Intermediate-dominated controllable biomimetic synthesis of gold nanoparticles in a quasi-biological system†
Ran
Cui‡
,
Ming-Xi
Zhang‡
,
Zhi-Quan
Tian
,
Zhi-Ling
Zhang
and
Dai-Wen
Pang
*
Key Laboratory of Analytical Chemistry for Biology and Medicine (Ministry of Education), College of Chemistry and Molecular Sciences, and State Key Laboratory of Virology, Wuhan University, Wuhan, 430072, P. R. China. E-mail: dwpang@whu.edu.cn; Fax: (+)0086-27-68754685
First published on 6th September 2010
Abstract
A new biomimetic strategy of creating a quasi-biological system (an aqueous solution containing electrolytes, peptide, enzyme and coenzyme) for the preparation of gold nanoparticles with uniform and tunable sizes has been put forward and validated, adopting environmentally-friendly reducing agents and a biocompatible capping ligand in aqueous solution at room temperature. The biomimetic synthetic route has the characteristics for good stability of the resulting AuNPs capped with glutathione via strong Au–S bond in aqueous solution, an appropriate composition of the intermediate with a redox potential favorable for the biomimetic reduction under mild conditions, suitable pH values to adjust the rate of the reduction, and the addition of enzyme catalyzing the reduction. By only adjusting the concentration of the reducing agent NADPH, a series of AuNPs with narrow size-distribution could be controllably synthesized. This method of rational utilization of biological processes could provide a new way for the sustainable development of nanotechnology.
Introduction
The interdisciplinary subject between nanotechnology and bioscience has opened up a new scientific field in the past decade. Nanotechnology greatly activates the research of approaches for bioscience. On the other hand, could we construct a new platform to expand nanotechnology through biology? Green synthesis of nanomaterials becomes a significant challenge to chemists with increasing environmental problems. Some key factors, such as environmentally-compatible media and reducing reagents and nontoxic capping ligands should be considered.1 Most approaches for the synthesis of nanomaterials are energy inefficient, requiring high temperature, pressure and often produce toxic byproducts. Fortunately, researchers have found help from biological systems. Microbial cells could produce nanocrystals in their specific domains based on their resistance to heavy ions.2–4 This finding inspired researchers to extract some active components or proteins from biological systems to produce different kinds of nanomaterials (Au, Ag, Fe3O4, TiO2, CoPt, CaCO3)2–10 in environmentally-friendly ways.In the previous work, the uniform fluorescent CdSe quantum dots with color controllability of photoluminescence could be synthesized in living yeast cells without combustible, explosive and toxic reagents.11 In this biosynthetic route for CdSe, we have found that glutathione (GSH), reduced nicotinamide adenine dinucleotide phosphate (NADPH), and glutathione reductase (GR) synergically reacted with Cd and Se elements and produced CdSe nanocrystals. The NADPH/GR system and GSH molecule play important roles in the physiological reaction which maintains a reducing environment in cells suffering from oxidation damage.12 These bioactive agents have also exhibited their abilities of reducing metal ions into nanostructures in vitro.13,14 Restricted by their relatively weak activity, NADPH and GR have not been widely used in the synthesis of nanomaterials so far. However, because of the intricate environment in the yeast cell, it was very difficult to purify the products from the cells. Therefore, it is necessary to find a method that retains the green characteristics of biosynthesis whilst avoiding the problems of purification at the same time.
Inspired by our biosynthesis using living cells, we created a relatively more simple quasi-biological system than the intricate environment in the yeast cell containing GSH, NADPH, GR, gold ions and other electrolytes to biomimetically synthesize gold nanoparticles (AuNPs) in an aqueous medium. By adjusting the composition of the intermediate product in the synthesis of AuNPs, we let bioactive reducing agent NADPH in the presence of GR work under mild conditions, successfully synthesizing GSH-capped gold nanoparticles with uniform and tunable sizes. Both the inner core and the outer capping shell of the products were characterized. Some factors influencing the formation process of AuNPs were investigated in detail.
