Biocidal properties of metal oxidenanoparticles and their halogenadducts
Johanna A.
Haggstrom
a,
Kenneth J.
Klabunde
*a and
George L.
Marchin
b
aDepartment of Chemistry, Kansas State University, Manhattan, Kansas 66506, USA. E-mail: kenjk@ksu.edu
bDivision of Biology, Kansas State University, Manhattan, Kansas 66506, USA
First published on 20th November 2009
Abstract
Nanosized metal oxide halogen adducts possess high surface reactivities due to their unique surface morphologies. These adducts have been used as reactive materials against vegetative cells, such as Escherichia coli as well as bacterial endospores, including Bacillus subtilis and Bacillus anthracis (Δ Sterne strain). Here we report high biocidal activities against gram-positive bacteria, gram-negative bacteria, and endospores. The procedure consists of a membrane method. Transmission electron micrographs are used to compare nanoparticle-treated and untreated cells and spores. It is proposed that the abrasive character of the particles, the oxidative power of the halogens/interhalogens, and the electrostatic attraction between the metal oxides and the biological material are responsible for high biocidal activities. While some activity was demonstrated, bacterial endospores were more resistant to nanoparticle treatment than the vegetative bacteria.
1. Introduction
Several laboratories have recently investigated the use of nanomaterials as biocides. Work has concentrated on the use of nanosized TiO2, ZnO and silver. Several products with incorporated nano-silver are on the market, including antibacterial clothes that kill bacteria and prevent odor. There are advantages to nanosized biocides. For example, since chemical properties change at the nanoscale, it is possible that there are many elements or compounds that are non-biocidal at micron size that may be biocidal at nanosize. It is also possible and that micron-sized materials that are somewhat biocidal become even more biocidal on the nanoscale. Nanoparticles can also be prepared in a variety of forms, such as powders, slurries and pellet form, which makes their use more convenient and widely applicable. Nanoparticles are generally easily stored which increases their flexibility.The biocidal properties of nanoparticleMgO–halogenadducts were reported earlier.1,2 Many more such nanosized metal-oxideadducts have now been prepared, and of particular interest are Al2O3, TiO2, and CeO2 systems, where the strength of interactions with halogens vary, affecting their stabilities and activities.3,4 Herein, we report the biocidal activities of these nanomaterials with several vegetative bacterial cells and endospores. Nanosized metal-oxide–halogenadducts possess high surface reactivities due to their unique surface morphologies. Several metal oxides were studied throughout this research, but the main focus was set on NanoActive® (NA) Al2O3 Plus, NA–TiO2 and NA–CeO2.
To determine the bactericidal activity a membrane technique was developed. The surface of nanomaterials gives them a significant advantage as disinfectants. Their high surface area, the presence of defect sites like corners and edges, and a general abrasive character influences their chemical reactivity.2 A solid interaction depends mainly on the surface morphology and the more of the surface exposed, the more interaction, leading to a hopefully better biocidal activity.
There are many types of microorganisms of interest that could be studied, such as bacteria, spores, viruses, fungi and so on. Our attention was focused on several mimics of biological warfare agents and also of common bacteria that represents different bacterial classes. The interaction of these prepared adducts with viruses will be discussed in a later report.
The organisms studied include Escherichia coli (E. coli), a gram-negative rod-shaped bacterium, Bacillus subtilis (B. subtilis) spores and the Δ Sterne strain of Bacillus anthracis (B. anthracis), an endospore-forming bacterium and a surrogate of anthrax.
