Antioxidant capacity in cultivated and wild Solanum species: The effect of wound stress
Christina B.
Wegener
* and
Gisela
Jansen
Julius Kuehn Institute, Federal Research Centre for Cultivated Plants, Institute for Resistance Research and Stress Tolerance, Experimental Station for Potato Research, Rudolf-Schick-Platz 3, OT Groß Lüsewitz, D-18190, Sanitz, Germany. E-mail: christina.wegener@jki.bund.de
First published on 5th October 2010
Abstract
Wild potatoes are of increasing interest as a gene pool in breeding. In this study, 23 genotypes of two cultivated (S. tuberosum subsp. andigena, S. phureja) and two wild Solanum species (S. chacoense, S. pinnatisectum) were evaluated for contents of soluble phenols and soluble proteins as well as their antioxidant capacity measured as ascorbic acid and trolox equivalent. Amounts of phenols present in tuber tissue ranged from 0.25 to 2.84 mg kg−1 fw. On average, S. pinnatisectum (pnt) exhibited 3.9-fold greater quantities of phenols in its tuber tissue than the other Solanum species. In pnt tissue, high phenol content coincided with high levels of soluble proteins and antioxidants. It is concluded that an involvement of individual accessions of pnt in breeding could be profitable for the antioxidant potential and thus for the nutritional value of new potato cultivars. The results also revealed that soluble phenols as well as proteins present in tuber tissue substantially contributed to the total antioxidant capacity of potatoes. Moreover, it was found that quantities of soluble phenols, proteins and antioxidants increased notably upon wounding the tubers, a fact which underlines the role of all these components in wound stress responses of potatoes.
Introduction
A high level of tolerance to biotic and abiotic stress as well as enhancing health-related quality traits like antioxidants, vitamins and anticancer compounds are the most important topics for plant breeding in the future.1 Also breeding of potatoes is focused on these traits.2–4 Particularly, plant phenols comprising hydroxycinnamates, flavonoids, tannins, lignin etc. are of great importance in this context. The latter are mainly derived from cinnamic acid synthesised via the phenylpropanoid metabolism of plants.5 Phenolic compounds are not only associated with expression of disease resistance in plants,6 they also act as radical scavengers7 and are thus part of the plant antioxidant system diminishing undesired effects of oxidative stress on metabolism and cells, as caused by various environmental stresses.8 When consumed in the diet, plant phenols are incorporated in an antioxidant network protecting animal and human cells against oxidative damage.9,10 In addition, they are involved in a variety of essential physiological functions associated with acclimation of plants to stressful environments,7 and similar to reactive oxygen species (ROS), plant phenols are inducible by environmental stresses.11 Accordingly, it has been argued that adaptation of plants to environmental stresses coincides with an improvement of the nutritional value.12 In this context, the rich genetic resource comprised by wild potatoes could be an interesting source,1 which should increasingly be exploited in the future in order to improve the stress tolerance and with it the nutritional value of cultivated potatoes. In previous work a multitude of accessions of wild, tuber-bearing Solanum species and Andean cultivated potatoes as well as an international assortment of S. tuberosum subsp. tuberosum has been evaluated with respect to quality traits like concentrations of dry matter, crude protein and starch in tuber tissue including starch quality characteristics.13In the present study, two cultivated (S. tuberosum subsp. andigena, adg; S. phureja, phu) and two wild, tuber-bearing Solanum species (S. chacoense, chc; S. pinnatisectum, pnt), each represented by several accessions and genotypes (Table 1) were assessed for contents of soluble phenols and proteins in tuber tissue as well as their antioxidant capacity measured as ascorbic acid (ACE) and trolox (TXE) equivalent. Furthermore, the effect of wounding, as one of the major stress factors for plants in nature,11 on the level of soluble phenols, proteins and antioxidants has been examined. Investigating the aftermath of wound stress on the level of antioxidants in tuber tissue is a novel aspect in context with wild potatoes. Apart from the investigation of stress-inducible responses as a prerequisite for the development of stress tolerant crops, whose plants are able to produce high yield under stress conditions,12 the effect of wounding the tubers is interesting with respect to general quality characteristics of potatoes which are offered on the markets or processed in the food industry, especially in the potato processing industry.
| Series | Species | Abbreviations | Number of genotypes |
|---|---|---|---|
| Tuberosa - cultivated | S. tuberosum subsp. andigena | adg | 5 |
| Tuberosa - cultivated | S. phureja | phu | 6 |
| Yungasensa | S. chacoense | chc | 6 |
| Pinnatisecta | S. pinnatisectum | pnt | 6 |
| Altogether | 23 |
Material and methods
Plant material
The experiments were carried out in Groß Lüsewitz, near the Baltic Sea. Seed tubers of two cultivated (adg, phu) and two wild, tuber-bearing Solanum species (chc, pnt), each represented by several accessions and genotypes (Table 1) were from the Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Potato Genebank, Groß Lüsewitz, Germany. The Solanum species used in the test series were chosen according to information about evaluation results on resistance properties to potato diseases available in the literature.14 Ten plants per genotype were grown in pots of 130 mm diameter under a shelter from April to October in 2008. Fertilizer, insecticides, fungicides and all other treatments were conducted according to local agronomic practice. After harvest, tubers were stored in a controlled environment at 5 °C. All analyses started in November and finished in December of the test year.Preparation of potato cylinder samples
Twenty tubers were taken as an average sample from each genotype and halved. A cork borer of 5 mm diameter was used to cut two cylinders from the outer region of each half of each tuber. In order to test the effect of wounding on concentrations of total soluble phenols (A), soluble proteins (B) and antioxidants (C), two cylinder samples were taken per genotype and assay: the first one was excised from (i) fresh tuber tissue and a second was prepared (ii) 24 h after wounding the tubers. Before preparing the second sample, the tuber halves of each experimental set were stored for 24 h at 20 °C with the wound-surface upward on moist filter papers placed in a plastic box which was covered with a glass plate.Assay of total soluble phenols
Preparation of extracts from (i) fresh and (ii) wounded tuber tissue for assaying total soluble phenols was carried out as detailed.15 A 1 mm thick slice was excised from the upper wound region of each cylinder cut from the tuber half as described above. The tissue slices were pooled, and 1 g of the slices was ground under liquid nitrogen using mortar and pestle. The homogenate was suspended in 4 mL of methanol (Roth, Karlsruhe, Germany). The suspension was stirred slightly and after 1 h centrifuged at 6000 × g for 10 min at 4 °C. The supernatant was removed and the plant material re-extracted. The total amount of phenols present in the combined extracts was determined using Folin-Ciocalteu reagent (Sigma-Aldrich, Taufkirchen, Germany) according to Cahill & Mc Comb.16 The absorbance was measured at 725 nm on a UV spectrophotometer (Kontron Instruments, Neufahrn, Germany). Standards were prepared from p-coumaric acid (Sigma-Aldrich, Taufkirchen, Germany). Amounts of soluble phenols (= coumaric acid equivalent) were expressed in grams per kilogram of fresh weight (fw). Extractions and measurements were performed in duplicate (SD ≤ 5%).Assay of soluble proteins
The tissue slices excised from the cylinders prepared from (i) fresh and (ii) wounded tubers as described for the assay of soluble phenols were pooled and 3 g of slices were ground under liquid nitrogen using a mortar and pestle. The homogenate was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min at 4 °C. Amounts of soluble proteins were determined in the supernatant (= cell sap fraction) by means of a Bradford assay using a RotiR-Quant reagent (Roth, Karlsruhe, Germany) according to the manufacturer recommendations. The absorbance was measured at 595 nm on a UV spectrophotometer (Kontron Instruments). Standards were prepared from bovine serum albumin (Cohn fraction V; Sigma-Aldrich, Taufkirchen, Germany). Amounts of soluble proteins were calculated as milligrams per millilitre of extract. Analyses were carried out in duplicate (SD ≤ 5%).