Results and discussion
Biomimetic controllable synthesis of AuNPs
By mixing mercapto compounds (RSH) with chloroauric acid (HAuCl4) in the molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or higher, stable Au(I)–SR complex is produced in solution (eqn (1)).15,16 The Au(I)–SR complex was proven to be the intermediates of gold nanoparticles prepared by Brust's method17,18 which could be isolated as precursors (polymeric complexes) to synthesize monolayer-protected AuNPs by strong reducing agents such as NaBH4.19,20
1 or higher, stable Au(I)–SR complex is produced in solution (eqn (1)).15,16 The Au(I)–SR complex was proven to be the intermediates of gold nanoparticles prepared by Brust's method17,18 which could be isolated as precursors (polymeric complexes) to synthesize monolayer-protected AuNPs by strong reducing agents such as NaBH4.19,20| Au(III) + 3RSH → Au(I)SR + RSSR | (1) |
According to the above route, GSH and chloroauric acid were mixed in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio to prepare Au(I)–SG complex solution. The pH of the solution was adjusted to 5.0, followed by adding a certain amount of NADPH and GR. The solution was allowed to react and monitored by an UV-vis spectrophotometer. After 2 h, no surface plasmon resonance (SPR) band of AuNPs was observed (curve 1 in Fig. 1). Moreover, the NADPH-related absorption peak at 340 nm indicated that NADPH had not reacted, implicating that the reducibility of NADPH/GR was too weak to reduce Au(I)–SG into AuNPs. Under the same conditions, when the molar ratio of GSH to chloroauric acid was decreased to 1
1 molar ratio to prepare Au(I)–SG complex solution. The pH of the solution was adjusted to 5.0, followed by adding a certain amount of NADPH and GR. The solution was allowed to react and monitored by an UV-vis spectrophotometer. After 2 h, no surface plasmon resonance (SPR) band of AuNPs was observed (curve 1 in Fig. 1). Moreover, the NADPH-related absorption peak at 340 nm indicated that NADPH had not reacted, implicating that the reducibility of NADPH/GR was too weak to reduce Au(I)–SG into AuNPs. Under the same conditions, when the molar ratio of GSH to chloroauric acid was decreased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the absorption peak at 340 nm vanished and an obvious SPR band at 528 nm appeared (curve 2 in Fig. 1), which clearly reflected the consumption of NADPH and the formation of AuNPs.
1, the absorption peak at 340 nm vanished and an obvious SPR band at 528 nm appeared (curve 2 in Fig. 1), which clearly reflected the consumption of NADPH and the formation of AuNPs.
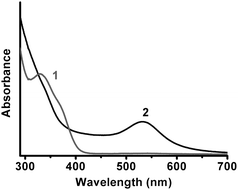 | ||
Fig. 1 UV-vis spectra of the mixtures of GSH and chloroauric acid reacted with NADPH/GR for 2 h at room temperature and pH 5.0. The molar ratios of GSH to chloroauric acid were (1) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (2) 1 1; (2) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
An urgent problem existing in the biological or biomimetic syntheses of nanomaterials was the difficulty of controlling the size of products.21 In previous reports, the size of AuNPs was mainly controlled by adjusting the molar ratio of Au ions to capping ligands.22–25 In this biomimetic method, by only adjusting the concentration of the reducing agent NADPH, a series of AuNPs with narrow size-distribution could be controllably synthesized (Fig. 2). Increasing the concentration of NADPH resulted in the size of synthesized AuNPs becoming smaller. According to the nucleation-growth mechanism, the nucleation rate as well as the number of Au nuclei increased as the concentration of reducing agent NADPH increased.26 Since the concentration of Au precursors was fixed, the size of AuNPs decreased as the concentration of NADPH increased.
 | ||
| Fig. 2 Representative TEM images of synthesized AuNPs (scale bar: 50 nm). The concentrations of NADPH used in the syntheses were (a) 2 mg mL−1; (b) 3 mg mL−1; (c) 4 mg mL−1, respectively. Size-distribution histograms of the synthesized AuNPs are given in the lower row. | ||
The surface condition of AuNP samples with mean diameter of about 12.6 nm (Fig. 2a) was characterized. The infrared spectrum of purified samples displayed peaks identical with those of glutathione (νmax/cm−1 3420 (NH), 1635 (CO), 1380 (CN)). In addition, the binding energy of S 2p from X-ray photoelectron spectroscopy (XPS) data of AuNPs samples was 162.4 eV (see Supplementary Data†), corresponding to sulfur atoms bound to gold nanoparticles. In thermogravimetric analysis, AuNPs samples lost 33% of weight after heating from 50 °C to 700 °C. The evidence clearly showed that the surface of AuNPs was capped by glutathione molecules with a relatively high coverage via strong Au–S bond, thus the products could be stored at room temperature for months without any aggregation.