Many studies have paid special attention to the mechanism by which bacteria and spores die, however, spores have been the most difficult to study. Antimicrobial compounds can be very specific in the way they affect the systems of the cell, eventually leading to death. Examples are the antibiotics like penicillins where the process of building the cell envelope is completely blocked or significantly slowed down, which leads to alterations of the cell membrane, hindering reproduction.5Halogens that have strong oxidizing powers however, have a more general activity that is not limited to the cell membrane, but that rather affects all systems in the cell.6–9
Most bactericides interact with major chemical pathways of the cell. Many antibiotics, for example, suppress the protein synthesis, whereas heavy-metal ions bind to the sulfhydryl groups of important enzymes, eventually leading to cell death. Another major example of cell death cause is damage to the cell membrane, which then could lead to cell leakage of the internal content. However, depending on the severity of the damage, the cell might not die if the surrounding environment is favorable. On the other hand, the cell would be more vulnerable to additional attack when the membrane is already slightly damaged. This process, where a cell or spore is damaged and more susceptible to death, is called sensitization. This concept becomes very important in the case of spores, where a combination of two agents is often much more efficient than one agent alone, and very often necessary.10–23
Nanoparticles have very different surface morphologies as compared to their micron-sized counterparts. This leads to an expectation that the nanoparticle surface–cell membrane interaction would be the major one that will determine the activity of the nanoparticles against cells and spores. In addition, the active halogen/interhalogen on the surface of the particles will carry an oxidizing power with them, further increasing the activity of the material. The size of the nanoparticles themselves however, is much too large to directly penetrate a healthy cell membrane.
In this study we have focused on determining whether nanoparticles, especially halogen and interhalogen adducts of metal oxides, are in fact promising for the use against vegetative cells and spores. It is of high importance that new solid formulations, displaying high biocidal activities, are formulated for potential use against warfare attacks, since the liquid forms currently available are not suitable for use on all types of surfaces. In addition, nanosized metal oxides are very easy to handle and they are considerably more environmentally friendly as compared to many other currently used biocides.
2. Methods
2.1 Preparation of halogen/interhalogen adducts
The procedure was carried out as described in the literature4 and is described in short below.
About 10 g of freshly activated powder contained in a 150 mL Schlenk tube was allowed contact with chlorine gas, or in the case of Br2 or ICl, connected to a Schlenk tube containing liquid Br2 or ICl. Normally two doses of halogen/interhalogen were transferred initially. The difference in pressure between the atmosphere above the metal oxide and in the halogen source allowed the transfer of Cl2 gas or Br2 and ICl vapors to the Schlenk tube containing the metal oxide. During this step, the outlet to the vacuum line was closed. As the color of the atmosphere above the powder changed to green-yellow (chlorine) or brown-red (bromine or iodine monochloride), the connection was disengaged and the powder was well shaken to encourage maximum contact. Another dose of halogen was transferred when the atmosphere above the powder had cleared up. Halogen dosage continued until the atmosphere above the powder did not clear up and halogen remained. The halogen container was then disconnected and the powder was again connected to the vacuum line and maintained until the pressure reached 50 × 10−3 Torr or below. This step took approximately 20 min and ensured the removal of excess halogen The freshly prepared halogenadduct was transferred to a Teflon®-sealed glass vial.
2.2 Preparation of bacterial cultures
Escherichia coli strain C3000 was obtained from ATCC. Bacillus subtilisspores were purchased from Raven Biological Labs.E. coli (either a small amount of previously prepared liquid bacterial culture or a single colony from a Petri dish) was added to a sterile flask, containing 25–100 mL Luria Broth (LB) nutrient. The solution was typically agitated under aerobic conditions for 12–24 h. on a shaker-thermostat at 37 °C. At that moment the flask contained a turbid suspension of bacteria cells with a concentration between 106–109 Colony Forming Units (CFU)/mL.
B. subtilis spore suspension was used as-received with the concentration already determined by Raven Biological Labs.
B. anthracis, Δ Sterne strain, was received from the Life Sciences Center, University of Missouri, Columbia (this strain is totally avirulent).
B. anthracis spores, Δ Sterne strain, were prepared using two different techniques. To prepare small amounts for testing procedures, 100 μL of B. anthracis culture was added to a nutrient agar plate and incubated for 5–15 days (depending on how thick the agar on the plate was), until no nutrients remained and sporulation had occurred. The spores were then suspended in 3 mL PBS buffer and centrifuged at 2500 rpm to obtain a pellet. The supernatant was discarded and the pellet was resuspended in 10 mL of PBS buffer, vortexed and centrifuged again. This washing procedure was repeated three times and finally the spores were stored at room temperature in PBS buffer until used.
2.3 Bacteriological test procedure
Vegetative cells or spores were diluted to a desirable concentration (normally in the order of 103–109 CFU/mL, depending on organism and nanoparticles used).For each experiment, sterile individually wrapped nitrocellulose water filter paper membranes (Millipore) with a pore diameter of 0.45 μm (for vegetative cells) or 0.22 μm (B. subtilis and B. anthracisspores) were used to retain the bacteria cells or spores.
