Colloidal synthesis and blue based multicolor upconversion emissions of size and composition controlled monodisperse hexagonal NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) Yb,Tm nanocrystals†
Yb,Tm nanocrystals†
Anxiang
Yin
,
Yawen
Zhang
*,
Lingdong
Sun
and
Chunhua
Yan
*
Beijing National Laboratory for Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications, PKU-HKU Joint Laboratory in Rare Earth Materials and Bioinorganic Chemistry, Peking University, Beijing, 100871, China. E-mail: yan@pku.edu.cn; ywzhang@pku.edu.cn; Fax: +86-10-6275-4179; Tel: +86-10-6275-4179
First published on 29th March 2010
Abstract
Monodisperse β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals with controlled size (25–150 nm), shape (sphere, hexagonal prism, and hexagonal plate), and composition (Yb: 20–40%, Tm: 0.2–5%) were synthesized from the thermolysis of metal trifluoroacetates in hot surfactant solutions. The upconversion (UC) of near-infrared light (980 nm) to ultra-violet (360 nm), blue (450 and 475 nm), red (650 and 695 nm) and infrared (800 nm) light in the β-NaYF4
Yb,Tm nanocrystals with controlled size (25–150 nm), shape (sphere, hexagonal prism, and hexagonal plate), and composition (Yb: 20–40%, Tm: 0.2–5%) were synthesized from the thermolysis of metal trifluoroacetates in hot surfactant solutions. The upconversion (UC) of near-infrared light (980 nm) to ultra-violet (360 nm), blue (450 and 475 nm), red (650 and 695 nm) and infrared (800 nm) light in the β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals has been studied by UC spectroscopy. Both the total intensity of UC emissions and the relative intensities of emissions at different wavelengths have shown a strong dependence on different particle sizes and different Tm3+ and Yb3+ concentrations. As a result, different overall output colors of UC emissions can be achieved by altering sizes and Yb3+/Tm3+ doping concentrations of the β-NaYF4
Yb,Tm nanocrystals has been studied by UC spectroscopy. Both the total intensity of UC emissions and the relative intensities of emissions at different wavelengths have shown a strong dependence on different particle sizes and different Tm3+ and Yb3+ concentrations. As a result, different overall output colors of UC emissions can be achieved by altering sizes and Yb3+/Tm3+ doping concentrations of the β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals. The intensity-power curves of a series of samples have proved that emissions at 360 and 450 nm can be ascribed to four-photon process (1D2 to 3H6 and 1D2 to 3H4, respectively), while emissions at 475 and 650 nm are three-photon processes (1G4 to 3H6 and 1G4 to 3H4, respectively) and emissions at 695 and 800 nm are two-photon ones (3F2 to 3H6 and 3F4 to 3H6, respectively). A UC saturation effect would occur under a certain excitation intensity of the 980 nm CW diode laser for the as-obtained β-NaYF4
Yb,Tm nanocrystals. The intensity-power curves of a series of samples have proved that emissions at 360 and 450 nm can be ascribed to four-photon process (1D2 to 3H6 and 1D2 to 3H4, respectively), while emissions at 475 and 650 nm are three-photon processes (1G4 to 3H6 and 1G4 to 3H4, respectively) and emissions at 695 and 800 nm are two-photon ones (3F2 to 3H6 and 3F4 to 3H6, respectively). A UC saturation effect would occur under a certain excitation intensity of the 980 nm CW diode laser for the as-obtained β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals, leading to the decrease of the slopes of the I–P curves. The results of our study also revealed that the successive transfer model instead of the cooperative sensitization model can be applied to explain the UC behaviors of the β-NaYF4
Yb,Tm nanocrystals, leading to the decrease of the slopes of the I–P curves. The results of our study also revealed that the successive transfer model instead of the cooperative sensitization model can be applied to explain the UC behaviors of the β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals. Further, an unexpected stronger emissions of four-photon process at 360 and 450 nm for ∼50 nm β-NaYF4
Yb,Tm nanocrystals. Further, an unexpected stronger emissions of four-photon process at 360 and 450 nm for ∼50 nm β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals than those for the bigger (∼150 nm) nanocrystals was observed and explained in terms of the effects of crystallite size, surface-to-volume ratio and homogeneity of the doping cations.
Yb,Tm nanocrystals than those for the bigger (∼150 nm) nanocrystals was observed and explained in terms of the effects of crystallite size, surface-to-volume ratio and homogeneity of the doping cations.
Introduction
Upconversion (UC) phosphors, especially rare-earth-based (RE-based) UC phosphors, have been a hotspot of material research for several decades because of their wide and important applications in many realms, such as solid-state lasers, IR imaging1,2 and bioimaging.1c,3 Compared to those traditional biological labels, for example, organic dyes4 and semiconductors,5 RE-based UC fluorescent (from near-infrared (NIR) to visible) nanoparticles possess several inherent advantages, including weak autofluorescence backgrounds, strong penetration abilities for NIR radiation, resistances to photobleaching, and low toxicity, etc.4,5,6 Among those UC materials, β-NaYF4 is considered to be one of the most efficient host materials for green/blue UC phosphors when sensitized by Yb3+ and activated by Er3+/Ho3+/Tm3+ ions due to the relatively low lattice phonon energy.1,7More recently, much attention has been focused on the size/shape/phase-controlled synthesis, UC properties and mechanisms, and bio-applications of the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er/Tm nanocrystals.8 For instance, Haase and co-workers8a reported the precipitation-based solution synthesis of NaYF4
Yb,Er/Tm nanocrystals.8 For instance, Haase and co-workers8a reported the precipitation-based solution synthesis of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er/Tm nanoparticles with bright UC emissions, revealing the application potential of UC nanocrystals in bio-imaging areas. Li et al.8b,i obtained NaYF4 nanocrystals with controllable size and morphology and high dispersibility by hydrothermal procedures. Yan et al.,8c Capobianco et al.,8d,e and Chow et al.8f developed the method of thermal decomposition of metal–organic complexes in hydrophobic high-boiling solvents to synthesize monodisperse NaYF4
Yb,Er/Tm nanoparticles with bright UC emissions, revealing the application potential of UC nanocrystals in bio-imaging areas. Li et al.8b,i obtained NaYF4 nanocrystals with controllable size and morphology and high dispersibility by hydrothermal procedures. Yan et al.,8c Capobianco et al.,8d,e and Chow et al.8f developed the method of thermal decomposition of metal–organic complexes in hydrophobic high-boiling solvents to synthesize monodisperse NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er/Tm nanocrystals with controllable size, shape and phase. Yan et al.8j,k also studied the nucleation and growth kinetics, and multicolor UC emissions and mechanisms of NaYF4
Yb,Er/Tm nanocrystals with controllable size, shape and phase. Yan et al.8j,k also studied the nucleation and growth kinetics, and multicolor UC emissions and mechanisms of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er nanocrystals, revealing the relationships between the outcome UC emissions and size, shape, phase and composition of the nanocrystals. Liu and co-workers1c,9a realized the tuning of the outcome UC light color of NaYF4
Yb,Er nanocrystals, revealing the relationships between the outcome UC emissions and size, shape, phase and composition of the nanocrystals. Liu and co-workers1c,9a realized the tuning of the outcome UC light color of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er nanocrystals via incorporating Tm3+ cations and altering the doping concentrations. Veggel et al.9f obtained transparent UC NPs-polymer composite materials via dispersing NaYF4 nanoparticles into PMMA through an in situ polymerization method. Zhang and co-workers9b employed NaYF4
Yb,Er nanocrystals via incorporating Tm3+ cations and altering the doping concentrations. Veggel et al.9f obtained transparent UC NPs-polymer composite materials via dispersing NaYF4 nanoparticles into PMMA through an in situ polymerization method. Zhang and co-workers9b employed NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er@SiO2 as fluorescent labels in cell imaging. Also, they achieved multicolor UC fluorescence by using NaYF4
Yb,Er@SiO2 as fluorescent labels in cell imaging. Also, they achieved multicolor UC fluorescence by using NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er nanocrystals as the energy donator in the FRET process to organic dyes and QDs.
