100 g dried herb, respectively. The Broomweed and Chinese Leucas had similar levels of total phenolic and flavonoid contents, with total phenolic contents of 558.45 ± 53.62 and 517.24 ± 10.38 gallic acid equivalents/100 g dried herb respectively, and the total flavonoid content of 340.59 ± 4.37 and 342.79 ± 19.31 mg catechin equivalents/100 g dried herb, respectively. Statistical analysis revealed that the total phenolic and flavonoid contents of Broomweed and Chinese Leucas showed no significant difference (p > 0.05), but were higher than the contents of the dried herbs of Horsetail and Indian Wikstroemia, whose total phenolic contents were around 170.11 ± 1.72 and 158.196 ± 0.69 gallic acid equivalents/100 g dried herbs respectively, whereas flavonoid contents were 178.16 ± 0.59 and 117.35 ± 1.56 mg catechin equivalents/100 g dried herbs, correspondingly. We thus concluded that Inulae Flos possessed significantly higher phenolic and flavonoid contents than those of other herbal tea hot water extracts (p < 0.05). Compared to the most widely consumed tea beverages such as green tea and black tea, Inulae Flos in relativity possesses an intermediate amount of polyphenolic content and flavonoid among the three, whereas those of green tea are the highest and black tea the lowest.24 The structure identification of effective compounds in the hot water extract of Inulae Flos was conducted by Fourier Transform Mass Spectrometry. Mass spectra gave two distinct ion peaks at m/z 453.2 and 555.1 [M + H+], and it is believed that these two peaks represented glycosylated naringenin and amentoflavone, respectively. To confirm the assumption, HPLC (Agilent Technologies 1200 series) analysis was carried out after an acid treatment of crude extract was performed. Such pre-treatment was to ensure the release of the free forms of polyphenolic and flavonoid from what is commonly present in plants such as glycosides or esters, or those that are bound to cell walls.25 Two major peaks were shown in the chromatograph of Inulae Flos, whose retention times are identical to those of naringenin and amentoflavone standards (data not shown), confirming the presence of naringenin and amentoflavone in the hot water extract of Inulae Flos.Assessment of antioxidant capacity
Three assay systems, namely the DPPH˙ and ABTS˙+ radical-scavenging assays and the FRASC assay were selected to evaluate the antioxidant activities of the five herbal tea hot water extracts. While DPPH˙ and ABTS˙+ approaches have been applied widely to measure the antioxidant activities of polyphenolics, FRASC assay is used to determine specifically the ascorbic acid content presented in the samples. The addition of ascorbate oxidase to a parallel sample removed the ascorbic acid, leaving a background value.26 As illustrated in Fig. 1, Inuale Flos showed the greatest antioxidant capacity (p < 0.05) than that of the other four herbal tea extracts as determined by these three assays.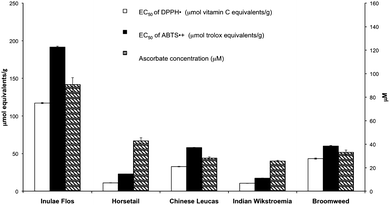 | ||
| Fig. 1 Assessment of the antioxidant activities of the herbal tea extracts using 3 approaches (DPPH˙ radical-scavenging assay, ABTS˙+ radical-scavenging assay, and FRASC assay). | ||
Cytotoxicity
MTT assay was used to investigate the cytotoxicity of the five herbal teas prior to the experiments of their anti-inflammatory effects on LPS-induced RAW 264.7 cells. MTT is reduced by mitochondrial dehydrogenase to form formazan, an insoluble purple compound. We measured the cytotoxicity in terms of the intensity of the purple compound. Dead cells, on the other hand, did not form any purple formazan because the enzyme was degraded and lack of regular function. Among the five herbal teas, the Inulae Flos exhibited the highest cytotoxic effect on RAW 264.7 cells, as evident by its lowest IC50 of 389.8 μg mL−1 listed in the inset table of Fig. 2. The macrophage cells were then treated separately with 0, 100, 200 300, 400, 500 and 600 μg mL−1 of the five herbal tea extracts to determine the maximum concentration that RAW 264.7 cells could tolerate and exhibit at least 80% viability. Cytotoxicity for each group was expressed as a percentage of the control group (without co-treatment with herbal tea extracts). As shown in Fig. 2, 200 μg mL−1 was chosen as the maximum concentration for all five herbal tea extracts, at where it exhibited 80% cell viability. Moreover it was also found that a greater level of Inulae Flos might exert an antiproliferative effect on RAW 264.7 cells.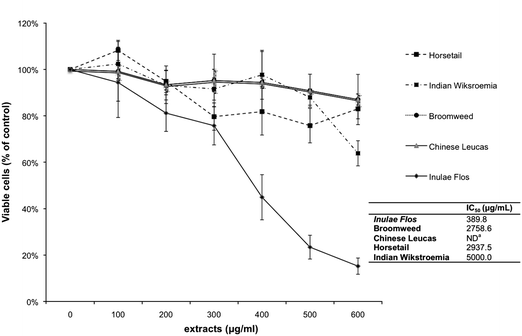 | ||