Au(I) intermediate: a key factor in the bio-reduction process by NADPH/GR
The preparation process implied that the intermediate in the synthesis of AuNPs in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GSH/HAuCl4 molar ratio was different from the Au(I)–SG complex formed in 3
1 GSH/HAuCl4 molar ratio was different from the Au(I)–SG complex formed in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GSH/HAuCl4 molar ratio. It is well-known that Au(I) is a soft Lewis acid that can form complexes with polar ligands, such as halide anions27,28 and organothiolate anions. Based on previous references, in the presence of GSH molecules, Au(III) ions could be reduced to Au(I) ions, and then Au(I) and GS− could form the stable Au(I)–SG complex. But when the amount of GSH decreased, accordingly the relative amount of GS− decreased. Since there were a few kinds of anions in the quasi-biological system, Au(I) ions might have coordinated with other polar ligand besides GS−, and formed a less stable Au(I) complex which could be reduced to AuNPs by NADPH/GR. In order to understand the mechanism of the formation of AuNPs in the quasi-biological system, it was of great importance to analyze the composition of the intermediates.
1 GSH/HAuCl4 molar ratio. It is well-known that Au(I) is a soft Lewis acid that can form complexes with polar ligands, such as halide anions27,28 and organothiolate anions. Based on previous references, in the presence of GSH molecules, Au(III) ions could be reduced to Au(I) ions, and then Au(I) and GS− could form the stable Au(I)–SG complex. But when the amount of GSH decreased, accordingly the relative amount of GS− decreased. Since there were a few kinds of anions in the quasi-biological system, Au(I) ions might have coordinated with other polar ligand besides GS−, and formed a less stable Au(I) complex which could be reduced to AuNPs by NADPH/GR. In order to understand the mechanism of the formation of AuNPs in the quasi-biological system, it was of great importance to analyze the composition of the intermediates.
By adjusting the pH value to 2.5–3.0, the complexes formed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GSH/HAuCl4 ratio were purified from the solution. Based on previous work by Briñas et al.,29 Au(I) ions were bridged by ligands and formed a polymeric complex. Their hydrodynamic radii were related to pH values, which were determined by the ionization states of GSH30 at different pH values. In the pH range of 2.12(pK1) to 3.53(pK2), the net charge of GSH is zero. Without intermolecular electrostatically repulsive force, the metal-metal interaction of Au(I) ions, called aurophilic attraction, increased.31 Thus the complex tended to aggregate and formed an insoluble product, which could be separated by centrifugation. The complexes formed in 1
1 GSH/HAuCl4 ratio were purified from the solution. Based on previous work by Briñas et al.,29 Au(I) ions were bridged by ligands and formed a polymeric complex. Their hydrodynamic radii were related to pH values, which were determined by the ionization states of GSH30 at different pH values. In the pH range of 2.12(pK1) to 3.53(pK2), the net charge of GSH is zero. Without intermolecular electrostatically repulsive force, the metal-metal interaction of Au(I) ions, called aurophilic attraction, increased.31 Thus the complex tended to aggregate and formed an insoluble product, which could be separated by centrifugation. The complexes formed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios had different colors (pale yellow for 1
1 ratios had different colors (pale yellow for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and white for 3
1 and white for 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), suggesting that these complexes had different compositions.
1), suggesting that these complexes had different compositions.
The complexes were dissolved in alkaline solution and reacted with NADPH/GR under the same conditions mentioned above, which is denoted as the ex situ method in order to distinguish from the original method denoted as the in situ method. As expected, the results (Fig. 3) obtained by the ex situ method were quite similar to those by the in situ method, with little difference in the UV-vis spectra. The in situ and ex situ experiments for the GSH/HAuCl4 molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were also performed, obtaining the same results as the 3
1 were also performed, obtaining the same results as the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (data not shown). It could be hypothesized that the composition of complexes acted as a key factor in the formation of AuNPs.
1 ratio (data not shown). It could be hypothesized that the composition of complexes acted as a key factor in the formation of AuNPs.