Before the experiments were performed, the Fisher Scientific filtering system, including removable funnels, was washed with 70% ethanol to avoid contamination from other organisms. Everything was rinsed with distilled water several times, to ensure complete removal of ethanol. Each filter paper used was moistened before use by submerging it into a beaker containing distilled water. The filter paper was then positioned in the filtering system and approximately 30 mL distilled water was added. With the filtering system still off, the desired amount of bacteria or spores was added (normally 100 μL). The filtering system was started, removing the water, while the bacteria or spores were retained on the filter paper. The filter paper was then carefully removed using special tweezers and allowed to partially dry on a clean paper towel with the inoculated side of the filter upwards. The filtering system contained three funnels so this step was repeated until the desired number of inoculated filter papers was obtained.
To avoid sticking of the nanoparticles to the filter paper membranes, they needed to be partially or completely dried. In the case of vegetative cells, the filters were only partially dried (about 15 min). In the case of spores, the filter membranes were completely dried (45 min). For each experiment a control filter paper that underwent the exact same treatment, except for the addition of nanoparticles was always used. After drying, nanoparticles were added directly to the filter paper, completely covering the inoculated area. The particles were left for 30 min, and then carefully shaken off. To get rid of the possible remaining halogens/interhalogens, the filter was put in the filtering system and washed with a small amount of 5 wt% sodium thiosulfate solution. Without this step, false good results could arise from the remaining halogen suppressing the growth of survived bacterial cells or spores. The control filter paper underwent this treatment as well. The filter papers were then deposited directly onto agar plates and incubated for 24 h. The colonies that were formed were then counted and the results averaged. Each nanoparticle formulation and control was performed in triplicate and the experiment was later repeated at least one more time and the results averaged. The results were compared to the control plates and log reduction values were calculated. The log-kill values all have standard deviation values less than one and most of them have a standard deviation less than ±0.5.
2.4 Transmission electron microscopy (TEM)
TEM images were recorded on a Philips CM 100, operating at 100 kV.TEM images of E. coli cells, were prepared using the following procedure: 100 μL of an E. coli suspension was inoculated into 50 mL LB broth in a shaker at 37 °C for 6 h. The entire fresh culture was centrifuged and re-suspended in PBS buffer and kept for further treatments.
TEM images of B. anthracis, Δ Sterne strain cells, were prepared using the following procedure: one single colony from the agar plate, containing B. anthracis cells, was inoculated into 50 mL LB broth in a shaker at 37 °C and allowed to age for 11 h. 35 mL of this overnight culture was centrifuged and the precipitated solid re-suspended in PBS buffer.
After re-suspension of the different cells (E. coli and B. anthracis) and spores (B. subtilis and B. anthracis) in PBS buffer, they were centrifuged again, and each one of the obtained pellets was dissolved in 2 mL DI water, vortexed and divided into two equal parts in eppendorf tubes. One of the tubes served as the control sample and the other one was used for nanoparticle treatment. NA–Al2O3/ICl3 Plus was added to one of the tubes, vortexed and left to interact for 60 min. Both tubes were then centrifuged, decanted and the solid washed five times with 0.1 M freshly prepared ascorbic acid solution. The pellets were then washed with distilled water, centrifuged and decanted. The control and nanoparticle-treated pellet cells/spores were then immersion fixed with 2% glutaraldehyde and left overnight with constant rotation. The samples were then washed with sodium cacodylate buffer a total of three times, each time for five minutes and with constant rotation in order to remove all the glutaraldehyde. During each washing, the pellet was resuspended in the buffer. The pellets were resuspended and post-fixed with 0.1 mL of 1% osmium tetraoxide solution, under constant rotation for 1.5 h. The pellets were then washed three times for five minutes each with sodium cacodylate buffer under constant rotation, each time the pellets were resuspended. The samples were then gradually dehydrated with increasing concentrations of acetone, each concentration for five minutes. 0.5 mL each of 50, 60, 70, 80, 90, 95 and 100% acetone was used to resuspend the pellet and this step was also done under constant rotation. Further, the pellets were gradually embedded in resin: firstly a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resin: acetone mixture was used and secondly a 1
1 resin: acetone mixture was used and secondly a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 acetone: resin mixture was used, each for about 10–15 min. Finally, approximately 250 μL pure resin was added to each pellet and the eppendorf tube was sealed and put in an oven, upright, to polymerize. The samples were later cut with a microtome diamond knife and viewed in the electron microscope.
