Structural and electronic properties of carbon nanotube-reinforced epoxy resins
Kelvin
Suggs
and
Xiao-Qian
Wang
*
Department of Physics and Center for Functional Nanoscale Materials, Clark Atlanta University, Atlanta, Georgia 30314, USA. E-mail: xwang@cau.edu
First published on 20th November 2009
Abstract
Nanocomposites of cured epoxy resin reinforced by single-walled carbon nanotubes exhibit a plethora of interesting behaviors at the molecular level. We have employed a combination of force-field-based molecular mechanics and first-principles calculations to study the corresponding binding and charge-transfer behavior. The simulation study of various nanotube species and curing agent configurations provides insight into the optimal structures in lieu of interfacial stability. An analysis of charge distributions of the epoxy functionalized semiconducting and metallic tubes reveals distinct level hybridizations. The implications of these results for understanding dispersion mechanism and future nano reinforced composite developments are discussed.
Epoxy resins serve as key elements in the field of high-performance materials, coatings, and composites.1–3 The availability of a wide variety of epoxy systems, along with different physical performances associated with various cross-linking degrees, makes these thermosetting polymers extremely suitable for applications such as structural, medical, or aerospatial. Although epoxy resins are inherently brittle, curing processing in the manufacture of high-performance composites offers high modulus, stiffness and thermal stability.1–3 In this regard, remarkable progress has been made in the curing of epoxy resins, and several studies have been performed to investigate the curing mechanism and the influence of various parameters on processing and material properties.1,4 On the other hand, carbon nanotubes have been employed as reinforcers, which is connected to their unique properties and outstanding performance.5,6
Of particular interest is the use of single-walled carbon nanotubes (SWNTs) as reinforcement agents for epoxy resins.4–6 Both covalent7 and non-covalent8,9 functionalization of SWNTs have been investigated experimentally as key techniques for disentangling their bundles and improving their interfacial adhesion to the matrix.10–12 In non-covalent functionalization, block copolymers have been shown to be excellent promoters of wetting and adhesion, offering a suitable alternative in nanotube interfacial engineering. The integration of polymer-wrapped SWNTs into polymeric matrices typically leads to a more homogeneous distribution of the reinforcer.7 EPON 862 (diglycidyl ether of bisphenol F) epoxy resins are specific type of polymers known for their stability at high temperature and are thus typically used as polymer matrices for advanced applications in aerospace, adhesives, packaging, and in manufacturing circuit boards.4,5 Nano-reinforced composites of EPON and SWNTs are promising lightweight materials suitable for the aerospace industry.
SWNTs can be either semiconducting or metallic depending on the chiral vector, which corresponds to the direction of a scrolled graphene sheet.4,12,13 Stronger than steel,13 many applications have been proposed to leverage the unique properties of SWNTs including quantum wires, tips for scanning probe microscopy, and molecular diodes. SWNTs can be produced with reasonable quality by several techniques. However, the mechanical and electronic properties of SWNTs are highly sensitive to their diameter and chirality. Slice-cutting, mechanical stretching, and melt spinning are promising approaches employed to control the alignment of SWNTs.4 In addition to the dispersion enhancement of SWNTs in the thermoset, functionalization has a dramatic effect on the behavior of epoxy resins, which leads to significant improvement in the mechanical properties of the nanocomposites.1–3 The increase in storage modulus and glass transition temperature boosts the use of the polymer and adds more stiffness to the polymer to resist failure.
There are many unsolved issues with respect to the interfacial strength and mechanical properties of carbon nanotube/polymer composites.2,4,14,15 Depending on the polymer matrix and processing conditions, large variations in experimentally measured properties have been reported, which implies that the properties of SWNT/polymer composites are highly polymer-specific. Moreover, due to van der Waals (vdW) interactions, the nanotubes tend to aggregate, forming bundles or ropes and further agglomerate when being dispersed in the polymer matrix.16 The surface of SWNTs yields the high viscosity of nanotube/polymer mixtures, which plays an important role in the efficiency of the dispersion. The important factors that influence these properties of epoxy resins are the cross-link density and the molecular chain structure.1,14 Consequently, it is highly desirable to understand mechanisms that lead to the increased toughness and thermal resistance of the epoxy resin.