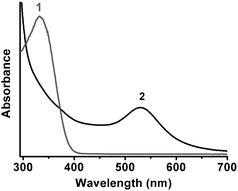 | ||
Fig. 3 UV-vis spectra of the complexes reacting with NADPH/GR for 2 h at room temperature and pH 5.0. The complexes were isolated from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (curve 1) and 1 1 (curve 1) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures (curve 2). 1 mixtures (curve 2). | ||
These three kinds of complexes prepared in mixtures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GSH/HAuCl4, abbreviated as the 1
1 GSH/HAuCl4, abbreviated as the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, 2
1 complex, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex and 3
1 complex and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, were characterized by XPS. As summarized in Table 1, besides Au and GSH-related elements, the survey and narrow scans clearly showed the presence of Cl (Cl 2p: 197.9 eV) in the 1
1 complex, were characterized by XPS. As summarized in Table 1, besides Au and GSH-related elements, the survey and narrow scans clearly showed the presence of Cl (Cl 2p: 197.9 eV) in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, which was absent in the 2
1 complex, which was absent in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 3
1 or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, respectively. The Au 4f7/2 binding energies of three complexes were close, confirming that Au atoms in these complexes were all in the form of Au(I) consistent with that reported in the reference (84.3 ± 0.1 eV).32 The S 2p binding energies (ca. 162.8 eV) corresponded to the coordination of negatively charged GS− to Au(I) and excluded the possibilities of free GSH (>163 eV) or disulfide (>164 eV).32,33 All the XPS spectra are available in the Supplementary Data.† The molar ratios of Au/SG in the 2
1 complexes, respectively. The Au 4f7/2 binding energies of three complexes were close, confirming that Au atoms in these complexes were all in the form of Au(I) consistent with that reported in the reference (84.3 ± 0.1 eV).32 The S 2p binding energies (ca. 162.8 eV) corresponded to the coordination of negatively charged GS− to Au(I) and excluded the possibilities of free GSH (>163 eV) or disulfide (>164 eV).32,33 All the XPS spectra are available in the Supplementary Data.† The molar ratios of Au/SG in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes close to 1 indicated that the 2
1 complexes close to 1 indicated that the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes were the same in the structure of polymeric complex [Au(I)SG]n. The molar ratio of Au
1 complexes were the same in the structure of polymeric complex [Au(I)SG]n. The molar ratio of Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SG in the 1
SG in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of nearly 2 implied the coordination of Au(I) with another kind of ligand except GS−. Combining with the XPS data, it could be concluded that both GS− and Cl− coordinated with Au(I) when equimolar chloroauric acid and GSH were mixed together and formed a polymeric complex.
1 complex of nearly 2 implied the coordination of Au(I) with another kind of ligand except GS−. Combining with the XPS data, it could be concluded that both GS− and Cl− coordinated with Au(I) when equimolar chloroauric acid and GSH were mixed together and formed a polymeric complex.
| Samples | Binding energy/eVa | Molar ratio | |||
|---|---|---|---|---|---|
| Au 4f7/2 | S 2p | Cl 2p | S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Clb Clb |
Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GS− GS−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
|
| a The binding energy of XPS data was referenced to the C 1s of aliphatic carbon at 284.8 eV. b S/Cl molar ratio was calculated using peak intensities and sensitivity factors from XPS experiments. c The content of Au was determined by ICP-OES; the content of GS− was from element analysis data of C, N, S. | |||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex 1 complex |
84.2 | 162.8 | 197.9 | 0.93 | 1.91 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex 1 complex |
84.4 | 162.7 | N | N | 0.89 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex 1 complex |
84.4 | 162.8 | N | N | 0.89 |
The standard redox potential (φ) of metal ions was directly affected by the coordination with ligands, as shown in eqn (2)–(4).
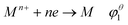 | (2) |
 | (3) |
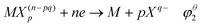 | (4) |
According to the Nernst equation (eqn (5)), the higher the formation constant of the complex is, the lower the standard redox potential of metal ions, namely big formation constant makes metal ions in the complex difficult to reduce.