| Fig. 2 MTT assays performed to measure the survival rate of RAW 264.7 cells after treatment with different concentrations of the five herbal teas' hot water extracts. The cell growth of the treated group was standardized with respect to the untreated control group in terms of A/A−10 (%), where A is the value of A570 generated by the MTT assay at a given concentration of a herbal tea extract, and A0 is that obtained from the untreated control group. The inset table shows the IC50 values for the cytotoxicity of the five dried herbs. (aNot detected.) | ||
Evaluation of anti-inflammatory effect
Three pro-inflammatory cytokines, namely tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) were evaluated in activated inflammatory cells. They are responsible for inducing neutrophil proliferation, fever and acute inflammatory phase, increasing the expression of adhesion factors on endothelial cells, and enabling the transmigration of leukocytes. Incidentally, it has been well understood that nitric oxide is not only a signaling molecule but also plays an important role in regulating inflammation through the up-regulation of leukocyte and endothelial adhesion molecules.22,27,28 Consequently, the quantitative assessment of the pro-inflammatory cytokines and nitric oxide can be regarded as the biomarkers for studying inflammatory response.The anti-inflammatory effects of the five herbal tea extracts in this study were evaluated through a Griess reagent method for determining NO production, and ELISA assays for screening in vitro levels of pro-inflammatory cytokine, TNF-α, IL-6, IL-1β. The percentage of inhibition for each was expressed as the ratio of NO production (measured at 540 nm) for each experimental group to that of LPS-induced cell control group. As shown in Fig. 3(a), only the Inulae Flos extract (with various concentrations: 50, 100, and 200 μg mL−1) demonstrated a dose-dependent suppression on NO production (p < 0.05) with an EC50 of 139.74 μg mL−1. However, no significant change was observed for the other 4 herbal tea extracts, and therefore their EC50 were undeterminable.
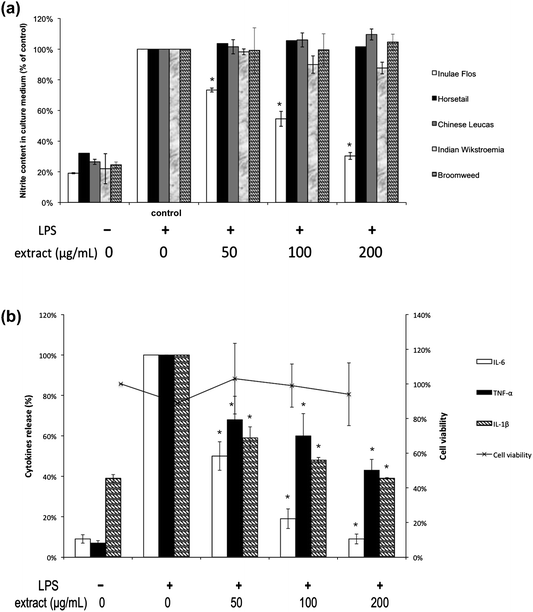 | ||
| Fig. 3 (a) Inhibitory effects of the five herbal teas’ hot water extracts on nitric oxide production. Significant difference from the control value: *p < 0.05. (b) Inhibitory effect of Inulae Flos extract on the production of LPS-induced pro-inflammatory cytokines. Significant difference from the control value: *p < 0.05. | ||
Furthermore, the percentage of inhibition for each herbal tea extract was expressed as the ratio of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) production (measured at 540 nm, reference at 650 nm) of each experimental group to that of the LPS-induced cell control group. Pro-inflammatory cytokines are secreted mostly by activated macrophages, and are involved in the up-regulation of inflammatory reactions. As shown in Fig. 3(b), LPS-induced cell groups treated with 50, 100, and 200 μg mL−1 of Inulae Flos extract led to the suppression of the production of IL-6 at 50%, 19%, and 9% of the control group respectively, while TNF-α was lowered to 68%, 60%, and 43% of control respectively, and IL-1β was reduced to 59%, 48%, and 39% of control respectively. The treatment of Inulae Flos extract applied on the LPS-induced cells demonstrated the reduced production of the selected cytokines in a concentration-dependent manner (p < 0.05) without observed cytotoxicity (cell viability > 80%).