Here we present a comprehensive investigation of structural and electronic properties of epoxy modifications through noncovalent functionalized SWNTs.12 We employ a combination of force-field-based molecular dynamics12,18,17 and local density-functional calculations.20 Force-field-based molecular dynamics17 were used to pre-select molecular geometries, and first-principles calculations12 were employed to determine the electronic structure of the nano-reinforced composites. A fundamental issue is how the self-organized dynamic structure of functional molecular systems affects the interactions of the nano-reinforced composites. Our results show that the π–π stacking between the aromaticmacrocycle and the surface of the SWNTs manifests itself via increased interfacial binding. First-principles calculations on the electronic structures further reveal that there exists distinct level hybridization behavior for metallic and semiconducting nanotubes.
The SWNTs involved in the present study were constructed based on the sp2 hybridization model. The initial value of 1.42 Å for the nearest-neighbor C–C bond length was used. The geometric structures of the SWNTs were fully relaxed in molecular dynamics through intensive simulated annealing.12 The optimized structures with use of MM+ force fields18 have diameters in good agreement with the predictions from the rolling graphene model.19 The molecular and chemical structures of the EPON resin 862 are shown in Fig. 1, along with two prototype curing agents, diethyltoluenediamine (DETDA) and diethyltoluenamine (DETA).15,14 The consensus is that DETDA represents characteristic features of the curing agent W.15
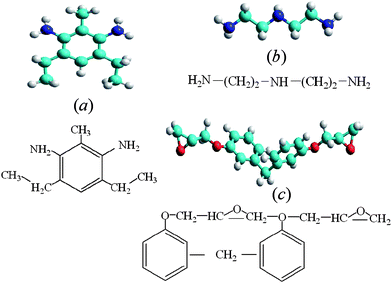 | ||
| Fig. 1 Molecular and chemical structures of (a) diethyltoluenediamine (DETDA), (b) diethyltoluenamine (DETA) curing agents, and (c) the EPON resin 862, respectively. Colored with cyan, blue, red, and white are carbon, nitrogen, oxygen, and hydrogen atoms, respectively. | ||
The terminal groups of the EPON resin 862 can open up and connect to curing agents. The formation of a chain-like or cross-linked structure15 is reminiscent of the cured state of the nano-reinforced composites. In order to explore the nature at the molecular level of nanotube composites, we have performed intensive geometry optimization studies of conformations via force-field-based molecular dynamics.12,18 Whereas our molecular dynamics simulation confirms that the EPON resin 862 self-assembles onto the surface of the SWNTs, the cured state of the EPON resin 862 adopts energetically favorable configurations by wrapping around the tube helically. Illustrated in Fig. 2 is the optimized structure of a SWNT wrapped with chain-like cured EPON 862. A careful examination of the dynamical structural and energetic changes reveals that electrostatic interactions within the EPON backbone are primarily responsible for the wrapping into a helical structure. The aromatic rings in the EPON resins 862 are planar to the surface of the SWNT, and the structural changes proceed through a rearrangement of the torsional angles. As seen in Fig. 2, the optimized structure tends to align the backbone along the nanotube surface in order to maximize the interaction between π-bonds. The backbone torsion in helix formation is important for the cured resin to successfully wrap around the tube. Furthermore, as the binding of conjugated polymers is the combination of electrostatic and vdW interactions, the binding is stronger than vdW binding for nonconjugated polymers, which is expected to yield improved dispersion in conjugated polymers.