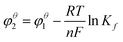 | (5) |
Au(I) is a typical Lewis soft acid and has a higher affinity with Lewis soft base GS− than with hard base Cl−. As in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, strong coordination of Au(I) with GS− lowered the standard redox potential of Au(I). Such Au(I) complexes could not be reduced by the NADPH/GR system under the mild conditions. However, attributed to the simultaneous coordinations of Au(I) with GS− and with Cl−, the standard redox potential of Au(I) in the 1
1 complexes, strong coordination of Au(I) with GS− lowered the standard redox potential of Au(I). Such Au(I) complexes could not be reduced by the NADPH/GR system under the mild conditions. However, attributed to the simultaneous coordinations of Au(I) with GS− and with Cl−, the standard redox potential of Au(I) in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was higher than that of the 2
1 complex was higher than that of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, providing an appropriate prerequisite for the reduction of Au(I) to AuNPs by the NADPH/GR system. This explained why the complexes with the same valence of Au had significantly different results when treated with NADPH/GR. In general, most ligands act as capping molecules or shape-controlled factors in nanomaterials synthesis. The present results also suggested that the ligand could tune the activity of metal ions as precursors for synthesis.
1 complexes, providing an appropriate prerequisite for the reduction of Au(I) to AuNPs by the NADPH/GR system. This explained why the complexes with the same valence of Au had significantly different results when treated with NADPH/GR. In general, most ligands act as capping molecules or shape-controlled factors in nanomaterials synthesis. The present results also suggested that the ligand could tune the activity of metal ions as precursors for synthesis.
The factors influencing the reduction process
In a typical ex situ synthesis, the time course plot of absorption intensity at 528 nm of reaction solution evidently illustrated that the reduction of added Au(I) complex into AuNPs by NADPH/GR was very slow (Fig. 4). In the beginning, the absorbance at 528 nm (A528nm) increased remarkably, indicating that the formation of AuNPs was at a relatively fast rate; afterwards the consumption of materials slowed down the formation rate, giving rise to little change in A528nm.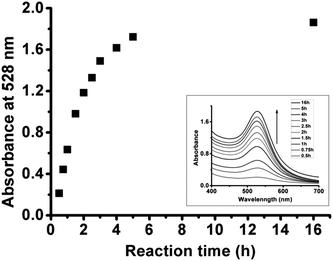 | ||
| Fig. 4 Time-dependent plot of absorption intensity at 528 nm of reaction solution of Au(I) complex with NADPH/GR at room temperature at pH 5.0. The inset is time-dependent UV-vis spectra of reaction solution. | ||
Since NADPH is a kind of hydride ion donor, the reducibility of NADPH could be significantly affected by pH values (see Supplementary Data†). Thus, pH values could also affect the formation rate of AuNPs. The time-dependent plots of absorption intensity at 528 nm under different pH conditions (4.5–6.5) are shown in Fig. 5. At pH 4.5, the absorbance increased quickly in the beginning, and changed slowly as Au(I) complex was gradually consumed. The trend of absorbance change at pH 5.5 was similar to that at pH 4.5, but the rate of absorbance change was slower than that at pH 4.5. When the pH was at 6.5, no obvious SPR band appeared within 5 h, indicating that AuNPs could not be produced at such a pH value. The addition of GR made both the rates of absorbance change at pH 4.5 and 5.5 increase evidently, but still did not change the absorbance of the solution at pH 6.5. The Au(I) complex tended to aggregate at pH values between 2 to 4. Therefore, the reducing products also aggregated into dark-blue precipitation after reacting for 0.5 h in this pH range. Furthermore, it was found that the reduction of Au(I) complex did not happen at pH values above 6.5 (data not shown).
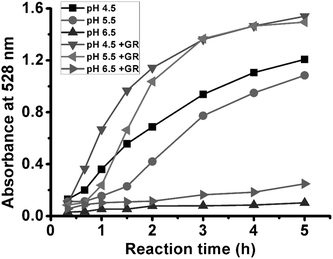 | ||
| Fig. 5 Time-dependent plots of absorption intensity at 528 nm of reaction solutions of different pH values. | ||
Compared to the natural process, namely reduction of oxidized glutathione (GSSG) catalyzed by GR, the biomimetic reduction of the Au(I) complex by NADPH/GR system was similar in some aspects. Both of them exhibited a pH dependence consistent with that of the reducibility of the NADPH/GR system. In other words, low pH values and addition of GR could accelerate both kinds of reduction processes in a certain pH range. In the reduction of GSSG, GR loosely bound with NADPH via non-covalent interactions and specifically recognized the substrate GSSG, giving rise to acceleration of the reduction process. Since the resulting Au(I) complex had a similar structure with GSSG (Glu-Cys-Gly unit), it implied that NADPH/GR might also recognize the Au(I) complex and facilitate the electron transfer between NADPH and Au(I) ion. Therefore, the existence of GR could increase the rate of the biomimetic reduction. However, the reduction of GSSG was a synergistic effect of NADPH and GR, occurring in a wide pH range (see Supplementary Data†). However, the biomimetic reduction of Au(I) complex was determined by the reducibility of NADPH which was only reactive in a narrow pH range (below 6). GR was a kind of catalytic additive which was not indispensable but increased the rate of the reduction to a certain extent.