Inhibitory effects of Inulae Flos hot water extracts on the activity of mushroom tyrosinase
An enzyme activity assay was performed to determine the kinetics and mode of the inhibitory effect for Inulae Flos hot water extracts. We observed a dose-dependent inhibitory effect of Inulae Flos hot water extracts on the oxidation of DL-DOPA by mushroom tyrosinase (Fig. 4(a)); the IC50 was 4.35 mg mL−1, which was equivalent to 26.8 mg mL−1 in terms of dry herb weight.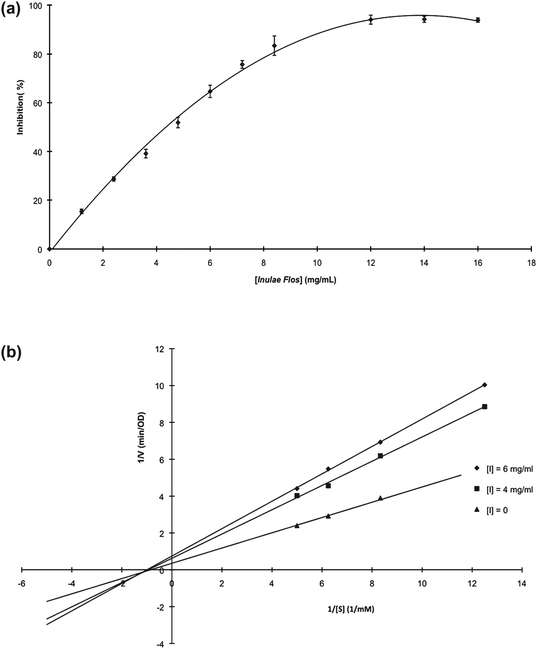 | ||
| Fig. 4 (a) Dose-dependent inhibitory effect of Inulae Flos on mushroom tyrosinase. (b) Lineweaver–Burk plots for the inhibition of Inulae Flos on mushroom tyrosinase for the catalysis of DL-DOPA. | ||
The inhibition kinetics of the Inulae Flos were subsequently analyzed by a Lineweaver–Burk plot. As seen in Fig. 4(b), the three lines, which were obtained from the uninhibited enzyme and two different concentrations of Inulae Flos extracts, intersected on the horizontal axis, indicating that Inulae Flos hot water extract-mediated inhibition of the concentration of DL-DOPA to dopachrome is based on a noncompetitive inhibitory effect. The kinetic parameters, such as Km and Vmax, and inhibition rate constants (KI) for the mushroom tyrosinase/Inulae Flos system, were calculated to be 1.48 mM, 3.48 OD (optical density)/min, and 10.56 mg mL−1 respectively.
Melanogenesis is the process of protecting skin cells from the attack of free radicals resulting from various environmental factors (i.e., UV radiation) or from potential cellular injury caused by aging or cancer. However, hyper-pigmentation may cause negative impacts on agriculture commodities or on health and medical conditions. The search for tyrosinase inhibitor alternatives are therefore of great focus. We found that Inulae Flos water extracts noncompetitively inhibited the activity of tyrosinase, indicating that some constituents present in the extract would bind to both the enzymes (E) and the enzyme-substrates (ES). Noncompetitive inhibitors for tyrosinase, such as 3,4-dihydroycinnamic acid, 4-hydroxy-3-methoxycinnamic acid (isolated from Pulstillla cernua roots), and oxyresveratrol were previously reported.14,29,30 We thereby suggested that amentoflavone and naringenin found in Inulae Flos water extract revealed structural commonness of the presence of a resorcinol moiety as shown in Fig. 5.