2 acetone: resin mixture was used, each for about 10–15 min. Finally, approximately 250 μL pure resin was added to each pellet and the eppendorf tube was sealed and put in an oven, upright, to polymerize. The samples were later cut with a microtome diamond knife and viewed in the electron microscope.
3. Results and discussion
3.1 Log-kills of E. coli, B. subtilisspores, B. anthracisvegetative cells and spores
Table 1 summarizes the results obtained using the prepared metal oxideadducts against E. coli, B. subtilis and B. anthracis. The results will be discussed for each organism in separate sections below.| Nanoparticle formulation | E. coli | B. subtilis spores | B. anthracis veg. cells | B. anthracis spores |
|---|---|---|---|---|
| NA–Al2O3 | 5.8 | 0 | N/T | N/T |
| NA–Al2O3/Cl2 | 8.6 | 4.4 | 8 | 6.8 |
| NA–Al2O3/Br2 | 8.6 | 5.2 | 8 | 6.8 |
| NA–Al2O3/l2 | 8.6 | 1 | N/T | N/T |
| NA–Al2O3/lCl | 8.6 | 4.3 | 8 | 6.8 |
| NA–Al2O3/lBr | 8.6 | 5.2 | 8 | 6.8 |
| NA–Al2O3/lCl3 | 8.6 | 6 | 8 | 6.8 |
| NA–TiO2 | 7.6 | 0 | N/T | N/T |
| NA–TiO2/Cl2 | 7.6 | 3.6 | N/T | N/T |
| NA–TiO2/Br2 | 7.6 | 3.6 | N/T | N/T |
| NA–TiO2/l2 | 8.6 | 1 | N/T | N/T |
| NA–TiO2/lCl | 8.6 | 6 | 8 | 6.8 |
| NA–TiO2/lBr | 8.6 | 4.3 | 8 | 6.8 |
| NA–TiO2/lCl3 | 8.6 | 6 | 8 | 6.8 |
| NA–CeO2 | 6.9 | 0 | N/T | N/T |
| NA–CeO2/Cl2 | 7.6 | 0.3 | N/T | N/T |
| NA–CeO2/Br2 | 8.6 | 3.6 | N/T | N/T |
| NA–CeO2/l2 | 8.6 | 1.3 | N/T | N/T |
| NA–CeO2/lCl | 8.6 | 3.6 | N/T | N/T |
| NA–CeO2/lBr | 8.6 | 5 | N/T | N/T |
| NA–CeO2/lCl3 | 8.6 | 5 | N/T | N/T |
Further, the halogenated/interhalogenated adducts of NA–TiO2 also performed very well, most of them having a complete kill of the cells present. However, the chlorinated and brominated adducts were not as efficient as the other halogenadducts of NA–TiO2. The reason for this could be the stability of those adducts ; it is very likely that the halogens (Cl2 and Br2) have escaped from the TiO2 surface early during the 30 min interaction, leading to lower kills. It should also be noted that the standard deviation for the ‘naked’ TiO2 was slightly larger (in the order of ±0.9) than for the chlorine and bromineadducts (both less than ±0.5). It was difficult obtaining reproducible data for the ‘naked’ TiO2 and since the average value is reported and the standard deviation was quite large, it is possible that with more experiments, the average value could have been lower. This is more likely, as the chlorinated and brominated adducts should have a higher activity due to the oxidative power of the halogens.
The adducts prepared from NA–CeO2 were also tested and the results can be seen in Table 1. Again, it can be seen that the chlorinated adduct was not quite as efficient as the other adducts and that the ‘naked’ CeO2 was slightly less active than the ones containing halogen/interhalogen.
It should be noted that all the ‘naked’ metal oxides studied were quite active by themselves, considering that they contained no halogens or other corrosive chemicals. The reason for the unusual activity of the nanoparticles is most likely their abrasive character and high surface area, leading to a very close contact with the cells.