To further investigate the mechanism, the biomimetic reduction process was performed at a different ionic strength. The addition of KNO3 did not change the position of the SPR band, but kinetically affected the reduction process. As shown in Fig. 6, the rate of the reduction process decreased as the concentration of KNO3 increased. The NADPH molecules were negatively charged due to the existence of phosphate groups. Before NADPH reduced the Au(I) complex into AuNPs, they may firstly interacted with Au(I) ions via an electrostatic force. High ionic strength suppressed the electrostatic interaction between NADPH and Au(I) ions, thus decreasing the rate of the reduction process. It also implied that the resulting AuNPs had very good colloidal stability under the condition of high salt concentration even in 0.5 mol L−1 KNO3. Furthermore, a less negative charge coenzyme that reduces nicotinamide adenine dinucleotide (NADH), which was similar to NADPH except for a phosphate group, was used as the reducing agent for the biomimetic reduction and demonstrated a slower rate compared to NADPH (data not shown). The above result strongly confirmed our deduction about the behavior of NADPH in the biomimetic reduction.
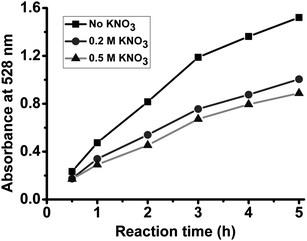 | ||
| Fig. 6 Time-dependent plots of absorption intensity at 528 nm of reaction solutions with different salt concentrations. | ||
Conclusion
This work demonstrated a biomimetic method for synthesizing gold nanoparticles in a completely green route. By simulating the environment in living cells, a quasi-biological system was created for the preparation of water-soluble AuNPs with uniform and tunable sizes under mild conditions. Through adjusting the constituents in the system, Au(I) ions coordinated with GS− and Cl− ions, and formed a special kind of Au(I) complex as the intermediate of the reaction. The simultaneous coordination of Au(I) with GS− and Cl− provided an appropriate redox potential of Au(I), making the biomimetic reduction possible under mild conditions. NADPH molecules firstly interacted with Au(I) ions via an electrostatic force and then reduced them to AuNPs. The size of AuNPs could be easily controlled by adjusting the concentration of NADPH. Due to the pH-dependence of the reducibility of NADPH coupled with GR, the rate of the reduction could be controlled by adjusting the pH values in a certain pH range. The addition of GR evidently accelerated the reduction, confirming the regulating role of GR in such a biomimetic process. The resulting AuNPs capped with glutathione via a strong Au–S bond, characterized by XPS and FTIR, showed good stability in aqueous solution even with high salt concentration. This biomimetic strategy of creating a quasi-biological system for the preparation of nanomaterials under mild conditions may provide an environmentally-friendly way for sustainable chemistry.Experimental
Chemicals
Ultrapure water was used as solvent in all experiments. Chloroauric acid (HAuCl4·4H2O), sodium hydroxide (NaOH), hydrochloric acid (HCl) were of analytical grade and were not purified before use. Reduced glutathione (GSH) and oxidized glutathione (GSSG) were purchased from Amresco. NADPH (reduced β-nicotinamide adenine dinucleotide 2′-phosphate tetrasodium salt) was purchased from Biomol. Yeast glutathione reductase (GR) was purchased from Calbiochem with specific activity of ≥100 units/mg protein. Amicon Centrifugal Filter Units were purchased from Millipore.Synthesis of gold nanoparticles
1 g of chloroauric acid (HAuCl4·4H2O) was dissolved in 100 mL of ultrapure water to get 1% aqueous HAuCl4 solution. 7.5 mg of GSH (24.4 μmol) was dissolved in 0.25 mL of H2O and then mixed with 1 mL of 1% HAuCl4 solution (24.4 μmol) and 9 mL of H2O under vigorous stirring. The pH of the solution was adjusted to 5.0 by 10 mol L−1 NaOH solution. For preparing gold nanoparticles with an average diameter of 12.6 nm, 2 mg of NADPH and about 2 units of GR were mixed in 0.1 mL of H2O and quickly added to the complex solution under gentle stirring for 16 h at room temperature. The product was purified by Amicon Centrifugal Filter Unit (MWCO 30 kDa) to remove free agents and then dispersed in ultrapure water and stored at 4 °C. AuNPs were freeze-dried to get powder for further characterizations.General procedure for the preparation of gold complexes