Yb,Er nanocrystals as the energy donator in the FRET process to organic dyes and QDs.
Compared to the massive studies on the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er nanocrystals with green UC emissions, researches on the NaYF4
Yb,Er nanocrystals with green UC emissions, researches on the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals with blue emissions are still scarce, though they are important and necessary supplement to the NIR to visible light UC nanomaterials. For example, Nann et al.9d exploited NaYbF4
Yb,Tm nanocrystals with blue emissions are still scarce, though they are important and necessary supplement to the NIR to visible light UC nanomaterials. For example, Nann et al.9d exploited NaYbF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tm nanocrystals as one of the probes for multiplexing detections. Zhang et al. studied the core–shell effects9b and the bio-applications9c of NaYF4
Tm nanocrystals as one of the probes for multiplexing detections. Zhang et al. studied the core–shell effects9b and the bio-applications9c of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er and NaYF4
Yb,Er and NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals. Prasad et al.9e introduced NaYF4
Yb,Tm nanocrystals. Prasad et al.9e introduced NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanoparticles as an in vitro and in vivo NIR-NIR UC probes. Further compared to the systematic studies of UC emissions and mechanisms of bulk NaYF4
Yb,Tm nanoparticles as an in vitro and in vivo NIR-NIR UC probes. Further compared to the systematic studies of UC emissions and mechanisms of bulk NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm materials,11 the fundamental understanding of the size/shape/phase modulated UC properties and mechanisms of NaYF4
Yb,Tm materials,11 the fundamental understanding of the size/shape/phase modulated UC properties and mechanisms of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals is still rather inadequate.
Yb,Tm nanocrystals is still rather inadequate.
In this article, we report the controlled synthesis, tuning of the UC emission colors, and the inherent UC mechanisms of monodisperse NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals with different dopant ratios (Yb: 20–40%, Tm: 0.2–5%), sizes (25–150 nm), and shapes (sphere, hexagonal prism, and hexagonal plate).
Yb,Tm nanocrystals with different dopant ratios (Yb: 20–40%, Tm: 0.2–5%), sizes (25–150 nm), and shapes (sphere, hexagonal prism, and hexagonal plate).
Experimental section
A Schlenk line system and commercially available reagents were used in the synthesis procedure. Rare-earth oxides (RE = Y, Yb, and Tm), oleic acid (OA; 90%, Alpha), oleylamine (OM; >80%, Acros), 1-octadecene (ODE; >90%, Acros), trifluoroacetic acid (99%, Acros), CF3COONa (>97%, Acros), absolute ethanol, and cyclohexane were used as received. RE(CF3COO)3 were prepared via the literature method.8c,10Synthesis of α-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Yb,Tm nanocrystals
Yb,Tm nanocrystals
1 mmol of CF3COONa and the appropriate proportion of Y(CF3COO)3, Yb(CF3COO)3, and Tm(CF3COO)3 were added to a mixed solution of OA (10 mmol), OM (10 mmol), and ODE (20 mmol) in a three-necked flask at room temperature. Then, the slurry was degassed and heated to 140 °C with vigorous magnetic stirring for 30 min in a temperature-controlled electromantle to remove water and oxygen, and thus to form an optically transparent, colorless or slightly yellow solution. Next, the solution was heated to 300 °C at a heating rate of 20 °C min−1 under a high purified N2 atmosphere. The solution became a bit turbid and was maintained at this temperature for 30 min under N2 atmosphere. The solution was left to be air-cooled with gentle stirring for 5–10 min after it became transparent again. Then, an excess amount of ethanol was poured into the solution at room temperature. The resultant turbid mixture was separated by a centrifuge; and the products were collected. The as-precipitated nanocrystals, without any size-selections, were washed several times with cyclohexane and ethanol, and then dried at 80 °C in air for 24 h.
Synthesis of β-NaYF4 : Yb,Tm nanocrystals
The synthesis procedure of β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals was similar to the synthesis of α-NaYF4
Yb,Tm nanocrystals was similar to the synthesis of α-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals except that (a) quantitative CF3COONa and α-NaYF4:Yb,Tm nanocrystals were added to a mixed solution of OA (20 mmol), and ODE (20 mmol) and, (b) the reaction temperature was increased to 320–330 °C and maintained for 30–45 min (see Table S1 in the ESI†). These obtained nanocrystals could be easily redispersed in various nonpolar organic solvents (e.g., cyclohexane) and showed good redispersibility even after aging for a long time.
Yb,Tm nanocrystals except that (a) quantitative CF3COONa and α-NaYF4:Yb,Tm nanocrystals were added to a mixed solution of OA (20 mmol), and ODE (20 mmol) and, (b) the reaction temperature was increased to 320–330 °C and maintained for 30–45 min (see Table S1 in the ESI†). These obtained nanocrystals could be easily redispersed in various nonpolar organic solvents (e.g., cyclohexane) and showed good redispersibility even after aging for a long time.
Instrumentation
X-ray diffraction (XRD) patterns of the dried powders were recorded on a Rigaku D/MAX-2000 diffractometer (Japan) with a slit of 1/2° at a scanning rate of 4° min−1 using Cu-Kα radiation (λ = 1.5418 Å). Samples for transmission electron microscopy (TEM) analysis were prepared by drying a drop of colloid solution of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals in cyclohexane on copper grids coated by amorphous carbon. Particle sizes and shapes were examined by a TEM (200CX, JEOL, Japan) operated at 160 kV. High resolution TEM (HRTEM) and EDS analysis was performed on a FEG-TEM (Tecnai F30, Philips, USA) operated at 300 kV. UC emission spectra of all samples (1 wt % NaYF4
Yb,Tm nanocrystals in cyclohexane on copper grids coated by amorphous carbon. Particle sizes and shapes were examined by a TEM (200CX, JEOL, Japan) operated at 160 kV. High resolution TEM (HRTEM) and EDS analysis was performed on a FEG-TEM (Tecnai F30, Philips, USA) operated at 300 kV. UC emission spectra of all samples (1 wt % NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystal dispersion in cyclohexane) were measured on a Raman spectrometer (Jobin-Yvon HR800, France) with an external tunable 2 W 980 nm diode laser as the excitation source under the same conditions.