000 × g for 10 min at 4 °C. Amounts of soluble proteins were determined in the supernatant (= cell sap fraction) by means of a Bradford assay using a RotiR-Quant reagent (Roth, Karlsruhe, Germany) according to the manufacturer recommendations. The absorbance was measured at 595 nm on a UV spectrophotometer (Kontron Instruments). Standards were prepared from bovine serum albumin (Cohn fraction V; Sigma-Aldrich, Taufkirchen, Germany). Amounts of soluble proteins were calculated as milligrams per millilitre of extract. Analyses were carried out in duplicate (SD ≤ 5%).
Assay of the antioxidant activity
The tissue cylinders prepared from (i) fresh and (ii) wounded tuber tissue as described above were cut into 3 mm slices, after the peel region was removed from each cylinder by means of a scalpel. The slices were pooled, and 3 g of the tissue slices were ground under liquid nitrogen by mortar and pestle. The homogenate was suspended in a solution of 85% (v/v) ice cold ethanol. The suspension was stored on ice, occasionally shaken, and after 1 h centrifuged for 10 min at 8000 × g and 4 °C. The supernatant was removed and used for measurement of the antioxidant activity on a Photochem instrument, utilizing an ACW-kit for water soluble and ACL-kit for lipid soluble antioxidants, as detailed.15 The Photochem instrument as well as kit reagents were supplied by the AnalytikJena AG (Germany). This automated photochemiluminescent (PCL) method is based on a photochemical generation of free radicals combined with their detection by chemiluminescence as described recently.17 The antioxidant activity was calculated by means of an ascorbic acid calibration curve for hydrophilic antioxidants and a trolox calibration curve for lipid soluble antioxidants, using the Photochem software package. Results were expressed in microgram equivalents in antioxidant activity of the reference compound, i.e. as ascorbic acid (ACE) and trolox equivalents (TXE) per milligram of fresh weight, respectively. Measurements were performed in duplicate with SD ≤ 5%.Statistical methods
Conventional statistical methods were used for the analyses of the data. The differences between pnt and the other Solanum species with respect to soluble phenols, proteins and antioxidants comprising ACE and TXE were assessed using unpaired t-test, at the 0.05 level of significance. Differences in all these traits between extracts derived from (i) fresh tuber tissue and those prepared (ii) 24 h after wounding the tubers were valued by means of t-test for paired samples, and P < 0.05 was considered significant. Correlation coefficients (Pearson) were calculated between amounts of the individual substances, i.e. soluble phenols, proteins, water (ACE) and lipid soluble antioxidants (TXE), found in fresh tissue and those detected 24 h after wounding the tubers. Moreover, correlations were assessed between soluble phenols and antioxidant capacity including ACE and TXE as well as between soluble proteins and both types of antioxidant activity. In addition, the relationship between phenols and antioxidant capacity as well as between proteins and the latter were examined by regression analyses.Results
Soluble phenols
The Solanum genotypes involved in the tests differed considerably in their contents of soluble phenols (Table 2A). Within potato samples prepared from (i) fresh tuber tissue the amounts of phenols ranged from 0.25 to 2.84 mg kg−1 fw, while comparative values for (ii) wounded tuber tissue ranged from 0.36 to 2.60 mg kg−1 fw. Of the 23 genotypes involved in the study, pnt 31598-2 exhibited the highest levels of soluble phenols in its fresh and wounded tuber tissue with values of ≥2.60 g kg−1 fw, followed by pnt 31598-3. All accessions of S. tuberosum subsp. andigena had less soluble phenols in their fresh tissue with values of <0.50 g kg−1 fw (Table 2A). On average, the wild Solanum species S. pinnatisectum displayed the highest phenol contents (Table 2A). This was observed in samples from fresh tissue, which had 3.9-times the phenol concentrations of the other Solanum species, and also in samples taken 24 h after wounding (×2.5).| (A) Soluble phenols (g kg−1 fw) | (B) Soluble proteins (mg mL−1) | |||
|---|---|---|---|---|
| Fresh tissue | Wounded tissue | Fresh tissue | Wounded tissue | |
| a Significance of the difference between fresh and wounded tuber tissue (n = 23). b Correlation between amounts of soluble phenols and proteins detected in fresh tissue and those found 24 h after wounding (23 genotypes); ns, not significant P ≥ 0.05. | ||||
| adg | ||||
| 31881-1 | 0.43 | 0.82 | 2.80 | 3.32 |
| 31881-2 | 0.39 | 0.90 | 7.28 | 8.04 |
| 34155-1 | 0.34 | 0.76 | 3.44 | 3.76 |
| 34155-3 | 0.48 | 0.81 | 11.48 | 11.92 |
| 34155-5 | 0.34 | 0.70 | 4.96 | 5.08 |
| Average | 0.40 | 0.80 | 5.99 | 6.42 |