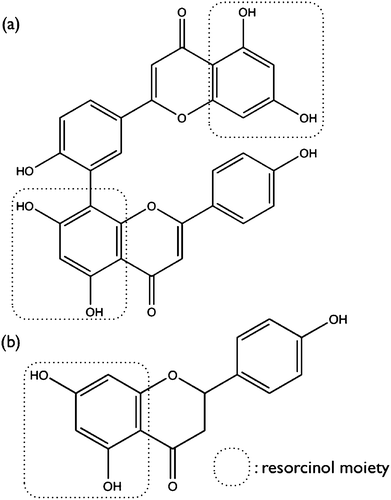 | ||
| Fig. 5 Chemical structures of amentoflavone (a) and naringenin (b). | ||
Conclusions
The hot water extracts of five herbal teas which are commonly used for relieving pain and inflammation in Asian countries were subjected to phytochemical characterization and bioactivity evaluation. Data obtained herein (summarized in Table 1) suggested that water extracts isolated from Inulae Flos possessed the greatest amount of total polyphenolics and flavonoids, and was the most effective antioxidant among the studied tea types for scavenging free radicals. In addition, the hot water extracts of Inulae Flos demonstrated a dose-dependent behavior in suppressing the production of nitric oxide and pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β) in LPS-induced RAW 264.7 macrophages. Furthermore, Inuale Flos water extract containing amentoflavone and naringenin might also be a potentially useful alternative as an anti-browning agent or tyrosinase inhibitor that could be applied in fresh-cut produce and meat industries, or as cosmeceutical products for treating hyper-pigmentation or skin-whitening. In summary, Inulae Flos herbal tea extract, with respect to its possession of the most abundant antioxidant, its high effectiveness in suppressing inflammation among the studied herbal teas, and its inhibitory effect on the production of melanin, may serve as a potential dietary nutraceutical supplement to keep human beings healthy. Furthermore, it holds promise for becoming a natural food additive as an anti-browning agent.| (a) | Inulae Flos | Horsetail | Chinese Leucas | Broomweed | Indian Wikstroemia |
|---|---|---|---|---|---|
| a mg gallic acid equivalents/100 g dried herb. b mg catechin equivalents/100 g dried herb. c μmol vitamin C equivalents/g in terms of EC50. d μmol trolox equivalents/g in terms of EC50. e μM of ascorbate concentration. f demonstrated inhibitory effect in dose-dependent manner. g not detected. h not tested. | |||||
| Total phenolic contenta | 1452.35 ± 21.33 | 170.11 ± 1.72 | 517.24 ± 10.38 | 558.45 ± 53.62 | 158.196 ± 0.69 |
| Total flavonoid contentb | 1220.75 ± 48.35 | 178.16 ± 0.59 | 342.79 ± 19.31 | 340.59 ± 4.37 | 117.35 ± 1.56 |
| DPPH˙ scavenging abilityc | 117.1 ± 0.8 | 10.98 ± 0.22 | 32.65 ± 0.38 | 10.48 ± 0.15 | 43.27 ± 0.81 |
| ABTS˙+ scavenging abilityd | 191.6 ± 1.2 | 22.83 ± 0.09 | 57.93 ± 0.29 | 17.23 ± 0.25 | 59.95 ± 0.72 |
| FRASC assaye | 141.7 ± 9.2 | 66.86 ± 4.11 | 43.97 ± 1.54 | 39.97 ± 1.03 | 51.60 ± 3.08 |
| Inhibitory effect on nitrate contents | +f | NDg | ND | ND | ND |
| Anti-inflammatory effects | + | —h | — | — | — |
| (b) | |
|---|---|
| Km | 1.48 mM |
| Vmax | 3.48 optical density/min |
| Kl | 10.56 mg mL−1 |
| Inhibition type | noncompetitive |
Acknowledgements
This study was supported by the National Science Council (NSC) in Taiwan, under grants NSC 97-2113-M-260-006-MY2 (Wu), 98-2627-M-260-002 (Wu), and 98-2113-M-007-013-MY3 (Ho). The authors thank Mr. Allan Yeh for his editorial assistance.References
- M. Liu, X. Q. Li, C. Weber, C. Y. Lee, J. Brown and R. H. Liu, Antioxidant and Antiproliferative Activities of Raspberries, Journal of Agricultural and Food Chemistry, 2002, 50(10), 2926–2930 CrossRef CAS.
- K. Wolfe, X. Wu and R. H. Liu, Antioxidant Activity of Apple Peels, Journal of Agricultural and Food Chemistry, 2003, 51(3), 609–614 CrossRef CAS.