To summarize the results obtained during E. coli testing, the activities of all of the prepared halogen/interhalogen adducts were very high, most of them displaying a complete kill of all E. coli cells present, even at high concentrations of bacteria (up to 8.6 log-kills). It seems that the NA–Al2O3 Plus adducts have the highest activity against E. coli cells, so they most likely would have the greatest potential in performing well against other microorganisms such as spores and viruses. Interestingly, even the non-halogenated metal oxides performed very well, with log-kills of over 5. This is very impressive for materials that are relatively environmentally friendly and contains no harsh chemicals that could potentially destroy sensitive equipment.
Further, the log-kill values for the NA–TiO2adducts can be observed in Table 1. As expected, the iodinated adduct had a low log-kill, as did the ‘naked’ NA–TiO2. Again, the interhalogenated adducts performed very well with results of up to a log-kill of 6 for the ICl3adduct . The chlorinated and brominated adducts had an intermediate activity. Several of the TiO2adducts are somewhat unstable, in the sense that the halogen leaves the surface quickly, possibly causing this lower activity of the Cl2 and Br2adducts . Again, the interhalogens have a very strong oxidizing power leading to very high log-kills.
The adducts prepared from NA–CeO2 were also tested as can be seen in Table 1. It is worth noting that the performance of the chlorinated adduct was very low, in fact, even lower than the iodinated adduct . Within the NA–CeO2 series, there is also no adduct that had a log-kill of 6 or higher, as there were in both the NA–Al2O3 Plus and NA–TiO2 series. The bare NA–CeO2 was inefficient, as expected. The amounts of halogen/interhalogen adsorbed on the NA–CeO2 are low, possibly leading to some of these lower log-kill values. In particular, the chlorinated adduct contains a low amount of halogen, as determined by TGA, explaining the low activity of this adduct .
To summarize, many of the halogenated adducts performed very well against the B. subtilisspores; however, the bare metal oxides did not seem to have any noticeable activity against the spores, as expected. Further, the iodinated adducts of all three metal oxides also displayed low activities.
For this study, only the most promising adducts were chosen to be tested. The adducts chosen to be used included NA–Al2O3/Cl2 Plus, NA–Al2O3/Br2 Plus, NA–Al2O3/ICl Plus, NA–Al2O3/IBr Plus and NA–Al2O3/ICl3 Plus, as well as NA–TiO2/ICl, NA–TiO2/IBr and NA–TiO2/ICl3. Although there were some of the NA–CeO2adducts that were indeed very efficient, they were excluded due to the fact that NA–CeO2 is a heavier metal oxide and hence less environmentally friendly, it is more expensive and more dusty, leading to higher risks of inhalation problems.
B. anthracis vegetative cells were not expected to be very difficult to kill. The results of the adducts are summarized in Table 1. The highest concentration tested corresponds to a log-kill of 8, which means that all adducts tested displayed a complete kill of B. anthracisvegetative cells. This was not surprising considering that all of the tested adducts were also lethal towards E. coli cells of high concentrations
Table 1 further shows the results obtained from testing against the B. anthracisspores. These values are based on an assumption of a 70% conversion of cells to spores (based on counting cells versusspores on several TEM images taken from different parts of the grid). All the studied adducts displayed a complete kill of the spores used.