| Range | 0.34–0.48 | 0.70–0.90 | 2.80–11.48 | 3.32–11.92 |
| Significancea | P < 0.001 | P < 0.05 | ||
| chc | ||||
| 30161-12 | 0.61 | 0.77 | 12.32 | 11.16 |
| 30161-17 | 0.25 | 0.36 | 12.00 | 11.68 |
| 30180-3 | 0.56 | 0.73 | 11.00 | 11.32 |
| 30180-4 | 0.34 | 0.45 | 7.08 | 9.68 |
| 30180-13 | 0.64 | 0.79 | 12.68 | 12.76 |
| 30180-16 | 0.66 | 0.73 | 11.72 | 11.48 |
| Average | 0.51 | 0.64 | 11.13 | 11.35 |
| Range | 0.25–0.66 | 0.36–0.79 | 7.08–12.68 | 9.68–12.76 |
| Significancea | P < 0.001 | ns | ||
| phu | ||||
| 31455-1 | 0.31 | 0.48 | 10.16 | 11.68 |
| 31455-2 | 0.55 | 0.90 | 5.56 | 6.00 |
| 31455-4 | 0.52 | 1.14 | 6.24 | 7.20 |
| 31467-2 | 0.45 | 0.97 | 4.56 | 4.08 |
| 31567-4 | 0.67 | 0.84 | 11.48 | 14.36 |
| 31567-8 | 0.44 | 0.78 | 5.92 | 6.92 |
| Average | 0.49 | 0.85 | 7.32 | 8.37 |
| Range | 0.31–0.67 | 0.48–1.14 | 4.56–11.48 | 4.08–14.36 |
| Significancea | P < 0.01 | ns | ||
| pnt | ||||
| 31598-2 | 2.84 | 2.60 | 11.40 | 12.16 |
| 31598-3 | 2.12 | 2.10 | 13.52 | 14.40 |
| 31598-4 | 1.60 | 1.76 | 13.12 | 12.64 |
| 31606-1 | 1.58 | 1.53 | 11.96 | 11.48 |
| 31606-2 | 1.06 | 1.41 | 14.44 | 14.36 |
| 31606-3 | 1.74 | 1.87 | 12.84 | 12.80 |
| Average | 1.82 | 1.88 | 12.88 | 12.97 |
| Range | 1.06–2.84 | 1.41–2.60 | 11.40–14.44 | 11.48–14.40 |
| Significancea | ns | ns | ||
| All (n = 23) | ||||
| Average | 0.82 | 1.05 | 9.48 | 9.93 |
| Range | 0.25–2.84 | 0.36–2.60 | 2.80–14.44 | 3.32–14.40 |
| Significancea | P < 0.0001 | P < 0.05 | ||
| Correlationb | 0.97, P < 0.01 | 0.97, P < 0.01 | ||
In tissue samples prepared after 24 h, the amounts of soluble phenols were on average greater (+28%) than in those derived from fresh tuber tissue (Table 2A). The differences in phenols between fresh and wounded tissue were statistically significant, when all genotypes were regarded, and also within the species adg, chc and phu. But, they were not significant within S. pinnatisectum. Individual genotypes of this wild Solanum species, e.g. pnt 31598-2, had even slightly lower quantities of soluble phenols in their wounded tuber tissue (Table 2A). Interestingly, S. tuberosum subsp. andigena which had only small amounts of phenols in its fresh tissue, revealed the greatest increase in phenols after wounding among the species involved in this study. Of the 23 genotypes tested so far, the S. phureja accession phu 31455-4 displayed the highest raise in phenols after 24 h (Table 2A).
There was a clear relationship between amounts of phenols present in fresh tissue and those found 24 h after wounding the tubers (Table 2A). Furthermore, soluble phenols accumulated in fresh and wounded tuber tissue correlated with the antioxidant activity, including ACE and TXE, and also with soluble proteins as discussed below.
Soluble proteins
The Solanum genotypes also varied notably in their soluble protein concentrations (Table 2B). In potato samples taken from (i) fresh and (ii) wounded tuber tissue the quantities of proteins ranged from 2.80 to 14.44 mg mL−1 and from 3.32 to 14.40 mg mL−1, respectively. Among the 23 genotypes, pnt 31606-2 and pnt 31598-3 exhibited the greatest concentrations of soluble proteins in their fresh and wounded tuber tissue (Table 2B). On average, S. pinnatisectum revealed higher protein contents in both tissue types than the other three Solanum species had (Table 2B). Of the four species tested so far, S. tuberosum subsp. andigena had on average the lowest protein values in its fresh and wounded tuber tissue (Table 2B).Amounts of soluble proteins were greater on average in potato samples prepared 24 h after wounding than in those derived from fresh tuber tissue (Table 2B). This difference was statistically significant when all genotypes were regarded (P < 0.05, +5.0%) and within adg (P < 0.05, +7.2%). Within the other Solanum species the protein values were also increased in their tendency after wounding the tubers, however, these differences were statistically not significant. Among the genotypes tested so far, phu 31567-4 exhibited the greatest raise of proteins in response to wounding (Table 2B), whereas individual accessions of S. pinnatisectum (e.g. 31598-4) showed even smaller amounts of soluble proteins in their wounded tuber tissue.
There was a significant correlation between quantities of soluble proteins detected in fresh tissue and those found after wounding the tubers (Table 2B). Moreover, a correlation between soluble proteins and soluble phenols could be detected with respect to fresh tuber tissue (r = 0.52, P < 0.01, n = 23). In the case of wounded tissue, the correlation between these last two components was statistically not significant (r = 0.36, P ≥ 0.05, n = 23). Soluble proteins were also correlated with the ascorbic acid (ACE) as well as with the trolox equivalent (TXE) as mentioned below.