- L.-C. Wu, J.-a. A. Ho, M.-C. Shieh and I.-W. Lu, Antioxidant and Antiproliferative Activities of Spirulina and Chlorella Water Extracts, Journal of Agricultural and Food Chemistry, 2005, 53(10), 4207–4212 CrossRef CAS.
- L.-C. Wu, H.-W. Hsu, Y.-C. Chen, C.-C. Chiu, Y.-I. Lin and J.-a. A. Ho, Antioxidant and antiproliferative activities of red pitaya, Food Chemistry, 2006, 95(2), 319–327 CrossRef CAS.
- M. Aviram, N. Volkova, R. Coleman, M. Dreher, M. K. Reddy, D. Ferreira and M. Rosenblat, Pomegranate Phenolics from the Peels, Arils, and Flowers Are Antiatherogenic: Studies in Vivo in Atherosclerotic Apolipoprotein E-Deficient (E0) Mice and in Vitro in Cultured Macrophages and Lipoproteins, Journal of Agricultural and Food Chemistry, 2008, 56(3), 1148–1157 CrossRef CAS.
- F. Pinnen, I. Cacciatore, C. Cornacchia, P. Sozio, A. Iannitelli, M. Costa, L. Pecci, C. Nasuti, F. Cantalamessa and A. Di Stefano, Synthesis and Study of l-Dopa-Gluathione Codrugs as New Anti-Parkinson Agents with Free Radical Scavenging Properties, Journal of Medicinal Chemistry, 2007, 50(10), 2506–2515 CrossRef CAS.
- A. Mantovani, P. Allavena, A. Sica and F. Balkwill, Cancer-related inflammation, Nature, 2008, 454(7203), 436–444 CrossRef CAS.
- C. Anesini, G. E. Ferraro and R. Filip, Total Polyphenol Content and Antioxidant Capacity of Commercially Available Tea (Camellia sinensis) in Argentina, Journal of Agricultural and Food Chemistry, 2008, 56(19), 9225–9229 CrossRef CAS.
- P.-D. Duh, G.-C. Yen, W.-J. Yen, B.-S. Wang and L.-W. Chang, Effects of Pu-erh Tea on Oxidative Damage and Nitric Oxide Scavenging, Journal of Agricultural and Food Chemistry, 2004, 52(26), 8169–8176 CrossRef CAS.
- G. Jie, Z. Lin, L. Zhang, H. Lv, P. He and B. Zhao, Free Radical Scavenging Effect of Pu-erh Tea Extracts and Their Protective Effect on Oxidative Damage in Human Fibroblast Cells, Journal of Agricultural and Food Chemistry, 2006, 54(21), 8058–8064 CrossRef CAS.
- T. M. Rababah, N. S. Hettiarachchy and R. Horax, Total Phenolics and Antioxidant Activities of Fenugreek, Green Tea, Black Tea, Grape Seed, Ginger, Rosemary, Gotu Kola, and Ginkgo Extracts, Vitamin E, and tert-Butylhydroquinone, Journal of Agricultural and Food Chemistry, 2004, 52(16), 5183–5186 CrossRef CAS.
- M. Richelle, I. Tavazzi and E. Offord, Comparison of the Antioxidant Activity of Commonly Consumed Polyphenolic Beverages (Coffee, Cocoa, and Tea) Prepared per Cup Serving, Journal of Agricultural and Food Chemistry, 2001, 49(7), 3438–3442 CrossRef CAS.
- P.-J. Tsai, T.-H. Tsai, C.-H. Yu and S.-C. Ho, Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea, Food Chemistry, 2007, 103(1), 181–187 CrossRef CAS.
- H. Li, K.-W. Cheng, C.-H. Cho, Z. He and M. Wang, Oxyresveratrol as an Antibrowning Agent for Cloudy Apple Juices and Fresh-Cut Apples, Journal of Agricultural and Food Chemistry, 2007, 55(7), 2604–2610 CrossRef CAS.
- Y. Luo, & V. Barbosa-Cánovas Gustavo. ( 1995). Inhibition of Apple-Slice Browning by 4-Hexylresorcinol. Enzymatic Browning and Its Prevention, vol. 600 (pp. 240–250): American Chemical Society Search PubMed.
- J. R. L. Walker. ( 1995). Enzymatic Browning in Fruits. Enzymatic Browning and Its Prevention, vol. 600 (pp. 8-22): American Chemical Society Search PubMed.