A typical method of preparing complex with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of GSH/HAuCl4 is presented. 22.4 mg of GSH (72.8 μmol) was dissolved in 0.75 mL of H2O and then mixed with 3 mL of 1% HAuCl4 solution (72.8 μmol) under vigorous stirring. The pH of solution was adjusted to 2.5–3.0 by 10 mol L−1 NaOH solution and precipitates quickly appeared. The product was collected by centrifugation, washed by H2O, and finally dried under vacuum. The complexes of 2
1 molar ratio of GSH/HAuCl4 is presented. 22.4 mg of GSH (72.8 μmol) was dissolved in 0.75 mL of H2O and then mixed with 3 mL of 1% HAuCl4 solution (72.8 μmol) under vigorous stirring. The pH of solution was adjusted to 2.5–3.0 by 10 mol L−1 NaOH solution and precipitates quickly appeared. The product was collected by centrifugation, washed by H2O, and finally dried under vacuum. The complexes of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 3
1 or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio were prepared in the same method except for changing the molar ratio of GSH/HAuCl4.
1 ratio were prepared in the same method except for changing the molar ratio of GSH/HAuCl4.
UV-vis spectroscopy
UV-vis spectra were recorded on a Shimadzu UV-2550 UV-vis spectrophotometer. All experimental data were corrected for ultrapure water background absorption.Characterization methods
X-Ray photoelectron spectroscopy (XPS) data were recorded on PHI Quantum 2000 using an aluminium anode (1486.6 eV) radiation source. The binding energy of XPS data was referenced to the C 1s of aliphatic carbon at 284.8 eV. Mass contents of C, N and S were obtained on an Elementar Vario EL III elemental analyzer. Inductively coupled plasma-optical emission spectroscopy (ICP-OES) data of Au content were obtained on Spectro Genesis EOP ICP spectrometer. Transmission electron microscopy (TEM) images were taken on a JEOL JEM-1230 transmission electron microscope at 100 kV. Samples for TEM were prepared by dropping 10 μL AuNP solution on a Formvar-coated copper grid and allowing the sample to dry. Infrared spectra were recorded on a Nicolet Nexus 670 Fourier transform infrared spectrometer. Thermogravimetric analysis was performed on a Perkin-Elmer Diamond DSC TG-DTA 6300 in air atmosphere from 50 °C to 700 °C.Acknowledgements
We would like to thank Shuiju Wang and Haiyan Shi at Xiamen University for XPS experiments, Professor Bing Hu for ICP-OES analysis, Xiuping Yuan for TEM experiments and Dongsheng Zhao for HRTEM experiments. This work was supported by the National Key Scientific Program (973) – Nanoscience and Nanotechnology (No. 2006CB933100), the Science Fund for Creative Research Groups of NSFC (No. 20621502 and 20921062) and the National Natural Science Foundation of China (No. 20833006).References
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940–13941 CrossRef CAS.
- R. R. Naik, S. J. Stringer, G. Agarwal, S. E. Jones and M. O. Stone, Nat. Mater., 2002, 1, 169–172 CrossRef CAS.
- M. F. Lengke, M. E. Fleet and G. Southam, Langmuir, 2007, 23, 2694–2699 CrossRef CAS.
- R. M. Kramer, C. Li, D. C. Carter, M. O. Stone and R. R. Naik, J. Am. Chem. Soc., 2004, 126, 13282–13286 CrossRef CAS.
- S. S. Shankar, A. Rai, B. Ankamwar, A. Singh, A. Ahmad and M. Sastry, Nat. Mater., 2004, 3, 482–488 CrossRef CAS.
- A. Bharde, D. Rautaray, V. Bansal, A. Ahmad, I. Sarkar, S. M. Yusuf, M. Sanyal and M. Sastry, Small, 2006, 2, 135–141 CrossRef CAS.
- S. L. Sewell and D. W. Wright, Chem. Mater., 2006, 18, 3108–3113 CrossRef CAS.