Antioxidant activity
The different genotypes of cultivated and wild Solanum species varied notably in their antioxidant capacity measured as ascorbic acid (ACE: Fig. 1) and trolox equivalent (TXE: Fig. 2). In potato samples taken from fresh tissue, the ACE and TXE values ranged from 0.06 to 4.22 μg mg−1 fw and from 0.08 to 3.98 μg mg−1 fw, respectively. In the case of wounded tuber tissue, the ascorbic acid and trolox equivalent ranged from 0.28 to 4.11 μg mg−1 fw and from 0.23 to 4.26 μg mg−1 fw, respectively. Among the genotypes tested here, pnt 31598-2 exhibited the highest ACE values in both types of tuber tissue (Fig. 1). The latter also reached the highest TXE in its fresh tissue (Fig. 2), whereas in wounded tuber tissue, pnt 31598-3 displayed the highest trolox equivalent. Of the four Solanum species analysed so far, S. pinnatisectum had on average the highest antioxidant potential comprising the ascorbic acid (Fig. 1, Table 3A) as well as the trolox equivalent (Fig. 2, Table 3B).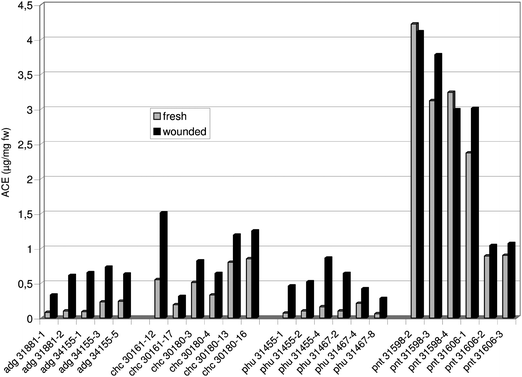 | ||
| Fig. 1 Antioxidant activity measured as ascorbic acid equivalent (ACE) in extracts prepared from (i) fresh tuber tissue and in those taken (ii) 24 h after wounding the tubers of different Solanum genotypes. | ||
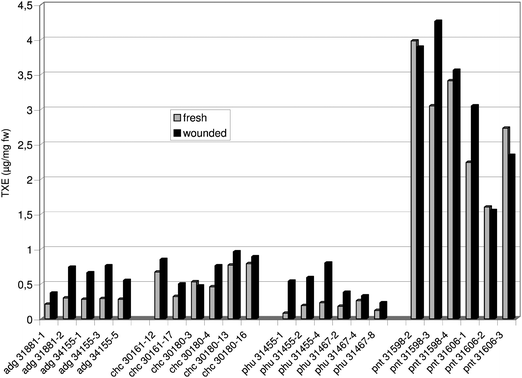 | ||
| Fig. 2 Antioxidant activity measured as trolox equivalent (TXE) in extracts prepared from (i) fresh tuber tissue and in those taken (ii) 24 h after wounding the tubers of different Solanum genotypes. | ||
| Species | (A) ACE (μg mg−1 fw) | (B) TXE (μg mg−1 fw) | ||
|---|---|---|---|---|
| Fresh tissue | Wounded tissue | Fresh tissue | Wounded tissue | |
| a Significance of the difference in ACE and TXE between fresh and wounded tuber tissue (n = 23). b Correlation between antioxidant activity detected in fresh tissue and that measured 24 h after wounding (23 genotypes); ns, not significant P ≥ 0.05. | ||||
| adg | ||||
| Average | 0.15 | 0.59 | 0.27 | 0.62 |
| Range | 0.08–0.24 | 0.33–0.73 | 0.21–0.30 | 0.37–0.76 |
| Significancea | P < 0.001 | P < 0.01 | ||
| chc | ||||
| Average | 0.54 | 0.95 | 0.59 | 0.74 |
| Range | 0.19–0.85 | 0.31–1.51 | 0.32–0.79 | 0.47–0.96 |
| Significancea | P < 0.05 | P < 0.05 | ||
| phu | ||||
| Average | 0.12 | 0.53 | 0.18 | 0.48 |
| Range | 0.06–0.21 | 0.28–0.86 | 0.08–0.26 | 0.23–0.80 |
| Significancea | P < 0.05 | P < 0.05 | ||
| pnt | ||||
| Average | 2.46 | 2.67 | 2.84 | 3.11 |
| Range | 0.89–4.22 | 1.04–4.11 | 1.60–3.98 | 1.55–4.26 |
| Significancea | n.s. | n.s. | ||
| All | ||||
| Average | 0.84 | 1.21 | 1.00 | 1.26 |
| Range | 0.06–4.22 | 0.28–4.11 | 0.08–3.98 | 0.23–4.26 |
| Significancea | P < 0.0001 | P < 0.01 | ||
| Correlationb | 0.98, P < 0.01 | 0.96, P < 0.01 | ||
Potato samples taken after 24 h had on average higher ACE (Table 3A, +44%) and TXE values (Table 3B, +26%) than those derived from fresh tuber tissue. These differences were statistically significant, when all genotypes were regarded and within Solanum species adg, chc and phu. Also S. pinnatisectum exhibited on average higher ACE (Table 3A) and TXE values after wounding its tubers (Table 3B), however, in this case the differences were statistically not significant. Individual genotypes of pnt had even slightly lower ACE (e.g. pnt 31598-4) and TXE levels (e.g. pnt 31606-3) in their wounded tissue. Of the 23 genotypes assayed in this study, chc 30161-12 exhibited the greatest raise in the ascorbic acid equivalent after wounding (Fig. 1), and pnt 31598-3 was most efficient in the enhancement of its trolox equivalent within 24 h (Fig. 2). Moreover, it was found that S. pinnatisectum differed significantly from the other three Solanum species (adg, chc and phu) with respect to concentrations of soluble phenols (Table 4A), soluble proteins (Table 4B) as well as antioxidants measured as ACE and TXE (Table 4C) - a result noticed in the case of fresh and wounded tuber tissue.
| Type of analyses | S. pinnatisectum (6 genotypes) | Other species (17 genotypes) | Significancea |
|---|---|---|---|
| a Significance of the difference between pnt and the other three Solanum species. | |||
| (A) Soluble phenols (g kg−1 fw) | |||
| Fresh tissue | 1.82 | 0.47 | <0.01 |
| Wounded tissue | 1.88 | 0.76 | <0.001 |
| (B) Soluble proteins (mg mL−1) | |||
| Fresh tissue | 12.88 | 8.28 | <0.0001 |
| Wounded tissue | 12.97 | 8.85 | <0.001 |
| (C) Antioxidant activity | |||
| ACE (μg mg−1) | |||
| Fresh tissue | 2.46 | 0.27 | <0.05 |
| Wounded tissue | 2.67 | 0.70 | <0.05 |
| TXE (μg mg−1) | |||
| Fresh tissue | 2.84 | 0.35 | <0.001 |
| Wounded tissue | 3.11 | 0.61 | <0.01 |
There was a clear correlation between the ACE values detected in fresh and those found in wounded tuber tissue (Fig. 1, Table 3A; r = 0.98, P < 0.01), a result which was similarly noticed with respect to TXE values (Fig. 2, Table 3B; r = 0.96, P < 0.01). The ACE was significantly correlated with the TXE in the case of both tissue types (Fresh tissue: r = 0.94, P < 0.01; Wounded tissue: r = 0.95, P < 0.01). Furthermore, ACE and TXE values were significantly correlated with soluble phenols and proteins (Table 5).