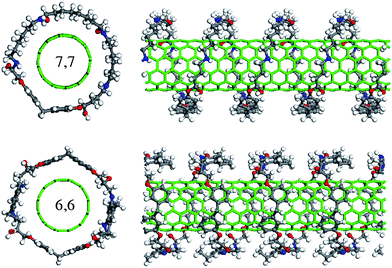 | ||
| Fig. 2 Top- and side-views of (top panel) an armchair (7,7) SWNT helically wrapped with a strand of DETA-cured EPON resin 862, and (bottom panel) an armchair (6,6) SWNT helically wrapped with DETDA-cured EPON resin 862, respectively. The carbon, oxygen, nitrogen, and hydrogen atoms are colored with gray, red, blue, and white, respectively. | ||
The capability of forming a helical conformation for cured EPON resins has important ramifications in dispersing nanotubes.12 Our molecular modeling suggests that the enhancement of nanotube solubilization can be attributed to the formation of self-assembled intermolecular structures associated with the inclusion of curing agents. This also conforms to the experimental findings that the cured epoxy resins exhibit higher fracture toughness and improved mechanical properties as compared to uncured epoxy resins.1,2,5 To further pursue this point, we illustrate in Fig. 3 the calculated interaction energy which is extracted from the difference between the energy of the composite and the energies for the cured EPON 862 and the SWNT as E = ESWNT + EEPON − Etotal, where Etotal is the total potential energy of the composite, ESWNT is the energy of the nanotube without the epoxy resin, and EEPON is the energy of the EPON resin without the nanotube. In other words, the interaction energy can be calculated as the difference between the energy of the optimized composite structure and the energy of the separate components.12
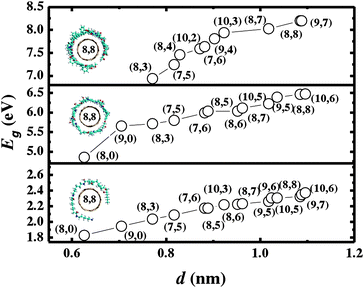 | ||
| Fig. 3 Calculated binding energies for tubes with diameters of 0.7–1.20 nm wrapped with DETA-cured (bottom panel), DETDA-cured (middle panel), and cross-linked (top panel) EPON resin 862. | ||
In addition to the helical wrapping conformations, the cured epoxy network can be formed through cross-linking of the EPON 862 resin with curing agents.14,15 During the curing reaction, the amine groups of curing agents react with the epoxide groups of epoxy resins. While for the chain-like conformation the curing reaction is limited at the –NH2 site of the curing agents, the cross-linking activity can happen at the NH site as well. As a result, the cross-link expands in all directions and forms a network of macromolecules. As seen from Fig. 3, for either helical wrapping or cross-linked conformations the interaction energy increases with the increase of the tube diameter, which is attributed to the planar epoxy resin conformation with respect to the surface of the carbon nanotube as well as a reduction in π-orbital misalignment.12 Furthermore, the interaction energy strongly correlates to the number of aromaticmacrocycles in the curing agents.
The cross-linked, DETDA-cured chain, and DETA-cured chain EPON resin 862 as shown in Fig. 3 have four, two, and one EPON resin 862, respectively. Consequently precaution needs to be taken for a comparison of the interacting energy for the three curing agent conformations. One can evaluate the binding interaction based on the interaction per unit length along the nanotube, or per EPON monomer. In either scenario, a careful extraction of the resulting interaction energy indicates that the greater the number of aromatic rings in the curing agent, the higher the binding energy. Specifically, DETA has no aromatic ring, while DETDA has one aromatic ring in the monomer conformation.15 As indicated in Fig. 2, both curing agents have similar wrapping pattern and pitch length (∼1.5 ± 0.3 nm). The resultant stronger affinity of DETDA over DETA implies greater stability of the former curing agent. It is thus reasonable to conjecture that curing agents with more aromaticmacrocycles are advantageous in forming more stable supramolecular structures, under the provision that the curing agent can successfully react with the epoxy resin. This is in conformity with experimental observation that the curing agent W is eminently employed in nano-reinforced composites.15
It is worth noting that DETA-cured EPON resin 862 can assume either chain-like or cross-linked conformations. Although the helically wrapped chain configuration is energetically preferred, the availability of additional reaction sites at NH for the cross-linked structure may lead to increased density for the nano-reinforced composite. As the curing reaction is a dynamic process, the interplay between the energy and molecular density is cumbersome and is beyond the scope of the present paper. However, we believe that the experimentally cured EPON resin 862 consists of both chain-like and cross-linked conformations.
Another important aspect of the nanotube reinforced epoxy resin is the charge transfer behavior. It is well known that classical molecular dynamics can not properly describe charge transfers.12 To this end, we have performed first-principles calculations based on density functional theory. A local density approximation to the exchange–correlation potential was used in the calculation.20 Periodic boundary conditions were employed with a supercell in the xy-plane large enough to eliminate the interaction between neighboring structures. A double numerical basis was sufficient to converge the grid integration of the charge density.