- M. T. Klem, D. Willits, D. J. Solis, A. M. Belcher, M. Young and T. Douglas, Adv. Funct. Mater., 2005, 15, 1489–1494 CrossRef CAS.
- M. B. Dickerson, K. H. Sandhage and R. R. Naik, Chem. Rev., 2008, 108, 4935–4978 CrossRef CAS.
- S.-H. Yu, H. Cölfen, J. Hartmann and M. Antonietti, Adv. Funct. Mater., 2002, 12, 541–545 CrossRef CAS.
- R. Cui, H.-H. Liu, H.-Y. Xie, Z.-L. Zhang, Y.-R. Yang, D.-W. Pang, Z.-X. Xie, B.-B. Chen, B. Hu and P. Shen, Adv. Funct. Mater., 2009, 19, 2359–2364 CrossRef CAS.
- J. B. Schulz, J. Lindenau, J. Seyfried and J. Dichgans, Eur. J. Biochem., 2001, 267, 4904–4911 CrossRef.
- Y. Xiao, V. Pavlov, S. Levine, T. Niazov, G. Markovitch and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 4519–4522 CrossRef CAS.
- D. Scott, M. Toney and M. Muzikár, J. Am. Chem. Soc., 2008, 130, 865–874 CrossRef CAS.
- A. K. Al-Sa'ady, C. A. McAuliffe, R. V. Parish and J. A. Sandbank, Inorg. Synth., 1985, 23, 191–194 CAS.
- A. C. Templeton, W. P. Wuelfing and R. W. Murray, Acc. Chem. Res., 2000, 33, 27–36 CrossRef CAS.
- S. Chen, A. C. Templeton and R. W. Murray, Langmuir, 2000, 16, 3543–3548 CrossRef CAS.
- Y.-S. Shon, C. Mazzitelli and R. W. Murray, Langmuir, 2001, 17, 7735–7741 CrossRef CAS.
- T. G. Schaaff, G. Knight, M. N. Shafigullin, R. F. Borkman and R. L. Whetten, J. Phys. Chem. B, 1998, 102, 10643–10646 CrossRef CAS.
- M. K. Corbierre and R. B. Lennox, Chem. Mater., 2005, 17, 5691–5696 CrossRef CAS.
- M. Eisenstein, Nat. Methods, 2005, 2, 6–7 CrossRef CAS.
- I. Hussain, S. Graham, Z. Wang, B. Tan, D. C. Sherrington, S. P. Rannard, A. I. Cooper and M. Brust, J. Am. Chem. Soc., 2005, 127, 16398–16399 CrossRef CAS.
- Z. Wang, B. Tan, I. Hussain, N. Schaeffer, M. F. Wyatt, M. Brust and A. I. Cooper, Langmuir, 2007, 23, 885–895 CrossRef CAS.
- S. Guo and E. Wang, Inorg. Chem., 2007, 46, 6740–6743 CrossRef CAS.
- F. Manea, C. Bindoli, S. Polizzi, L. Lay and P. Scrimin, Langmuir, 2008, 24, 4120–4124 CrossRef CAS.
- X. Ji, X. Song, J. Li, Y. Bai, W. Yang and X. Peng, J. Am. Chem. Soc., 2007, 129, 13939–13948 CrossRef CAS.
- N. Zheng, J. Fan and G. D. Stucky, J. Am. Chem. Soc., 2006, 128, 6550–6551 CrossRef CAS.
- X. Lu, H.-Y. Tuan, B. A. Korgel and Y. Xia, Chem.–Eur. J., 2008, 14, 1584–1591 CrossRef CAS.
- R. P. Briñas, M. Hu, L. Qian, E. S. Lymar and J. F. Hainfeld, J. Am. Chem. Soc., 2008, 130, 975–982 CrossRef CAS.
- N. W. Pirie and K. G. Pinhey, J. Biol. Chem., 1929, 84, 321–333 CAS.
- P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412–4456 CrossRef.
- M.-C. Bourg, A. Badia and R. B. Lennox, J. Phys. Chem. B, 2000, 104, 6562–6567 CrossRef CAS.
- J. Zhou, D. A. Beattie, R. Sedev and J. Ralston, Langmuir, 2007, 23, 9170–9177 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental methods and data. See DOI: 10.1039/c0nr00193g |
| ‡ R.C. and M.-X.Z. contributed equally to this work |
| This journal is © The Royal Society of Chemistry 2010 |