| Assay (2008) | Soluble phenols | Soluble proteins |
|---|---|---|
| a Significant: **P < 0.01, *P < 0.05. | ||
| Antioxidant activity | ||
| ACE | ||
| Fresh tissue | 0.93** | 0.52** |
| Wounded tissue | 0.85* | 0.45* |
| TXE | ||
| Fresh tissue | 0.96** | 0.57** |
| Wounded tissue | 0.91** | 0.50* |
Discussion
The evaluation of cultivated and wild, tuber-bearing Solanum species may lead to individual accessions bearing valuable traits and genes worth introducing into new potato cultivars by breeding. In this respect, several of the 23 genotypes tested in this study could be interesting candidates.Soluble phenols
The quantities of total soluble phenols found in tuber tissue of the wild and cultivated Solanum species involved in this study concurred with results reported elsewhere.18–20As expected, the different genotypes revealed a wide range in phenol contents of their tuber tissue (Table 2A). On the one side, there was the accession chc 30161-17 (S. chacoense) with its small amounts of soluble phenols, and on the other side, the two accessions pnt 31598-2 and 31598-3 (S. pinnatisectum) with their relatively high phenol contents (Table 2A). With it, these last two accessions reached similar high phenol levels as purple-fleshed potato breeding clones studied recently,15 whereas most of the other genotypes had less phenols in their tuber tissue and were thus comparable with actual, white/yellow fleshed potato cultivars.18 The fact that S. pinnatisectum exhibited significantly higher quantities of soluble phenols than the other three Solanum species (i.e. adg, chc and phu) had (Table 4A) concurred with former results, obtained in 2006 and 2007.21The effect of wound stress on soluble phenols
Wound stress is a continuous threat for potatoes in agricultural practice, during storage and transportation as well as in potato commerce because wounds are a major entree for plant pathogenic microorganisms causing tissue decay associated with quality decrease. Hence, a rapid wound healing is needed, and this process requires sufficient amounts of simple phenols in the wound area. It was important therefore, that soluble phenols were increased after wounding the tubers of wild and cultivated Solanum species (Table 2A). Especially, S. tuberosum subsp. andigena which had relatively low phenol contents in its fresh tissue displayed the greatest raise of phenols in response to wounding its tubers. The differences in phenols between fresh and wounded tuber tissue were statistically significant within the three Solanum species adg, chc and phu, but interestingly, not within S. pinnatisectum. For example, pnt 31598-2 had smaller phenol quantities in extract samples taken after 24 h than in those derived from fresh tuber tissue (Table 2A). This tendency might be based on the fact that S. pinnatisectum displayed considerably higher basic levels of phenols in its fresh tissue than the other Solanum species had. A similar differentiated wound-induced alteration of plant phenols was noticed in white/yellow and purple flesh potato cultivars and clones,15,22 and a weaker effect on phenols induced by wounding was also reported for a red-lettuce that was very rich in phenols.23Plant phenols are not only involved in pathogen defence due to their antimicrobial activities,24–27 they also function as precursors in the biosynthesis of suberin and lignin, both complex polymers associated with wound healing28 and defence responses.29,30 It has been suggested recently, that chlorogenic acid (CGA) as one of the major simple plant phenols31 could act as a reservoir that can rapidly be mobilized7 to form phenylpropanoid products such as lignin, phytoalexins and cell wall cross-linking compounds.32–34 This may explain why Solanum genotypes exhibiting small amounts of phenols in their tissue, like adg, increased their phenol concentrations much more in response to wounding than those with relatively high basic levels of phenols, such as pnt 31598-2 (Table 2A). Obviously, the latter had no compelling need to refill its reserves, in contrary, in tissue of pnt 31598-2 the phenol quantities were diminished after 24 h. Its tissue may have started previously to integrate portions of simple phenols into more complex polymers such as lignin or intermediates, whereas the other genotypes, like those of adg, aspired to fill up their phenol reserves in the meantime. It can be assumed therefore that in tuber tissue of pnt 31598-2 the wound response is advanced. Altogether these results underline the role of plant phenols within wound stress responses actually directed to healing damaged tissue as soon as possible and to prevent further damage.35 But besides their function within stress responses, plant phenols could contribute to the nutritional value of potatoes as discussed below.
Antioxidants
Regarding their consumption potatoes are considered as a significant antioxidant source in human nutrition.36 Different potato cultivars had antioxidant activities that excelled onion, carrots and bell pepper.18 Besides ascorbic acid, α-tocopherol and β-carotene, plant phenols such as CGA also play a role as potent antioxidants.18,37 It was to be expected therefore that similar to that observed with phenols, the 23 genotypes involved in this study varied notably in their antioxidant activity measured as ascorbic acid (ACE: Fig. 1) and trolox equivalent (TXE: Fig. 2). On average, S. pinnatisectum displayed the highest antioxidant capacity, and pnt also differed significantly in its antioxidant activity from the other three Solanum species (Table 4C), again a result which coincided with soluble phenols (Table 4A) and proteins (Table 4B). Similar high amounts of antioxidants were found in tuber tissue of pnt during another test performed in 2007.21The effect of wound stress on antioxidants
Investigating the effect of wound stress on the level of antioxidants was a novel aspect with respect to wild Solanum potatoes. It was important to find out that the antioxidant activity, including water (ACE: Fig. 1, Table 3A) and lipid soluble antioxidants (TXE: Fig. 2, Table 3B) raised on average 24 h after wounding the tubers of cultivated and wild Solanum species - a result that concurred with phenols (Table 2A) discussed above. The differences in ACE (Table 3A) and TXE (Table 3B) values between fresh and wounded tuber tissue were statistically significant when all genotypes were regarded and within the species adg, chc and phu. On average, also the wild Solanum species S. pinnatisectum increased its antioxidant potential in response to wounding (Table 3A and 3B). However, in the case of pnt the differences were statistically not significant. Similar to that observed with soluble phenols, there were several genotypes among pnt (e.g. pnt 31598-2) that revealed slightly reduced antioxidant activities after wounding. It was also interesting to note that the cultivated Solanum species adg and phu displayed greater enhancements of ACE (Fig. 1; Table 3A) and TXE (Fig. 2; Table 3B) values 24 h after wounding than the two wild potato species chc and pnt. However the latter, above all S. pinnatisectum exhibited on average multiple higher basic levels of water and lipid soluble antioxidants in the non-wounded tissue than adg and phu had (Table 3A). It seems that in the tuber tissue of pnt the level of antioxidants was sufficiently high and thus no tremendous increase of the latter was noticed. This tendency may also underline the role of antioxidants in wound stress responses of tuber tissue. A similar differentiated wound-induced alteration was found in context with plant phenols as discussed before.Apart from this fact, the enhancement of antioxidant capacity by wounding, especially within those Solanum species which had low concentrations of antioxidants in their fresh tissue (adg, phu) was not surprising, because there is growing evidence that plants respond to stress factors, such as wounding, by enhancing their radical scavenging capacity.7,9,38 A wound-induced increase of soluble phenols and antioxidant capacity in potatoes was also reported by Reyes & Cisneros-Zevallos,22 who in addition found an elevated activity of phenylalanine ammonia-lyase (PAL), an enzyme which is responsible for the synthesis of phenolic metabolites.5
The relationship between soluble phenols and antioxidant activity