- L.-C. Wu, Y.-C. Chen, J.-a. A. Ho and C.-S. Yang, Inhibitory Effect of Red Koji Extracts on Mushroom Tyrosinase, Journal of Agricultural and Food Chemistry, 2003, 51(15), 4240–4246 CrossRef CAS.
- M. Jiménez-Atiénzar, J. Escribano, J. Cabanes, F. Gandía-Herrero and F. García-Carmona, Oxidation of the flavonoid eriodictyol by tyrosinase, Plant Physiology Biochemistry, 2005, 43(9), 8 Search PubMed.
- K. B. Hicks, R. M. Haines, C. B. S. Tong, G. M. Sapers, Y. El-Atawy, P. L. Irwin and P. A. Seib, Inhibition of Enzymatic Browning in Fresh Fruit and Vegetable Juices by Soluble and Insoluble Forms of β-Cyclodextrin Alone or in Combination with Phosphates, Journal of Agricultural and Food Chemistry, 1996, 44(9), 2591–2594 CrossRef.
- N. Bai, C.-S. Lai, K. He, Z. Zhou, L. Zhang, Z. Quan, N. Zhu, Q. Y. Zheng, M.-H. Pan and C.-T. Ho, Sesquiterpene Lactones from Inula britannica and Their Cytotoxic and Apoptotic Effects on Human Cancer Cell Lines, Journal of Natural Products, 2006, 69(4), 531–535 Search PubMed.
- C. Choy, I. Benzie and P. Cho, Ascorbic Acid Concentration and Total Antioxidant Activity of Human Tear Fluid Measured Using the FRASC Assay, Investigative Ophthalmology & Visual Science, 2000, 41(11), 3293–3298 Search PubMed.
- A. Rossi, D. Rigano, C. Pergola, C. Formisano, A. Basile, P. Bramanti, F. Senatore and L. Sautebin, Inhibition of Inducible Nitric Oxide Synthase Expression by an Acetonic Extract from Feijoa sellowiana Berg. Fruits, Journal of Agricultural and Food Chemistry, 2007, 55(13), 5053–5061 CrossRef CAS.
- H. S. Mason and E. W. Peterson, Biochim. Biophys. Acta., 1965, 111, 134–146 CrossRef CAS.
- C. Astill, M. R. Birch, C. Dacombe, P. G. Humphrey and P. T. Martin, Factors Affecting the Caffeine and Polyphenol Contents of Black and Green Tea Infusions, Journal of Agricultural and Food Chemistry, 2001, 49(11), 5340–5347 CrossRef CAS.
- R. Ksouri, H. Falleh, W. Megdiche, N. Trabelsi, B. Mhamdi, K. Chaieb, A. Bakrouf, C. MagnÈ and C. Abdelly, Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents, Food and Chemical Toxicology, 2009, 47(8), 2083–2091 CrossRef CAS.
- L.-C. Wu, N.-C. Fan, M.-H. Lin, I.-R. Chu, S.-J. Huang, C.-Y. Hu and S.-Y. Han, Anti-inflammatory Effect of Spilanthol from Spilanthes acmella on Murine Macrophage by Down-Regulating LPS-Induced Inflammatory Mediators, Journal of Agricultural and Food Chemistry, 2008, 56(7), 2341–2349 CrossRef CAS.
- J.-Y. Bor, H.-Y. Chen and G.-C. Yen, Evaluation of Antioxidant Activity and Inhibitory Effect on Nitric Oxide Production of Some Common Vegetables, Journal of Agricultural and Food Chemistry, 2006, 54(5), 1680–1686 CrossRef CAS.
- K.-L. Liu, H.-W. Chen, R.-Y. Wang, Y.-P. Lei, L.-Y. Sheen and C.-K. Lii, DATS Reduces LPS-Induced iNOS Expression, NO Production, Oxidative Stress, and NF-kB Activation in RAW 264.7 Macrophages, Journal of Agricultural and Food Chemistry, 2006, 54(9), 3472–3478 CrossRef CAS.
- T. J. Y. Ha, M. S. Jang, D. S. Jang, S. U. Choi and K. H. Park, Inhibitory activities of flavanone derivatives isolated from Sophora flaVescens for melanogenesis, Bull. Korean Chem. Soc., 2001, 22, 11.
- H.-S. Lee, Tyrosinase Inhibitors of Pulsatilla cernua Root-Derived Materials, Journal of Agricultural and Food Chemistry, 2002, 50(6), 1400–1403 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