Yb,Tm nanocrystal dispersion in cyclohexane) were measured on a Raman spectrometer (Jobin-Yvon HR800, France) with an external tunable 2 W 980 nm diode laser as the excitation source under the same conditions.
Results and discussion
1. Characteristics of β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Yb,Tm nanocrystals
Yb,Tm nanocrystals
High-quality β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals (nanospheres, hexagonal nanoprisms, and hexagonal nanoplates) in a pure hexagonal structure (space group: P
Yb,Tm nanocrystals (nanospheres, hexagonal nanoprisms, and hexagonal nanoplates) in a pure hexagonal structure (space group: P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) ) with different Yb and Tm concentrations and different sizes ranging from 25 to 150 nm were selectively synthesized under 320–330 °C for 30–45 min, with using α-NaYF4
) with different Yb and Tm concentrations and different sizes ranging from 25 to 150 nm were selectively synthesized under 320–330 °C for 30–45 min, with using α-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm as the seeds and CF3COONa as the fluorine source (Table 1 and Table S1†). In the present synthesis, the transformation from the α-NaYF4
Yb,Tm as the seeds and CF3COONa as the fluorine source (Table 1 and Table S1†). In the present synthesis, the transformation from the α-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm seeds to the β-NaYF4
Yb,Tm seeds to the β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals was realized under the presence of excess Na+ and F− ions controllably released from CF3COONa.8j,k
Yb,Tm nanocrystals was realized under the presence of excess Na+ and F− ions controllably released from CF3COONa.8j,k
| Molar ratioa | Size/nmb | Structure | Space group | Morphology | |
|---|---|---|---|---|---|
| Yb (%) | Tm (%) | ||||
| a Molar ratio of reactants. b The standard deviation statistic from at least 50 particles. | |||||
| 30 | 0.2 | 24.4 ± 1.2 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere |
| 0.5 | 23.9 ± 0.8 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 47.3 ± 1.7 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Hexagonal prism | ||
| 150 × 70 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Hexagonal plates | ||
| 1 | 24.1 ± 0.8 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 2 | 24.4 ± 1.0 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 5 | 24.5 ± 0.8 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 20 | 0.2 | 23.7 ± 0.7 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere |
| 0.5 | 25.4 ± 1.0 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 1 | 22.8 ± 0.8 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 2 | 23.6 ± 0.8 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 5 | 23.9 ± 0.6 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 40 | 0.2 | 23.9 ± 0.8 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere |
| 0.5 | 23.1 ± 0.9 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 1 | 24.2 ± 1.0 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 2 | 25.2 ± 0.9 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
| 5 | 24.4 ± 1.0 | Hexagonal |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) |
Sphere | |
Shapes and sizes of β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals were revealed by TEM measurements. All the obtained β-NaYF4
Yb,Tm nanocrystals were revealed by TEM measurements. All the obtained β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals showed high size- and shape-uniformity. Small nanocrystals, with the size of about 25 nm, were all of the shape of sphere (for a typical example, see Fig. 1a), while those larger nanocrystals possessed the shape of hexagonal prisms (about 50 nm; Fig. 1b) or hexagonal plates (about 150 nm × 70 nm; Fig. 1c). Powder X-ray diffraction patterns (Fig. 2) were in good agreement with the JCPDS data (JCPDS Card #: 16–0334), revealing that all these nanocrystals are of hexagonal phase with the P
Yb,Tm nanocrystals showed high size- and shape-uniformity. Small nanocrystals, with the size of about 25 nm, were all of the shape of sphere (for a typical example, see Fig. 1a), while those larger nanocrystals possessed the shape of hexagonal prisms (about 50 nm; Fig. 1b) or hexagonal plates (about 150 nm × 70 nm; Fig. 1c). Powder X-ray diffraction patterns (Fig. 2) were in good agreement with the JCPDS data (JCPDS Card #: 16–0334), revealing that all these nanocrystals are of hexagonal phase with the P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) symmetry; while HRTEM images (Fig. 1d and e) showed that both the ∼25 nm spheres and the ∼50 nm prisms are all single crystalline. More TEM images of as-synthesized β-NaYF4
symmetry; while HRTEM images (Fig. 1d and e) showed that both the ∼25 nm spheres and the ∼50 nm prisms are all single crystalline. More TEM images of as-synthesized β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals with different Yb3+/Tm3+ concentrations are provided in the ESI† (see Fig. S1). EDS analysis of some typical samples reveal that the concentrations of different RE cations in the final nanocrystalline products agree well with the original molar ratios of the metal precursors (see Table 2), indicating that quantitative doping of Yb3+ and Tm3+ ions into NaYF4 lattice was achieved by the present synthesis method.
Yb,Tm nanocrystals with different Yb3+/Tm3+ concentrations are provided in the ESI† (see Fig. S1). EDS analysis of some typical samples reveal that the concentrations of different RE cations in the final nanocrystalline products agree well with the original molar ratios of the metal precursors (see Table 2), indicating that quantitative doping of Yb3+ and Tm3+ ions into NaYF4 lattice was achieved by the present synthesis method.