In addition, results of the present tests reflect a close relationship between antioxidants and soluble phenols. Thus, a significant correlation could be observed between the ascorbic acid equivalent and soluble phenols as well as between the trolox equivalent and the latter (Table 5). In addition, quantities of soluble phenols showed a linear relationship with ACE (fresh: R2 = 0.86; wound: R2 = 0.72) and TXE values (fresh: R2 = 0.93; wound: R2 = 0.82). A similar clear relationship between phenols and antioxidants was reported for purple and white/yellow fleshed potato breeding clones and cultivars15 as well as for Andean potato cultivars studied elsewhere.19 Altogether, these results underline the notion that amounts of phenols in tuber tissue can be an indicator for the antioxidant capacity of potatoes,39 and vice versa. Plant phenols are well known to function as efficient antioxidants7 due to a hydrogen-donating activity of the phenolic hydroxyl groups.9,40 For example, L-tyrosine, a monophenolic amino acid was found to exhibit strong antiradical activities.41,42 Moreover, caffeic and chlorogenic acid which are both among the major free phenolics in potatoes,36 and especially concentrated in the peel, correlated with the 1,1-diphenyl-2-picryl-hydrazyl free radical (DPPH·) scavenging activity.43 In this context, it should be mentioned that pnt 31598-2 with its high amounts of antioxidants (ACE: Fig.1; TXE: Fig. 2) was also found recently to have high content of chlorogenic acid in its tuber tissue (2.68 g kg−1 fdm).21 In potato peel extracts caffeic- and chlorogenic acid contributed to about 57% of total antioxidant activity measured as TXE,44 and both phenolic acids exceeded the antioxidant activity of α-tocopherol as reported elsewhere.45 Moreover, caffeic acid exhibited a stronger inhibiting activity on lipid peroxidation than trolox, an analogue of α-tocopherol,43 and also methanolic potato extracts had a better antioxidant capacity than the latter.46 Potato peel extracts containing high quantities of gallic-, caffeic acid and CGA inhibited lipid peroxidation and protected human erythrocyte membrane proteins from oxidative damage,47 while a potato peel powder was demonstrated to ameliorate oxidative stress in streptozotocin diabetic rats.48 All this indicates, that high amounts of simple plant phenols are associated with high antioxidant activities. Accordingly, plant phenols seem to have the ability to positively affect the antioxidant system in plant, animal and human cells. In this context it should also be mentioned, that caffeic acid and CGA can be absorbed in the small intestine of humans49 - i.e. part of hydroxycinnamic acids from food will enter into the blood circulation and could hereby induce biological effects in the human body. For example, plant phenols are well known to be anti-carcinogenic, anti-inflammatory, vasodilatory, antibacterial, immune-stimulating, anti-allergic etc.40,50 due to their radical scavenging activities.Soluble proteins
As observed in context with soluble phenols (Table 2A) and antioxidants (Fig. 1 and 2), the 23 Solanum genotypes varied in their contents of total soluble proteins (Table 2B). It was important to note, that also in this respect S. pinnatisectum exceeded on average the other three Solanum species involved in the tests (Table 2B, Table 4B). In particular, its two accessions pnt 31606-2 and pnt 31598-3 had significant soluble protein concentrations (Table 2B). On the other hand, the species S. tuberosum subsp. andigena exhibited on average the lowest amounts of proteins in its fresh tuber tissue - a result which was in agreement with soluble phenols.The effect of wound stress on soluble proteins
Wounding of plant tissue is normally associated with an increase in wound-related proteins within a few hours.51,52 For example, protein kinases, proteinase inhibitors,53 pathogenesis related proteins,51 PAL and peroxidase54 were found to be induced in plant tissue by wounding. It was to be expected therefore, that the protein contents increased on average 24 h after wounding the tubers (Table 2B). However, the increase of soluble proteins was less significant than that observed in the case of phenols (Table 2A) and antioxidants (Fig. 1 and 2; Table 3A and 3B). Only small differences with respect to proteins between wounded and non-wounded potato tubers were also reported by Logemann et al.52 Nevertheless, the enhancement of proteins by wounding, above all in tuber tissue of adg (Table 2B), a species with a relatively low level of soluble proteins in its fresh tissue, may underline their importance in wound stress responses of potatoes.The relationship between soluble proteins and antioxidant activity
It was interesting to note that soluble proteins correlated not only with soluble phenols (fresh tissue: r = 0.52), but also with water and lipid soluble antioxidants (Table 5). It appears therefore, that besides plant phenols, soluble proteins also accumulated in tuber tissue to support the antioxidant capacity of potatoes. Although, their effect seems to be less strong than that of phenols, a notion which is reflected by the results of regression analyses (fresh tissue, ACE: R2 = 0.27 and TXE: R2 = 0.31; wounded tissue, ACE: R2 = 0.21 and TXE: R2 = 0.25).Patatin, used as a trivial name for a family of glycoproteins, accounts for 30–40% of total soluble proteins accumulated in potatoes.55 Besides its function as a major storage protein in tuber tissue, it was reported to exhibit lipid acyl hydrolase and acyltransferase activity with a large number of lipid substrates,56 and moreover, patatin has been demonstrated by a series of in vitro tests to possess efficient radical scavenging activities.57 Especially, the cystein and tryptophan residues in the patatin molecule are assumed to mediate its antioxidant activity.57 It is imaginable therefore, that patatin contributed partly to the antioxidant potential of cultivated and wild Solanum species. Logemann et al.52 reported that wounding leads to suppression of patatin transcription in favour of defence and repair mechanisms, a fact which may explain why soluble proteins (Table 2B) raised less strong upon wounding than soluble phenols (Table 2A) and antioxidants (Fig. 1 and 2; Table 3A and 3B) which are both associated with plant defence responses.6,26
Characteristics of S. pinnatisectum
All these results imply that an involvement of S. pinnatisectum, a Mexican wild diploid Solanum species, with its relatively high contents of soluble phenols (Table 2A) and proteins (Table 2B) as well as water (Fig. 1) and lipid soluble antioxidants (Fig. 2) in potato breeding could be useful in order to improve the efficiency of the antioxidant system in tuber tissue of new cultivars. In addition, S. pinnatisectum was found recently to have high quantities of starch in its tuber tissue.13 However, tubers of this Solanum species are relatively small, i.e. about 10 to 15 mm diameter, and several backcrosses with cultivated potatoes will be necessary to get an acceptable tuber size. Moreover, this Solanum species could contain higher levels of glycoalkaloids (GA) including α-solanin and α-chaconine than are found in S. tuberosum.58 Apart from undesirable flavor,59 GA's are known to be poisoning.60,61 Therefore, the quantities of GA in parental genotypes and progenies derived from such crosses have to be checked in order not to exceed the accepted concentrations of <200 mg kg−1 fresh weight.62 Ergo - a transfer of desired properties from pnt to new potato cultivars by conventional breeding is not very easy.Conclusions
The results revealed that soluble phenols and soluble proteins present in tuber tissue substantially contribute to the total antioxidant capacity of potatoes. It was important to note, that (1) soluble phenols and (2) soluble proteins as well as (3) water and (4) lipid soluble antioxidants increased notably upon wounding the tubers of cultivated and wild Solanum species, a result which underlines the importance of all these components in wound stress responses of potatoes. Furthermore, it was interesting to find out that S. pinnatisectum exhibited significantly higher concentrations of total soluble phenols and proteins as well as water and lipid soluble antioxidants in its fresh and wounded tuber tissue than the other Solanum species had. Accordingly, an involvement of individual S. pinnatisectum accessions, e.g. pnt 31598-2 and 31598-4, in potato breeding could be profitable for the antioxidant potential and with it the nutritional value of new potato cultivars. This last aspect could be of great importance for the potato processing industry.Abbreviations
| fw | fresh weight |
| fdm | freeze-dried matter |
| ACE | ascorbic acid equivalent |
| TXE | trolox equivalent |
| PCL | photochemiluminescence |
| CGA | chlorogenic acid |
| GA | glycoalkaloids |
Acknowledgements
Ilona Schollenberg is thanked for excellent technical assistance.References
- J. B. Bamberg and A. H. del Rio, The canon of potato science: Genetic diversity and genebanks, Potato Res., 2007, 50, 207–210 CrossRef.