An investigation was performed on the band structure and the charge density distributions for two SWNTs, (8,8) and (14,0), functionalized with DETA-cured EPON resin 862. The nano-reinforced composite structures were fully optimized using a first-principles approach. All structures were relaxed with forces less than 0.01 eV Å−1. The armchair (8,8) tube is metallic, whereas the zigzag (14,0) tube is semiconducting. The diameters of the two tubes are very close, 1.085 and 1.096 nm for (8,8) and (14,0), respectively.19 Shown in Fig. 4 are the extracted band structures and the corresponding charge density distribution of near-gap states at the band center (Γ-point). Specifically, we label the valence band minimum (VBM) as band “−1” and the conduction band maximum (CBM) as “1”. The corresponding states are labeled in Fig. 4 by red arrows. In connection to the distinct level hybridization behavior, our first-principles calculation results show that the metallic (8,8) tube is energetically preferred over the semiconducting (14,0) by 0.4 eV. It is worth mentioning that the difference in binding energy between the two nearly identical diameter tubes is dormant in classical force-field based molecular dynamics calculations, which demonstrates the necessity of quantum calculations in accurate descriptions of interfacial interactions.
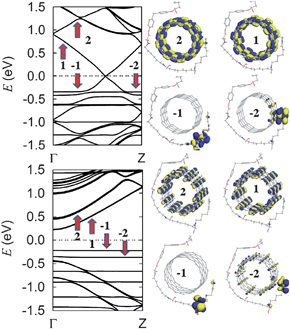 | ||
| Fig. 4 Calculated band structures and charge densities of near-gap states for (top panel) metallic (8,8) and (bottom panel) semiconducting (14,0) tubes, respectively. | ||
As is readily observable in Fig. 4, the band structures of the nano-reinforced composite is the superposition of dispersion bands originated from the SWNT and the flat bands that are attributed to the cured epoxy resins. The band alignment is such that the flat bands do not change the property of the near-gap conduction bands. By contrast, the flat band becomes the VBM of the nano-reinforced composite for semiconducting tubes, while flat bands strongly hybridize with the π-band of the metallic (8,8) tube. In accordance with the distinct flat and dispersion feature, dispersion bands have charges primarily confined on the tube, while flat bands have charges mainly concentrated on the epoxy resin. In conformity with non-covalent functionalization, the metallic and semiconducting behavior of the pristine tube remains intact. It is worth mentioning that we have found similar electronic structure characteristics for SWNTs functionalized with DETDA-cured EPON resin 862, particularly the alignment of the EPON HOMO-derived flat band and the distinct level hybridization feature.
For semiconducting nanotubes, the charge distribution of VBM and CBM is consistent with a type-I alignment of the bands.21 However, for metallic nanotubes the charge distribution exhibits a type-II alignment.21 In the latter cases, the charge density of HOMO-derived band of the epoxy resin is inhomogeneous due to the level hybridization and the associated charge transfer from the epoxy resin to the tube. Our results indicate that EPON resins 862 with curing agents are charge donors to SWNTs, resulting in complete charge separation of HOMO-derived band for semiconducting nanotubes. In contrast, for metallic tubes, the charge transfer induces considerable level hybridization, which leads to charged dipoles.21
In summary, our calculation results indicate that the EPON resin 862 with curing agents can adopt helically wrapped conformations around SWNTs. The number of aromatic rings in the curing agent influences the overall stability of the nano-reinforced composite. Furthermore, metallic and semiconducting SWNTs have distinct level hybridization behavior. The implication of the distinct level hybridization is that the utilization of single type of SWNTs would allow an effective tailoring of the charge transfer behavior that is relevant to the energetic responses. Notwithstanding the fact that this is not practical for the time being in view of the formidable cost associated with the separation of SWNTs at the industrial scale,11 we believe that our results provide insight to the charge transfer behavior of graphene-reinforced epoxy resins as well. In lieu of the rapid progress in this field, the combination of computational simulation and experimental characterization will be very fruitful in the future development of nano-reinforced composite materials.
Acknowledgements
The authors thank E. Mintz for stimulating discussions. This work was supported in part by the NSF (Grant No. DMR-0934142), NASA (Grant No. NCC3-1044), and Army Research Office (Grant No. W911NF-06-1-0442).References
- E. Bekyarova, E. T. Thostenson, A. Yu, M. E. Itkis, D. Fakhrutdinov, T. W. Chou and R. C. Haddon, Functionalized single-walled carbon nanotubes for carbon fiber-epoxy composites, Langmuir, 2007, 23, 3970 CrossRef CAS.