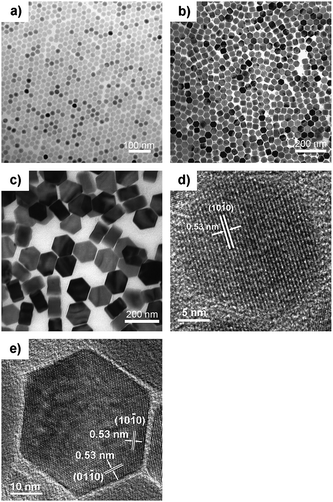 | ||
| Fig. 1 TEM images of β-NaYF4 : 30%Yb,0.5%Tm nanocrystals of different sizes and shapes: (a) 23.9 ± 0.8 nm nanospheres; (b) 47.3 ± 1.7 nm nanoprisms; and (c) ∼150 nm × ∼70 nm nanoplates. HRTEM images of β-NaYF4 : 30%Yb,0.5%Tm nanocrystals: (d) 23.9 ± 0.8 nm nanospheres; (e) 47.3 ± 1.7 nm nanoprisms. | ||
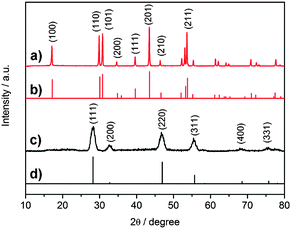 | ||
Fig. 2 XRD pattern of (a) as-obtained β-NaYF4 : 30%Yb,0.5%Tm nanocrystals (47.3 ± 1.7 nm nanoprisms); (b) JCPDS Card # 16–0334; (c) as-obtained α-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals, and (d) JCPDS Card # 77–2042. Yb,Tm nanocrystals, and (d) JCPDS Card # 77–2042. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals
Yb,Tm nanocrystals
| Molar ratioa | Size/nm | EDS results (atomic ratio)b | |||
|---|---|---|---|---|---|
| Yb (%) | Tm (%) | Y (%) | Yb (%) | Tm (%) | |
| a Molar ratio of reactants. b Average of three independent areas on the copper grid for each sample. | |||||
| 20 | 0.5 | 25.4 ± 1.0 | 79.6 | 19.8 | 0.6 |
| 30 | 0.5 | 23.9 ± 0.8 | 68.2 | 31.2 | 0.6 |
| 47.3 ± 1.7 | 69.3 | 30.1 | 0.6 | ||
| 1 | 24.1 ± 0.8 | 72.2 | 26.8 | 1.1 | |
| 2 | 24.4 ± 1.0 | 64.1 | 33.5 | 2.3 | |
| 40 | 0.5 | 23.1 ± 0.9 | 61.9 | 37.4 | 0.7 |
2. UC properties of β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Yb,Tm nanocrystals
Yb,Tm nanocrystals
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Yb,Tm nanocrystals.
As shown in Fig. 3a, in the case of β-NaYF4 : 30%Yb,0.5%Tm nanocrystals, the near-ultra-violet to visible emission bands of the UC spectra of Yb3+/Tm3+ transitions include 360 (1D2 – 3H6), 450 (1D2 – 3H4), 475 (1G4 – 3H6), 650 (1G4 – 3H4) and 695 nm (3F2 – 3H6). The infrared emission at around 800 nm is much stronger than those of ultra-violet and visible light, which enables the as-obtained β-NaYF4
Yb,Tm nanocrystals.
As shown in Fig. 3a, in the case of β-NaYF4 : 30%Yb,0.5%Tm nanocrystals, the near-ultra-violet to visible emission bands of the UC spectra of Yb3+/Tm3+ transitions include 360 (1D2 – 3H6), 450 (1D2 – 3H4), 475 (1G4 – 3H6), 650 (1G4 – 3H4) and 695 nm (3F2 – 3H6). The infrared emission at around 800 nm is much stronger than those of ultra-violet and visible light, which enables the as-obtained β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals to be excellent candidates for NIR-NIR bioprobes9d (Fig. 3b and Fig. S2 of the ESI†).
Yb,Tm nanocrystals to be excellent candidates for NIR-NIR bioprobes9d (Fig. 3b and Fig. S2 of the ESI†).
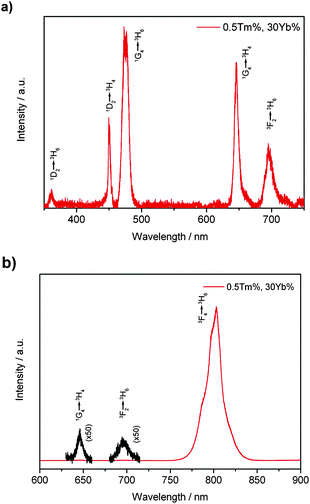 | ||
| Fig. 3 UC spectra of β-NaYF4 : 30%Yb,0.5%Tm nanocrystal dispersion (23.9 ± 0.8 nm) in cyclohexane (1 wt%) pumped by a 980 nm laser: (a) UV and visible emissions and (b) red and IR emissions. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Yb,Tm nanocrystals with different doping ratios of Yb3+ and Tm3+.
In the research work of bulk GdF3
Yb,Tm nanocrystals with different doping ratios of Yb3+ and Tm3+.
In the research work of bulk GdF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm11a and YF3
Yb,Tm11a and YF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm11b materials, Hewes et al.11a and Ostermayer et al.11b studied the UC luminescence mechanism of Yb3+ and Tm3+ system, pointing out the four-, three- and two-photon processes involved in the UC light and the effects of different doping ratios of Yb and Tm cations on the UC emissions.
Yb,Tm11b materials, Hewes et al.11a and Ostermayer et al.11b studied the UC luminescence mechanism of Yb3+ and Tm3+ system, pointing out the four-, three- and two-photon processes involved in the UC light and the effects of different doping ratios of Yb and Tm cations on the UC emissions.
The UC excitation and emission behavior of as-synthesized β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals are similar to those of bulk materials, and can be also artificially altered by changing different doping ratios. As seen from Fig. 4a, the UC intensities for the β-NaYF4 : 20%Yb,5%Tm sample is rather weak. With the concentration of Yb3+ fixed at 20%, as the molar ratio of Tm3+ decreases from 5% to 0.2%, the intensities of the emissions at around 360 and 450 nm increase more quickly than those at 475 and 650 nm. This result shows that the increase of Tm3+ concentration from 0.2% to 5% tends to quench the blue UC emissions. The same effects of different doping ratios of Tm3+ also take place when the molar ratio of Yb3+ is fixed at 30% and 40% (see Fig. S4 of the ESI†). As a result, by increasing the doping ratio of Tm3+, the color of the overall emission light can be tuned from bright blue to purple, and to dark red for the β-NaYF4 : 20%Yb,0.2–5%Tm nanocrystals (see Fig. 4b–f). As shown in Fig. 4b to f, after mild sonication, those colloid solutions which have been aged for more than half a year can be easily redispersed in cyclohexane showing relatively low scattering effects under the laser beam. The effect of different doping ratios of Yb3+ cations is shown in Fig. 5 and Fig S5.† With the molar ratio of Tm3+ ions fixed at 0.5% or 1%, the relative intensities of emissions at around 360, 450 and 475 nm gradually increase as the molar ratio of Yb3+ increases from 20% to 30% and to 40%, while that at around 650 nm is slightly changed.
Yb,Tm nanocrystals are similar to those of bulk materials, and can be also artificially altered by changing different doping ratios. As seen from Fig. 4a, the UC intensities for the β-NaYF4 : 20%Yb,5%Tm sample is rather weak. With the concentration of Yb3+ fixed at 20%, as the molar ratio of Tm3+ decreases from 5% to 0.2%, the intensities of the emissions at around 360 and 450 nm increase more quickly than those at 475 and 650 nm. This result shows that the increase of Tm3+ concentration from 0.2% to 5% tends to quench the blue UC emissions. The same effects of different doping ratios of Tm3+ also take place when the molar ratio of Yb3+ is fixed at 30% and 40% (see Fig. S4 of the ESI†). As a result, by increasing the doping ratio of Tm3+, the color of the overall emission light can be tuned from bright blue to purple, and to dark red for the β-NaYF4 : 20%Yb,0.2–5%Tm nanocrystals (see Fig. 4b–f). As shown in Fig. 4b to f, after mild sonication, those colloid solutions which have been aged for more than half a year can be easily redispersed in cyclohexane showing relatively low scattering effects under the laser beam. The effect of different doping ratios of Yb3+ cations is shown in Fig. 5 and Fig S5.† With the molar ratio of Tm3+ ions fixed at 0.5% or 1%, the relative intensities of emissions at around 360, 450 and 475 nm gradually increase as the molar ratio of Yb3+ increases from 20% to 30% and to 40%, while that at around 650 nm is slightly changed.