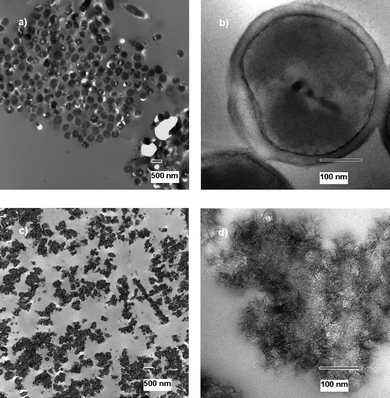
3.2 Transmission electron micrographs—to visualize the mechanism of action
Transmission electron microscope images were obtained to further visualize that the cells were in fact destroyed, rather than just prevented from reproduction. For future application in decontamination and as possible first-response weapons against terrorist attacks it is very important that the materials are bactericidal and not just bacteristatic. The images of treated cells/spores were prepared using exposure of the cells/spores to NA–Al2O3/ICl3 for 60 min, as explained previously in the experimental section.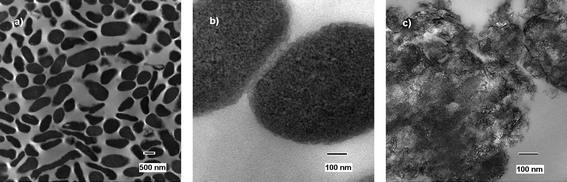 | ||
| Fig. 1 TEM micrographs of untreated (a and b) and treated (c) E. coli cells. | ||
The treated cells can be seen in Fig. 1c) and the difference between treated and untreated cells can easily be observed. The metal oxide can be observed in the image containing dead cell parts, DNA parts and proteins, indicating the mechanism in which the cells have died. The metal oxide appears to have an affinity for the cells, the abrasive character further increases biocidal effect, and the oxidative power of the halogen kills the cell.
 | ||
| Fig. 2 TEM micrographs of untreated (a and b) and treated (c and d) B. subtilisspores. | ||
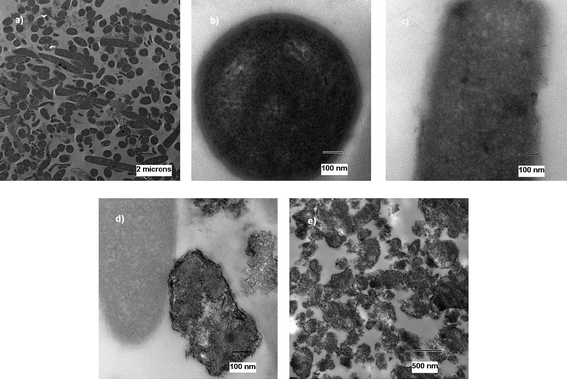 | ||
| Fig. 3 TEM micrograph of untreated (a and b) and treated (c, d and e) B. anthracis cells. | ||
Further, Fig. 4a shows one single healthy B. anthracisspore, displaying its very tough and thick protective coat. The treated spores are shown in Fig. 4b and 4c. Fig. 4b shows very well the proposed mechanism in which the death occurs. The spore is not completely healthy anymore, but it can be seen how it is surrounded by metal oxide material. This picture represents the perfect ‘intermediate’ stage of the process in which the spores die. One step further is represented in Fig. 4c. Only DNA, proteins and parts of the spores remain.
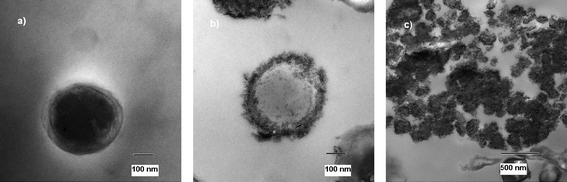 | ||
| Fig. 4 TEM micrograph of untreated (a) and treated (b and c) B. anthracisspores. | ||
To summarize, in order to kill spores, a material normally needs to have some functional group or chemical attached to it that is very aggressive and most often not environmentally friendly. The search for the ultimate environmentally safe biocide continues, but it is rather difficult to find a material that is completely environmentally friendly and at the same time kills several microorganisms, including vegetative cells and the more dormant spores.
It is noteworthy that these experiments were performed using the filter paper membrane method, which includes having a “solid–solid,” mostly dry, interaction. These results may have been even better if performed in a water suspension where the direct contact between cells/spores and nanoparticles might be better, leading to higher log-kills. Although utilizing this ‘solid–solid’ interaction method, the results obtained were very satisfactory, including log-kills of spores over 6 in several cases. The vegetative cells of E. coli and B. anthracis had little resistance against all of the halogenated and interhalogenated adducts , in many cases leading to complete kills of the high concentrations used during testing. It is also worth noting that the non-halogenated metal oxides had some biocidal activity against the E. coli cells, with log-kill values over 5 for all of the metal oxides.