- S. C. Chiro, G. Olteanu and L. E. Asanache, History, statistics and trends of the Romanian potato industry, Potato Res., 2008, 51, 217–222 CrossRef.
- J. Van Gijssel, The potential of potatoes for attractive convenience food: Focus on product quality and nutritional value, in Potato in Progress, ed. by A. J. Haverkort and P. C. Struik. Wageningen Academic Publishers, Wageningen, 2005, pp. 27–32 Search PubMed.
- T. R. Tarn, Breeding for quality improvement: Market fitness and nutritional quality, in Potato in Progress, ed. by A. J. Haverkort and P. C. Struik. Wageningen Academic Publishers, Wageningen, 2005, pp. 66–75 Search PubMed.
- K. Hahlbrock and D. Scheel, Physiology and molecular biology of phenylpropanoid metabolism, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1989, 40, 347–369 CrossRef CAS.
- R. L. Nicholson and R. Hammerschmidt, Phenolic compounds and their role in disease resistance, Annu. Rev. Phytopathol., 1992, 30, 369–389 Search PubMed.
- S. C. Grace, Phenolics as antioxidants, in Antioxidants and reactive oxygen species in plants, ed. by Smirnoff N, Blackwell Publishing Ltd, Oxford, 2005, pp.141–168 Search PubMed.
- G. Noctor and C. H. Foyer, Ascorbate and glutathione: Keeping active oxygen under control, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1998, 49, 249–279 CrossRef CAS.
- R. Blomhoff, Dietary antioxidants and cardiovascular disease, Curr. Opin. Lipidol., 2005, 16, 47–54 CrossRef CAS.
- B. L. Halvorsen, M. H. Carlsen, K. M. Phillips, S. K. Bohn, K. Holte, D. R. Jacobs and R. Blomhoff, Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States, Am. J. Clin. Nutr., 2006, 84, 95–135 CAS.
- R. A. Dixon and N. L. Paiva, Stress-induced phenylpropanoid metabolism, Plant Cell, 1995, 7, 1085–1097 CrossRef CAS.
- M. A. K. Jansen, K. Hectors, N. M. O'Brien, Y. Guisez and G. Potters, Plant stress and human health: Do human consumers benefit from UV-B acclimated crops?, Plant Sci., 2008, 175, 449–458 CrossRef CAS.
- G. Jansen, W. Flamme, K. Schüler and M. Vandrey, Tuber and starch quality of wild and cultivated potato species and cultivars, Potato Res., 2001, 44, 137–146 CrossRef CAS.
- J. G. Hawkes, Origins of cultivated potatoes and species relationships, in Potato Genetics, ed. by J. E. Bradshaw and G. R. Mackay. CAB International, Wallingford, 1994, pp. 3–42 Search PubMed.
- C. B. Wegener, G. Jansen, H. U. Jürgens and W. Schütze, Special quality traits of coloured potato breeding clones: Anthocyanins, soluble phenols and antioxidant capacity, J. Sci. Food Agric., 2009, 89, 206–215 CrossRef CAS.
- D. M. Cahill and J. A. McComb, A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophthora cinamomi, Physiol. Mol. Plant Pathol., 1992, 40, 315–332 Search PubMed.
- I. N. Popov and G. Lewin, Photochemiluminescent detection of antiradical activity; IV: testing of lipid soluble antioxidants, J. Biochem. Biophys. Methods, 1996, 31, 1–8 CrossRef CAS.
- M. S. Al-Saikhan, L. R. Howard and J. C. Miller, Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum L.), J. Food Sci., 1995, 60, 341–343 CrossRef CAS.
- C. M. Andre, M. Ghishlain, P. Bertin, M. Oufir, M. Del Rosario Herrera and L. Hoffmann, Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients, J. Agric. Food Chem., 2007, 55, 366–378 CrossRef CAS.
- J. A. Vinson, Y. Hao, X. Su and L. Zubik, Phenol antioxidant quantity and quality in foods: Vegetables, J. Agric. Food Chem., 1998, 46, 3630–3634 CrossRef CAS.
- C. B. Wegener and G. Jansen, Antioxidants in wild and cultivated potato species, in Crop Plant Resistance to Biotic and Abiotic Factors, ed. by F. Feldmann, D. V. Alford and C. Furk. DPG Verlag, Braunschweig, 2009, pp. 91–95 Search PubMed.
- L. F. Reyes and L. Cisneros-Zevallos, Wounding stress increases the phenolic content and antioxidant capacity of purple-flesh potatoes (Solanum tuberosum L.), J. Agric. Food Chem., 2003, 51, 5296–5300 CrossRef CAS.
- F. Ferreres, M. I. Gil, M. Castaner and F. A. Tomás-Barberán, Phenolic metabolites in red pigmented lettuce (Lactuca sativa). Changes with minimal processing and cold storage, J. Agric. Food Chem., 1997, 45, 4249–4254 CrossRef.
- A. S. Ghanekar, S. R. Padwal-Desai and G. B. Nadkarni, The involvement of phenolics and phytoalexins in resistance of potato to soft rot, Potato Res., 1984, 27, 189–199 CrossRef CAS.
- A. Kumar, V. S. Pundhir and K. C. Gupta, The role of phenols in potato tuber resistance against soft rot caused by Erwinia carotovora ssp, carotovora. Potato Res., 1991, 34, 9–16 Search PubMed.
- G. D. Lyon and F. M. McGill, Inhibition of growth of Erwinia carotovora in vitro by phenolics, Potato Res., 1988, 31, 461–467 CrossRef CAS.
- J. Weber, O. Olsen, C. Wegener and D. von Wettstein, Digalacturonates from pectin degradation induce tissue responses against potato soft rot, Physiol. Mol. Plant Pathol., 1996, 48, 389–401 Search PubMed.
- N. G. Lewis and E. Yamamoto, Lignin: Occurrence, biogenesis and biodegradation, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1990, 41, 455–496 CrossRef CAS.
- R. T. V. Fox, J. G. Manners and A. Myers, Ultrastructure of tissue disintegration and host reactions in potato tubers infected by Erwinia carotovora var atroseptica., Potato Res., 1972, 15, 130–145 CrossRef.