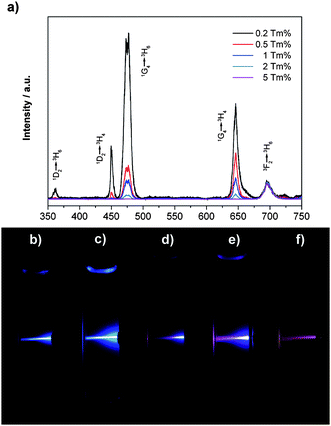 | ||
| Fig. 4 UC spectra of β-NaYF4 : 20%Yb,0.2–5%Tm nanocrystal dispersions (∼25 nm) in cyclohexane (1 wt%), normalized at the emission peak of ∼695 nm (a); and digital photos of the UC photoluminescence of the β-NaYF4 : 20%Yb,0.2–5%Tm nanocrystal dispersions in cyclohexane after storing for more than 6 months at RT: (b) 0.2%Tm; (c) 0.5%Tm; (d) 1%Tm; (e) 2%Tm; (f) 5%Tm. | ||
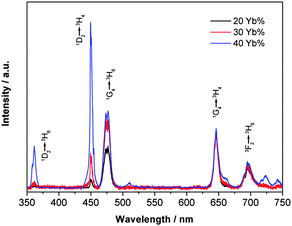 | ||
| Fig. 5 UC spectra of β-NaYF4 : 20–40%Yb,0.5%Tm nanocrystal (∼25 nm) dispersion in cyclohexane (1 wt%), normalized at the emission peak of ∼650 nm pumped by a 980 nm laser. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Yb,Tm nanocrystals with different sizes.
Different particle sizes also result in the differences of relative intensities of each UC emission peak and thus the different overall color of the total UC emissions for the as-obtained β-NaYF4
Yb,Tm nanocrystals with different sizes.
Different particle sizes also result in the differences of relative intensities of each UC emission peak and thus the different overall color of the total UC emissions for the as-obtained β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals. As shown in Fig. 6, when the particle sizes increase from 25 to 50 nm, the relative intensities of UV and blue emission (360 and 450 nm) increase dramatically, while the relative intensities of emissions at 475, 650 and 700 nm would not change so much, and thus the total color would become bluer as a result. When the size of β-NaYF4 : 30%Yb,0.5%Tm nanocrystals further increases to about 150 nm with the shape evolution from hexagonal prism to hexagonal plate, the relative intensity of two-photon process (695 nm) decreases significantly, while intensities of the four-photon processes (360 and 450 nm) also drop unexpectedly.
Yb,Tm nanocrystals. As shown in Fig. 6, when the particle sizes increase from 25 to 50 nm, the relative intensities of UV and blue emission (360 and 450 nm) increase dramatically, while the relative intensities of emissions at 475, 650 and 700 nm would not change so much, and thus the total color would become bluer as a result. When the size of β-NaYF4 : 30%Yb,0.5%Tm nanocrystals further increases to about 150 nm with the shape evolution from hexagonal prism to hexagonal plate, the relative intensity of two-photon process (695 nm) decreases significantly, while intensities of the four-photon processes (360 and 450 nm) also drop unexpectedly.
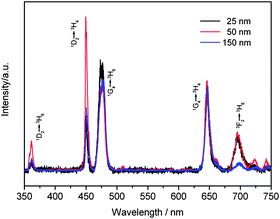 | ||
| Fig. 6 UC spectra of β-NaYF4 : 30%Yb,0.5%Tm nanocrystal dispersions (1 wt%) with different sizes (25–150 nm) pumped by a 980 nm laser. | ||
Compared to those smaller nanospheres or nanoprisms, UC properties of large hexagonal plates seem to be more similar to those of β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm crystals in micro-scale12 and macro-scale.2c In addition, as the particle size increases, the overall intensity of all UC emissions also increases for the as-obtained β-NaYF4
Yb,Tm crystals in micro-scale12 and macro-scale.2c In addition, as the particle size increases, the overall intensity of all UC emissions also increases for the as-obtained β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals, resulting in much brighter outcome light which can be seen by naked eyes. The reason for the variation in both relative and total emission intensities observed for the as-obtained β-NaYF4
Yb,Tm nanocrystals, resulting in much brighter outcome light which can be seen by naked eyes. The reason for the variation in both relative and total emission intensities observed for the as-obtained β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals was considered to be mainly ascribed to different surface states of nanocrystals with different sizes and shapes, as also revealed in the studies of the UC properties of β-NaYF4
Yb,Tm nanocrystals was considered to be mainly ascribed to different surface states of nanocrystals with different sizes and shapes, as also revealed in the studies of the UC properties of β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Er nanocrystals synthesized by similar methods.8j On the one hand, larger particle size, i.e. smaller surface-to-volume ratio, means the existence of lower density of surface quenching centers and thus leads to the enhanced total UC emissions for the bigger β-NaYF4
Yb,Er nanocrystals synthesized by similar methods.8j On the one hand, larger particle size, i.e. smaller surface-to-volume ratio, means the existence of lower density of surface quenching centers and thus leads to the enhanced total UC emissions for the bigger β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals.8a,j The unexpected stronger emissions of four-photon process at 360 and 450 nm for ∼50 nm β-NaYF4
Yb,Tm nanocrystals.8a,j The unexpected stronger emissions of four-photon process at 360 and 450 nm for ∼50 nm β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals than those for the bigger (∼150 nm) nanocrystals is interesting. The unexpected stronger four-photon process emissions in smaller β-NaYF4
Yb,Tm nanocrystals than those for the bigger (∼150 nm) nanocrystals is interesting. The unexpected stronger four-photon process emissions in smaller β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals is rather interesting. Herein, we assumed that the unexpected UC spectra were caused by different local structures around the photoactive sites with the reduction of crystalline size, the increase of surface-to-volume ratio and the inhomogeneity of dopant ions in relatively small nanocrystals.7f As mentioned above, in smaller particles, larger surface-to-volume ratio (s/v) would decrease the total intensity, as well as the relative intensity of emissions from higher energy levels (1D2 of Tm3+, for example); however, in smaller particles the variation of doped Tm3+ concentration in different nanocrystals (see EDS results in Table S2 of the ESI†) would also be more significant than in larger ones and thus there would be some nanocrystals with much lower Tm3+ concentrations which could give much stronger emissions from 1D2 to 3H6 as the self quenching of Tm3+ ions decreased dramatically,11bleading to the bluer emission. In our experiment, we further assumed that, when the particle size increased from about 25 nm (s/v = 0.24 nm−1, sphere) to 50 nm (s/v = 0.12 nm−1, hexagonal prism), the decreasing of surface-to-volume ratio would play a dominant role in affecting the UC emissions, and the intensity of four-photon process emission would become much stronger. However, when the nanocrystals grew further to ∼150 nm hexagonal plates (s/v = 0.06 nm−1, 150 nm × 70 nm, hexagonal plates), the decreasing of surface-to-volume ratio would not have obvious influence on the UC spectra as before. While the enhanced doping homogeneity in large nanocrystals would lead to fewer nanocrystals in which the Tm3+ doping concentrations were much lower than expected (see EDS results in Table S2 in the ESI†), and then resulted in the decrease of the relative intensity of four photon emissions. In addition, we supposed that as the particle size increased to hundreds of nanometres, the scattering effects of those particles in the colloidal solutions would become much stronger, which may also have strong effects on the acquired UC spectra.