Reasons for the excellent biocidal and sporicidal activity of these nanomaterials include their abrasive character, caused by their high surface area and many corners, edges and defect sites, the oxidative power of the halogens/interhalogens and the electrostatic attraction, as seen in the TEM pictures, caused by the overall negative surface charge of most microorganisms and the overall positive surface charge of many metal oxides. Another aspect was pointed out by a reviewer: the general order of effectiveness of Al2O3 ∼ TiO2 > CeO2halogenadducts might be attributed to oxide basicity, and the fact that cerium is more tolerant of valency changes, which could lower its biocidal power.
Acknowledgements
The partial support of the Kansas State University Targeted Excellence Program and the Women's Study Advance Program are acknowledged with gratitude. Partial support of the Army Research Office is also appreciated. Also, we thank Professor George C. Stewart of the University of Missouri for providing the avirulent Δ Sterne strain of B. anthracis.References
- O. Koper, J. Klabunde, G. Marchin, K. J. Klabunde, P. Stoimenov and L. Bohra, Curr. Microbiol., 2002, 44, 49–55 CrossRef CAS.
- P. K. Stoimenov, R. L. Klinger, G. L. Marchin and K. J. Klabunde, Langmuir, 2002, 18, 6679–6686 CrossRef CAS.
- P. K. Stoimenov, V. Zaikovski and K. J. Klabunde, J. Am. Chem. Soc., 2003, 125, 12907–12913 CrossRef CAS.
- J. A. Haggstrom, P. K. Stoimenov and K. J. Klabunde, Chem. Mater., 2008, 20, 3174–3183 CrossRef CAS.
- T. Nishino and S. Nakazawa, Jap. J. Microbiol., 1973, 17, 383–392 Search PubMed.
- N. D. Williams and A. D. Russell, FEMS Microbiol. Lett., 1993, 106, 183–186 CrossRef CAS.
- S. P. Gorman, E. P. Hutchinson, E. M. Scott and L. M. McDermott, J. Appl. Bacteriol., 1983, 54, 91–99 CAS.
- J. M. Newton, G. Henderson and J. A. Vickers, J. Appl. Bacteriol., 1967, 30, 484–487 CAS.
- I. M. Helander, H.-L. Alakomi, K. Latva-Kala, T. Mattila-Sandholm, I. Pol, E. J. Smid, L. G. M. Gorris and A. von Wright, J. Agric. Food Chem., 1998, 46, 3590–3595 CrossRef CAS.
- R. Tennen, B. Setlow, K. L. Davis, C. A. Loshon and P. Setlow, J. Appl. Microbiol., 2000, 89, 330–338 CrossRef CAS.
- N. D. Williams and A. D. Russell, FEMS Microbiol. Lett., 1992, 99, 277–280 CrossRef CAS.
- P. M. Foegeding and F. F. Busta, Appl. Environ. Microbiol., 1983, 45, 1374–1379 CAS.
- C. Barker and S. F. Park, Appl. Environ. Microbiol., 2001, 67, 1594–1600 CrossRef CAS.
- S. F. Bloomfield and M. Arthur, Lett. Appl. Microbiol., 1989, 8, 101–104 CrossRef CAS.
- S. F. Bloomfield and M. Arthur, J. Appl. Microbiol., 1992, 72, 166–172 CAS.
- S. F. Bloomfield and R. Megid, J. Appl. Microbiol., 1994, 76, 492–499 CAS.
- D. Coates and J. E. Death, J. Clin. Pathol., 1978, 31, 148–152 CrossRef CAS.
- C. M. Cousins and C. D. Allan, J. Appl. Bacteriol., 1967, 30, 168–174 CAS.
- S. P. Gorman, E. M. Scott and E. P. Hutchinson, Int. J. Pharm., 1983, 17, 291–298 CrossRef CAS.
- S. P. Gorman, E. M. Scott and E. P. Hutchinson, J. Appl. Bacteriol., 1984, 56, 295–303 CAS.
- S. P. Gorman, E. M. Scott and E. P. Hutchinson, J. Appl. Bacteriol., 1985, 59, 99–105 CAS.
- A. D. Russell, Clin. Microbiol. Rev., 1990, 3, 99–119 CAS.
- L. R. Wyatt and W. M. Waites, J. Gen. Microbiol., 1975, 89, 337–344 CAS.
- S. F. Bloomfield and R. Megid, J. Appl. Bacteriol., 1994, 76, 492–499 CAS.
| This journal is © The Royal Society of Chemistry 2010 |