- C. P. Vance, T. K. Kirk and R. T. Sherwood, Lignification as a mechanism of disease resistance, Annu. Rev. Phytopathol., 1980, 18, 259–288 Search PubMed.
- C. R. Brown, Antioxidants in potato, Am. J. Potato Res., 2005, 82, 163–172 Search PubMed.
- K. Yao, V. De Luca and N. Brisson, Creation of a metabolic sink for tryptophan alters the phenylpropanoid pathway and the susceptibility of potato to Phytophthora infestans, Plant Cell, 1995, 7, 1787–1799 CAS.
- S. C. Grace and B. A. Logan, Energy dissipation and radical scavenging by the plant phenylpropanoid pathway, Philos. Trans. R. Soc. London, Ser. B, 2000, 355, 1499–1510 CrossRef CAS.
- J. Días, A. Ros Barceló and F. M. De Cáceres, Changes in shikimate dehydrogenase and the end products of the shikimate pathway, chlorogenic acid and lignins, during the early development of seedlings of Capsicum annuum, New Phytol., 1997, 136, 183–188 CrossRef.
- J. León, E. Rojo and J. J. Sánchez-Serrano, Wound signalling in plants, J. Exp. Bot., 2001, 52, 1–9 CrossRef CAS.
- J. Lachman and K. Hamouz, Red and purple coloured potatoes as a significant antioxidant source in human nutrition - a review, Plant Soil Environ., 2005, 51, 477–482 Search PubMed.
- T. Byers and G. Perry, Dietary carotenes, vitamin C, and vitamin E as protective antioxidants in human cancers, Annu. Rev. Nutr., 1992, 12, 139–159 CrossRef CAS.
- S. C. Grace and B. A. Logan, Energy dissipation and radical scavenging by the plant phenylpropanoid pathway, Philos. Trans. R. Soc. London, Ser. B, 2000, 355, 1499–1510 CrossRef CAS.
- C. C. Teow, V. D. Truong, R. F. McFeeters, R. L. Thompson, K. V. Pecota and G. C. Yencho, Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colour, Food Chem., 2007, 103, 829–838 CrossRef CAS.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Structure-antioxidant activity relationships of flavonoids and phenolic acids, Free Radical Biol. Med., 1996, 20, 933–956 CrossRef CAS.
- I. Gülcin, Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa, Amino Acids, 2007, 32, 431–438 CrossRef CAS.
- F. W. P. C. Van Overveld, G. R. M. M. Haenen, J. Rhemrev, J. P. W. Vermeiden and A. Bast, Tyrosine as important contributor to the antioxidant capacity of seminal plasma, Chem.-Biol. Interact., 2000, 127, 151–161 CrossRef CAS.
- I. Gülcin, Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid), Toxicology, 2006, 217, 213–320 CrossRef.
- K. Nara, T. Miyoshi, T. Honma and H. Koga, Antioxidative activity of bound-form phenolics in potato peel, Biosci., Biotechnol., Biochem., 2006, 70, 1489–1491 CrossRef CAS.
- J. H. Chen and C. T. Ho, Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds, J. Agric. Food Chem., 1997, 45, 2374–2378 CrossRef CAS.
- R. G. O. Rumbaoa, D. F. Cornago and I. M. Geronimo, Phenolic content and antioxidant capacity of Philippine potato (Solanum tuberosum) tubers, J. Food Compos. Anal., 2009, 22, 546–550 CrossRef CAS.
- N. Singh and P. S. Rajini, Antioxidant-mediated protective effect of potato peel-extract in erytrocytes against oxidative damage, Chem.-Biol. Interact., 2008, 173, 97–104 CrossRef CAS.
- N. Singh, V. Kamath and P. S. Rajini, Protective effect of potato peel powder in ameliorating oxidative stress in streptozotocin diabetic rats, Plant Foods Hum. Nutr., 2005, 60, 49–54 CrossRef CAS.
- M. R. Olthof, P. C. H. Hollman and M. B. Katan, Chlorogenic acid and caffeic acid are absorbed in humans, J. Nutr., 2001, 131, 66–71 CAS.
- M. Valko, C. J. Rhodes, J. Moncol, M. Izakovic and M. Mazur, Free radicals, metals and antioxidants in oxidative stress-induced cancer, Chem.-Biol. Interact., 2006, 160, 1–40 CrossRef CAS.
- R. A. Mehta, B. L. Parsons, A. M. Mehta, H. L. Nakhasi and A. K. Mattoo, Differential protein metabolism and gene expression in tomato fruit during wounding stress, Plant Cell Physiol., 1991, 32, 1057–1065 CAS.
- J. Logemann, J. E. Mayer, J. Schell and L. Willmitzer, Differential expression of genes in potato tubers after wounding, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 1136–1140 CrossRef CAS.
- S. Seo, H. Sano and Y. Ohashi, Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase, Plant Cell, 1999, 11, 289–298 CAS.
- R. Borchert, Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue, Plant Physiol., 1978, 62, 789–793 CrossRef CAS.
- D. Racusen and M. Foote, A major soluble glycoprotein of potato tubers, J. Food Biochem., 1980, 4, 43–52 CrossRef CAS.
- D. L. Andrews, B. Beames, M. D. Summers and W. D. Park, Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector, Biochem. J, 1988, 252, 199–206 CAS.
- Y. W. Liu, C. H. Han, M. H. Lee, F. L. Hsu and W. C. Hou, Patatin, the tuber storage protein of potato (Solanum tuberosum L.) exhibits antioxidant activity in vitro, J. Agric. Food Chem., 2003, 51, 4389–4393 CrossRef CAS.
- P. Gregory, S. L. Sinden, S. F. Osman, W. M. Tingey and D. A. Chessin, Glycoalkaloids of wild, tuber-bearing Solanum species, J. Agric. Food Chem., 1981, 29, 1212–1215 CrossRef CAS.
- S. L. Sinden and K. L. Deahl, Effect of glycoalkaloids and phenolics on potato flavor, J. Food Sci., 1976, 41, 520–523 CrossRef CAS.
- I. Ginzberg, J. G. Tokuhisa and R. E. Veilleux, Potato steroidal glycolakaloids: Biosynthesis and genetic manipulation, Potato Res., 2009, 52, 1–15 CrossRef CAS.
- D. B. Smith, J. G. Roddick and J. L. Johnes, Potato glycoalkaloids: Some unanswered questions, Trends Food Sci. Technol., 1996, 7, 126–131 CrossRef CAS.
- J. P. T. Valkonen, M. Kekitalo, T. Vasara and L. Pietilä, Potato glycoalkaloids: A burden or a blessing?, Crit. Rev. Plant Sci., 1996, 15, 1–20 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