Yb,Tm nanocrystals is rather interesting. Herein, we assumed that the unexpected UC spectra were caused by different local structures around the photoactive sites with the reduction of crystalline size, the increase of surface-to-volume ratio and the inhomogeneity of dopant ions in relatively small nanocrystals.7f As mentioned above, in smaller particles, larger surface-to-volume ratio (s/v) would decrease the total intensity, as well as the relative intensity of emissions from higher energy levels (1D2 of Tm3+, for example); however, in smaller particles the variation of doped Tm3+ concentration in different nanocrystals (see EDS results in Table S2 of the ESI†) would also be more significant than in larger ones and thus there would be some nanocrystals with much lower Tm3+ concentrations which could give much stronger emissions from 1D2 to 3H6 as the self quenching of Tm3+ ions decreased dramatically,11bleading to the bluer emission. In our experiment, we further assumed that, when the particle size increased from about 25 nm (s/v = 0.24 nm−1, sphere) to 50 nm (s/v = 0.12 nm−1, hexagonal prism), the decreasing of surface-to-volume ratio would play a dominant role in affecting the UC emissions, and the intensity of four-photon process emission would become much stronger. However, when the nanocrystals grew further to ∼150 nm hexagonal plates (s/v = 0.06 nm−1, 150 nm × 70 nm, hexagonal plates), the decreasing of surface-to-volume ratio would not have obvious influence on the UC spectra as before. While the enhanced doping homogeneity in large nanocrystals would lead to fewer nanocrystals in which the Tm3+ doping concentrations were much lower than expected (see EDS results in Table S2 in the ESI†), and then resulted in the decrease of the relative intensity of four photon emissions. In addition, we supposed that as the particle size increased to hundreds of nanometres, the scattering effects of those particles in the colloidal solutions would become much stronger, which may also have strong effects on the acquired UC spectra.
3. UC mechanism of β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Yb,Tm nanocrystals
Yb,Tm nanocrystals
To confirm the multi-photon processes involved in the UC behaviors of our β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals, we carried out the power density dependent UC measurements. As shown in Fig. 7 and Fig. S3,† we can easily assign the emission bands into different multi-photon transition processes. The ultra-violet emission at about 360 nm and blue emission at about 450 nm are of four-photon processes, that is, the transition from 1D2 to 3H6 and 3H4, respectively. The blue emission at about 475 nm and red emission at about 650 nm are of three-photon processes, 1G4 to 3H6 and 1G4 to 3H4, respectively. And the two-photon UC processes include the emission band of about 695 nm (red, 3F3 to 3H6).
Yb,Tm nanocrystals, we carried out the power density dependent UC measurements. As shown in Fig. 7 and Fig. S3,† we can easily assign the emission bands into different multi-photon transition processes. The ultra-violet emission at about 360 nm and blue emission at about 450 nm are of four-photon processes, that is, the transition from 1D2 to 3H6 and 3H4, respectively. The blue emission at about 475 nm and red emission at about 650 nm are of three-photon processes, 1G4 to 3H6 and 1G4 to 3H4, respectively. And the two-photon UC processes include the emission band of about 695 nm (red, 3F3 to 3H6).
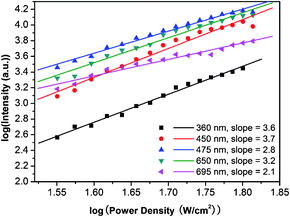 | ||
| Fig. 7 Power dependence of the UC emissions of β-NaYF4 : 30%Yb,0.5%Tm nanocrystal dispersions in cyclohexane (47.3 ± 1.7 nm) (pumped by a 980 nm laser). The straight lines are least-squares fits to the data points. | ||
During the characterization of UC emissions of β-NaYF4 : 30%Yb,0.5%Tm nanocrystals (23.9 ± 0.8 nm), we found that slopes of intensity–power (I–P) curves of near-UV and visible emission bands would decrease, meaning that a UC saturation effect would take place when the excitation power density of the 980 nm laser increased up to a certain value (see Fig. S6 in the ESI†). We considered the decrease of the I–P curve slopes to be the result of saturation effects of the intermediate levels of Yb3+ and Tm3+(esp. 2F5/2 of Yb3+, 1D2, 1G4 and 3F4 of Tm3+), which, as pointed out by Hewes et al.,11a can be easily affected by heating effects of the NIR laser beam and some other factors.
Our analyzed experiment results (see Fig. 7 and Fig. S2, S3 and S6 in the ESI†) indicate that the transitions from energy level 1D2 to 3H6 (ground state), 3H4 and 3H5 are four-photon processes, transitions from 1G4 to 3H6 and 3H4 are three-photon processes, and transitions from 3F2, 3F3 and 3F4 to 3H6 are two-photon processes, and thus suggest that the successive transfer model instead of the cooperative sensitization model,9d would be more acceptable, as shown in Fig. 8.