- S. J. V. Frankland, A. Caglar, D. W. Brener and M. Griebel, Molecular simulation of the influence of chemical crosslinks on the shear strength of carbon nanotube–polymer interfaces, J. Phys. Chem. B, 2002, 106, 3046 CrossRef CAS.
- J. Gao, G. Hou, Y. Wang, H. Li and Y. Liu, Curing and thermal properties of PEPEB liquid crystalline diepoxide/aromatic diamine, Polym.-Plast. Technol. Eng., 2006, 45, 947 CrossRef CAS.
- E. W. Wong, P. E. Sheehan and C. M. Lieber, Nanobeam mechanics: elasticity, strength, and toughness of nanorods and nanotubes, Science, 1997, 277, 1971 CrossRef CAS.
- P. M. Ajayan, L. S. Schadler, S. C. Giannaris and A. Rubio, Single-walled carbon nanotube-polymer composites: strength and weakness, Adv. Mater., 2000, 12, 750 CrossRef CAS.
- D. W. Steuerman, A. Star, R. Narizzano, H. Choi, R. S. Ries, C. Nicolini, J. F. Stoddart and J. R. Heath, Interactions between conjugated polymers and single-walled carbon nanotubes, J. Phys. Chem. B, 2002, 106, 3124 CrossRef CAS.
- M. Kanungo, H. Lu, G. G. Malliaras and G. B. Blanchet, Suppression of metallic conductivity of single-walled carbon nanotubes by cycloaddition reactions, Science, 2009, 323, 234 CrossRef CAS.
- D. A. Britz and A. N. Khlobystov, Noncovalent interactions of molecules with single walled carbon nanotubes, Chem. Soc. Rev., 2006, 35, 637 RSC.
- M. Zheng, A. Jagota, E. D. Semke, B. A. Diner, R. S. Mclean, S. R. Lustig, R. E. Richardson and N. G. Tassi, DNA-assisted dispersion and separation of carbon nanotubes, Nat. Mater., 2003, 2, 338 CrossRef CAS.
- R. J. Chen, Y. Zhang, D. Wang and H. Dai, Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization, J. Am. Chem. Soc., 2001, 123, 3838 CrossRef CAS.
- A. Nish, J.-Y. Hwang, J. Doig and R. J. Nicholas, Highly selective dispersion of single-walled carbon nanotubes, Nat. Nanotechnol., 2007, 2, 640 CrossRef CAS.
- O. O. Ogunro and X. Q. Wang, Quantum electronic stability in selective enrichment of carbon nanotubes, Nano Lett., 2009, 9, 1034 CrossRef CAS.
- M. M. J. Treacy, T. W. Ebbesen and J. M. Gibson, Exceptionally high Young's modulus observed for individual carbon nanotubes, Nature, 1996, 381, 678 CrossRef CAS.
- J. Gou, B. Fan, G. Song and A. Khan, Study of affinities between single-walled nanotube and epoxy resin using molecular dynamics simulation, Int. J. Nanosci., 2006, 5, 131 CrossRef CAS.
- H. B. Fan and M. M. F. Yuen, Material properties of the cross-linked epoxy resin compound predicted by molecular dynamics simulation, Polymer, 2007, 48, 2174 CrossRef CAS.
- J. Gou, B. Minaie, B. Wang, Z. Liang and C. Zhang, Computational and experimental study of interfacial bonding of single-walled nanotube reinforced composites, Comp. Mater. Sci., 2004, 31, 225 Search PubMed.
- R. R. Johnson, A. T. C. Johnson and M. L. Klein, Probing the structure of DNA-carbon nanotube hybrids with molecular dynamics, Nano Lett., 2008, 8, 69 CrossRef CAS.
- F. Jensen, Introduction to Computational Chemistry, 2nd ed, John Wiley & Sons, Hoboken, 2006 Search PubMed.
- M. Al-Haik, M. Y. Hussaini and H. Garmestani, Adhesion energy in carbon nanotube-polyethylene composite: effect of chirality, J. Appl. Phys., 2005, 97, 074306 CrossRef.
- DMol3, Accelrys Software Inc., San Diego, 2009 Search PubMed.
- A. Nduwimana and X.-Q. Wang, Charge carrier separation in modulation doped coaxial semiconductor nanowires, Nano Lett., 2009, 9, 283 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