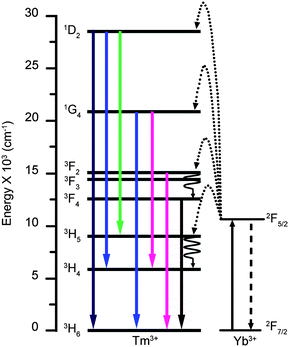 | ||
Fig. 8 Schematic energy level diagrams, UC excitation and emission schemes for the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm systems, showing two- (3F2 to 3H6 and 3F4 to 3H6), three- (1G4 to 3H6 and 1G4 to 3H4) and four-photon (1D2 to 3H6, 1D2 to 3H5 and 1D2 to 3H4) UC processes (pumped by a 980 nm laser). Yb,Tm systems, showing two- (3F2 to 3H6 and 3F4 to 3H6), three- (1G4 to 3H6 and 1G4 to 3H4) and four-photon (1D2 to 3H6, 1D2 to 3H5 and 1D2 to 3H4) UC processes (pumped by a 980 nm laser). | ||
As pointed out by Ostermayer et al.,11b the main factors determining the efficiency of the UC excitations and emissions lie in the Yb-to-Tm transfer probabilities and the quenching of Tm and Yb manifolds. A relatively low ratio of Tm3+ (0.03%) and a certain percentage of Yb3+ (around 30%) are preferred to get more efficient blue light emissions in bulk YF3 system. Our results indicate that higher percentage of Tm3+ would result in the dramatic reduction of the blue emissions (four-photon process at about 450 nm and three-photon process at about 475 nm) and the red emission at about 650 nm (three-photon process). And as the Tm3+ percentage rises to 5%, emissions of shorter wavelengths vanished dramatically, and the sole visible emission band left is the one at about 695 nm (Fig. 4 and Fig. S4 of the ESI†). The reason can be assigned to the self-quenching of Tm3+1D2 and 1G4 manifolds, which was much more sensitive to the concentration of Tm3+ compared to the case of Er3+ activated rare earth fluorides.11b Thus, as the Tm3+ percentage rises from 0.2% to 5%, the total color of the UC emission would turn from bright blue to dark red. Meanwhile, the proper increase of Yb3+ concentration would enhance the Yb-to-Tm transfer probabilities, and then result in the enhanced relative intensities of the blue emissions at 450 and 475 nm (especially the one at 450 nm) (see Fig. 5 and Fig. S5†). However, as the ratio of Yb3+ increases further, the quenching of Tm3+ manifolds by Yb3+ would become more significant and the blue emissions would become weaker instead of becoming stronger any more.11b Our experiments have proved that β-NaYF4 : 40%Yb,0.5%Tm nanocrystals are much better UC materials with blue emissions than β-NaYF4 : 20%Yb,0.5%Tm nanocrystals (see Fig. 5). In addition, it is observed with naked eyes that relatively larger β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals would result in brighter UC light due to the less surface defects as well as less influence of surface ligands.8j
Yb,Tm nanocrystals would result in brighter UC light due to the less surface defects as well as less influence of surface ligands.8j
As discovered above, a relatively low ratio of Tm3+ and about 40% or more of Yb3+ may result in the brighter and bluer emissions. Maybe a much lower percentage of Tm3+ would also lead to the enhancement of UC transitions of Yb3+/Tm3+; however, the segregations of Tm3+ cations in different particles would be a big problem in the nano-scale if only trace amount of Tm3+ was used in the synthesis procedure. And if for bio-imaging applications, a proper size of the nanocrystals should be chosen in order to balance the intensity of emissions and the colloidal dispersibility of the nanocrystals. Also, we noticed that when the concentration of Yb3+ raises to even higher stage, the UC emission process, as well as the mechanism beyond, would be significantly different from those discussed above, which would be one of the issues of our further studies.
Conclusions
Differently-sized, monodisperse, and single-crystalline β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals were obtained by the thermolysis of metal trifluoroacetates in hot surfactant solutions under a controllable and reproducible way. Multicolor UC emissions (blue, purple and red) can be observed for the as-synthesized β-NaYF4
Yb,Tm nanocrystals were obtained by the thermolysis of metal trifluoroacetates in hot surfactant solutions under a controllable and reproducible way. Multicolor UC emissions (blue, purple and red) can be observed for the as-synthesized β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals when excited by a 980 nm CW laser. Through investigating the effects of different concentrations of Tm3+ and Yb3+ cations and different particle sizes on the UC behaviors of the as-obtained nanocrystals, we found out that relatively low concentrations of Tm3+ (i.e. 0.2%) and high doping ratios of Yb3+ (i.e. 40%) were more favored to get purer and brighter blue emissions. We have also deduced the four-, three-, and two-photon processes of the UC excitation and emissions for our β-NaYF4
Yb,Tm nanocrystals when excited by a 980 nm CW laser. Through investigating the effects of different concentrations of Tm3+ and Yb3+ cations and different particle sizes on the UC behaviors of the as-obtained nanocrystals, we found out that relatively low concentrations of Tm3+ (i.e. 0.2%) and high doping ratios of Yb3+ (i.e. 40%) were more favored to get purer and brighter blue emissions. We have also deduced the four-, three-, and two-photon processes of the UC excitation and emissions for our β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals, and proved that the successive transfer model instead of the cooperative sensitization model would be applied to account for the UC mechanism of the present nanocrystals. The unexpected stronger emissions of a four-photon process at 360 and 450 nm for ∼50 nm β-NaYF4
Yb,Tm nanocrystals, and proved that the successive transfer model instead of the cooperative sensitization model would be applied to account for the UC mechanism of the present nanocrystals. The unexpected stronger emissions of a four-photon process at 360 and 450 nm for ∼50 nm β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals than those for the bigger (∼150 nm) nanocrystals was observed and explained in terms of the effects of crystallite size, surface-to-volume ratio and homogeneity of the doping cations. In prospect, this work has provided new scientific insights in understanding the fundamental aspects of the size, composition-dependent UC properties and mechanisms of NaYF4
Yb,Tm nanocrystals than those for the bigger (∼150 nm) nanocrystals was observed and explained in terms of the effects of crystallite size, surface-to-volume ratio and homogeneity of the doping cations. In prospect, this work has provided new scientific insights in understanding the fundamental aspects of the size, composition-dependent UC properties and mechanisms of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals with blue emissions in the nanometric regime, and promises various nanodevice applications (particularly for bio-imaging and bio-labeling) with the as-synthesized nanocrystalline UC phosphors.
Yb,Tm nanocrystals with blue emissions in the nanometric regime, and promises various nanodevice applications (particularly for bio-imaging and bio-labeling) with the as-synthesized nanocrystalline UC phosphors.
Acknowledgements
We gratefully acknowledge the financial support from the MOST of China (Grant No. 2006CB601104) and NSFC (Grant Nos. 20871006, 20821091, and 20671005).Notes and references
- (a) F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS; (b) J. F. Suyver, A. Aebischer, D. Biner, P. Gerner, J. Grimm, S. Heer, K. W. Kramer, C. Reinhard and H. U. Güdel, Opt. Mater., 2005, 27, 1111 CrossRef CAS; (c) F. Wang and X. G. Liu, Chem. Soc. Rev., 2009, 38, 976 RSC.
- (a) N. Menyuk, K. Dwight and J. W. Pierce, Appl. Phys. Lett., 1972, 21, 159 CAS; (b) J. L. Sommerdijk and A. Bril, Philips Tech. Rev., 1974, 34, 1 Search PubMed; (c) K. W. Kramer, D. Biner, G. Frei, H. U. Güdel, M. P. Hehlen and S. R. Luthi, Chem. Mater., 2004, 16, 1244 CrossRef; (d) E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CrossRef CAS; (e) M. Huang and F. Meng, Luminescence, 2005, 20, 276 CrossRef CAS.
- (a) S. Sivakumar, F. C. J. M. van Veggel and P. S. May, J. Am. Chem. Soc., 2007, 129, 620 CrossRef CAS; (b) F. van de Rijke, H. Zijlmans, S. Li, T. Vail, A. K. Raap, R. S. Niedbala and H. J. Tanke, Nat. Biotechnol., 2001, 19, 273 CrossRef; (c) G. S. Yi, H. C. Lu, S. Y. Zhao, G. Yue, W. J. Yang, D. P. Chen and L. H. Guo, Nano Lett., 2004, 4, 2191 CrossRef CAS; (d) L. Y. Wang, R. X. Yan, Z. Y. Huo, L. Wang, J. H. Zeng, J. Bao, X. Wang, Q. Peng and Y. D. Li, Angew. Chem., Int. Ed., 2005, 44, 6054 CrossRef CAS; (e) L. Wang and Y. Li, Chem. Commun., 2006, 2557 RSC.
- (a) W. N. Millar and L. E. Casida, Can. J. Microbiol., 1970, 16, 305 CrossRef CAS; (b) C. W. Griffin, T. R. Carski and G. S. Warner, J. Bacterial., 1961, 82, 534 Search PubMed; (c) J. L. Seifert, R. E. Connor, S. A. Kushon, M. Wang and A. Armitage, J. Am. Chem. Soc., 1999, 121, 2987 CrossRef CAS; (d) C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607 CrossRef CAS.
- (a) A. P. Alivisatos, K. P. Jonsson, X. G. Peng, T. E. Wilson, C. J. Loweth, M. P. Bruchez and P. G. Schultz, Nature, 1996, 382, 609 CrossRef CAS; (b) K. Konig, J. Microsc., 2000, 200, 83 CrossRef CAS; (c) D. R. Larson, W. R. Zipfel, R. M. Williams, S. W. Clark, M. P. Bruchez, F. W. Wise and W. W. Webb, Science, 2003, 300, 1434 CrossRef CAS; (d) W. Denk, J. H. Strickler and W. W. Webb, Science, 1990, 248, 73 CrossRef CAS.
- (a) H. J. M. A. Zijlmans, J. Bonnet, J. Burton, K. Kardos, T. Vail, R. S. Niedbala and H. Tanke, Anal. Biochem., 1999, 267, 30 CrossRef CAS; (b) J. A. Feijo and N. Moreno, Protoplasma, 2004, 223, 1 CrossRef; (c) S. R. Sershen, S. L. Westcott, N. J. Halas and J. L. West, J. Biomed. Mater. Res., 2000, 51, 293 CrossRef CAS; (d) R. W. Waynant, I. K. Ilev and I. Gannot, Philos. Trans. R. Soc. London, Ser. A, 2001, 359, 635 CrossRef CAS; (e) W. C. W. Chan and S. M. Nie, Science, 1998, 281, 2016 CrossRef CAS.
- (a) A. Bril, J. L. Sommerdijk and A. W. De Jager, J. Electrochem. Soc., 1975, 122, 660 CrossRef CAS; (b) J. F. Suyver, J. Grimm, K. W. Kramer and H. U. Güdel, J. Lumin., 2005, 114, 53 CrossRef CAS; (c) J. F. Suyver, J. Grimm, M. K. van Veen, D. Biner, K. W. Kramer and H. U. Güdel, J. Lumin., 2006, 117, 1 CrossRef CAS; (d) L. F. Liang, H. Wu, H. L. Hu, M. M. Wu and Q. Su, J. Alloys Compd., 2004, 368, 94 CrossRef CAS; (e) J. H. Burns, Inorg. Chem., 1965, 4, 881 CrossRef CAS; (f) A. Aebischer, M. Hostettler, J. Hauser, K. Kramer, T. Weber, H. U. Güdel and H. B. Burgi, Angew. Chem., Int. Ed., 2006, 45, 2802 CrossRef CAS; (g) J. F. Suyver, A. Aebischer, S. García-Revilla, P. Gerner and H. U. Güdel, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 125123 CrossRef.
- (a) S. Heer, K. Kompe, H. U. Güdel and M. Haase, Adv. Mater., 2004, 16, 2102 CrossRef CAS; (b) J. H. Zeng, J. Su, Z. H. Li, R. X. Yan and Y. D. Li, Adv. Mater., 2005, 17, 2119 CrossRef CAS; (c) H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS; (d) J. C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444 CrossRef CAS; (e) J. C. Boyer, L. A. Cuccia and J. A. Capobianco, Nano Lett., 2007, 7, 847 CrossRef CAS; (f) G. S. Yi and G. M. Chow, Adv. Funct. Mater., 2006, 16, 2324 CrossRef CAS; (g) Y. Wei, F. Lu, X. Zhang and D. Chen, Chem. Mater., 2006, 18, 5733 CrossRef CAS; (h) G. S. Yi and G. M. Chow, Chem. Mater., 2007, 19, 341 CrossRef CAS; (i) L. Y. Wang and Y. D. Li, Chem. Mater., 2007, 19, 727 CrossRef CAS; (j) H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13721 CrossRef CAS; (k) H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13730 CrossRef CAS; (l) F. Zhang, Y. Wan, Y. F. Shi, B. Tu and D. Y. Zhao, Chem. Mater., 2008, 20, 3778 CrossRef CAS.
- (a) F. Wang and X. G. Liu, J. Am. Chem. Soc., 2008, 130, 5642 CrossRef CAS; (b) H. S. Qian and Y. Zhang, Langmuir, 2008, 24, 12123 CrossRef CAS; (c) Z. Q. Li, Y. Zhang and S. Jiang, Adv. Mater., 2008, 20, 4765 CrossRef CAS; (d) O. Ehlert, R. Thomann, M. Darbandi and T. Nann, ACS Nano, 2008, 2, 120 CrossRef CAS; (e) M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano Lett., 2008, 8, 3834 CrossRef CAS; (f) J. C. Boyer, N. J. J. Johnson and F. C. J. M. van Veggel, Chem. Mater., 2009, 21, 2010 CrossRef CAS.
- J. E. Roberts, J. Am. Chem. Soc., 1961, 83, 1087 CrossRef CAS.
- (a) R. A. Hewes and J. F. Sarver, Phys. Rev., 1969, 182, 427 CrossRef CAS; (b) F. W. Ostermayer Jr., J. P. van der Ziel, H. M. Marcos, L. G. Van Uitert and J. E. Geusic, Phys. Rev. B: Solid State, 1971, 3, 2698 CrossRef.
- C. X. Li, Z. W. Quan, J. Yang, P. P. Yang and J. Lin, Inorg. Chem., 2007, 46, 6329 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: More TEM images and UC results of the β-NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb,Tm nanocrystals. See DOI: 10.1039/b9nr00397e Yb,Tm nanocrystals. See DOI: 10.1039/b9nr00397e |
| This journal is © The Royal Society of Chemistry 2010 |
