Adsorbed Tween 80 is unique in its ability to improve the stability of gold nanoparticles in solutions of biomolecules†
Yuyun
Zhao
,
Zhuo
Wang
*,
Wei
Zhang
and
Xingyu
Jiang
*
National Center for NanoScience and Technology, No. 11, Beiyitiao Zhongguancun, Beijing 100190, China. E-mail: xingyujiang@nanoctr.cn; wangz@nanoctr.cn; Fax: +86-10-82545631; Tel: +86-10-82545558
First published on 9th August 2010
Abstract
This report shows that, of the Tween series (Tween 20, Tween 40, Tween 60 and Tween 80) of nonionic surfactants adsorbed on gold nanoparticles (NPs), Tween 80 makes the NPs most stably dispersed in aqueous solutions with or without the presence of representative biological molecules, such as nucleic acids or proteins of different sizes, isoelectric points (pIs) and shapes. In addition, the stability of gold NPs already modified with poly(L-lysine)-graft-poly(ethylene glycol) (PLL-PEG) or hexa(ethylene glycol)-terminated undecanylthiol (HS(CH2)11EG6OH) is further improved in solutions of proteins when Tween 80 is co-adsorbed on the gold NPs. This strategy is the most effective when adsorption of Tween 80 on gold NPs precedes the coating of PLL-PEG or HS(CH2)11EG6OH on the NPs.
Introduction
Gold nanoparticles (NPs) are widely used in biological assays.1 Gold NPs, particularly in the presence of biological molecules, such as nucleic acids and proteins, have a tendency to aggregate. Although aggregation of gold NPs is useful for certain events of biomolecular recognition,2 NPs must be stably dispersed in biological fluids in most applications, for example, in drug delivery3 and diagnostic assays.4 Derivatives of polyethylene glycol (PEG) can stabilize dispersion and can be biocompatible for most applications when used to protect NPs, probably because PEG strongly resists nonspecific adsorption of proteins.5 Several laboratories have synthesized gold NPs coated by molecules with PEG moieties.3,6 Most of the reagents that introduce PEG groups onto gold NPs require custom synthesis or are difficult to obtain in biological laboratories. We wish to evaluate how commercially available surfactants that contain PEG groups, such as Tween 20, Tween 40, Tween 60 and Tween 80, could prevent aggregation of gold NPs when they modify the surfaces of these NPs, particularly in the presence of nucleic acids or proteins.7 We are also interested in how these surfactants could improve the dispersion of gold NPs already protected by molecules with PEG moieties in protein solutions. These surfactants are commonly used for biochemical analysis, but no one has, to our knowledge, systematically evaluated their abilities to keep gold NPs well dispersed in biological solutions. Researchers have employed Tween 20 to stabilize gold NPs within a range of pH values in aqueous solutions that do not contain biomolecules. Once unadsorbed Tween 20 was discarded from the system (there might be some Tween 20 left on the surface of NPs), however, aggregation occurred noticeably, presumably induced by the removal of unadsorbed, dissolved Tween 20.8 If unadsorbed, dissolved nonionic surfactant molecules exist in the solution, they tend to nonspecifically promote the desorption of pre-adsorbed biological molecules on the solid–solution interfaces and denature or deactivate some proteins.9 We are therefore also curious if, and to what extent, adsorbed surfactants can keep gold NPs dispersed in aqueous solutions with or without nucleic acids or proteins.This report evaluates the abilities of these commonly available nonionic surfactants in stabilizing gold NPs in aqueous solutions with or without the presence of biological molecules, such as DNAs (both thiol-derivatized and unmodifided) or proteins of different sizes, isoelectric points (pIs) and shapes. We studied the stability of pure gold NPs prepared by citrate reduction and also stabilized by citrate, as well as NPs modified with poly(L-lysine)-graft-poly(ethylene glycol) (PLL-PEG) and hexa(ethylene glycol)-terminated undecanylthiol (HS(CH2)11EG6OH). We used UV-Vis spectroscopy to quantify the dispersion of gold NPs modified with different molecules for up to 24 h; for convenience, we call these measurements flocculation parameters.10 We found that, after removing the unadsorbed, dissolved surfactants in the solution via two cycles of centrifugation-resuspension, Tween 80 retained the stability of gold NPs the most, even in the presence of DNA. We also found that, adsorption of Tween 80 further increased the abilities of HS(CH2)11EG6OH and PLL-PEG to prevent the aggregation of gold NPs in solutions of proteins.
Results and discussion
Stability of gold NPs modified with nonionic surfactants in aqueous solutions
Aggregation of gold NPs is accompanied with a change of color: optical absorption spectroscopy quantifies this process.11 Mie's scattering theory shows that a metallic sphere whose radius is much smaller than the wavelength of light will absorb a certain wavelength due to resonant excitation of surface plasmons.12 The resonance frequency of spherical gold NPs dispersed in aqueous solutions occurs around 520 nm. When particles become sufficiently close to each other due to aggregation, red-shifting and broadening in absorption occurs within 550–700 nm besides the characteristic absorption of dispersed particles near 520 nm.13We use this change in the optical absorption of the solution to identify changes in the state of aggregation of gold NPs. Dispersed gold NPs with 10 nm in diameter stabilized by citrate were prepared by reduction of chloroauric acid by sodium citrate.14 After the formation of gold NPs, we incubated them with each surfactant (Tween 20, Tween 40, Tween 60 and Tween 80, at the concentration of 1.84 mg mL−1, 20–100-fold of their critical micelle concentrations) at room temperature for 30 min and monitored their abilities to reduce the aggregation of gold NPs. The stability of gold NPs in these surfactants that underwent different cycles of centrifugation-resuspension was monitored by optical spectroscopy (Fig. 1). All graphs were normalized in intensity to the absorption peak of gold NPs stabilized by citrate.8
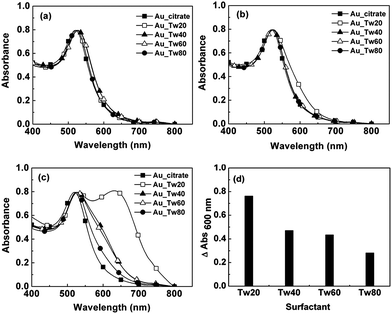 | ||
| Fig. 1 Optical absorption of Au NPs modified with each surfactant at a concentration of 1.84 mg mL−1. (a) Gold NPs with excess surfactants without any centrifugation. (b) Gold NPs mixed with the surfactants were centrifuged for 10 min at 13000 rpm and resuspended in 0.01 M PBS (pH 7.4). (c) Gold NPs mixed with the surfactants underwent two cycles of centrifugation-resuspension to remove the unadsorbed surfactants. The absorption spectrum of gold NPs only stabilized by citrate (abbr. Au_citrate) did not undergo centrifugation and is used as a control in the graphs. Gold NPs modified with Tween 20, Tween 40, Tween 60 and Tween 80 are abbreviated as Au_Tw20, Au_Tw40, Au_Tw60 and Au_Tw80, respectively. (d) Quantifies the increase in the absorbance of gold NPs at 600 nm corresponding to the data from graph (c). | ||
We first examined the stability of gold NPs with excess surfactants in aqueous solutions without centrifugation (i.e., in the presence of unadsorbed, dissolved surfactants in the solution) (Fig. 1a). The absorption peaks of gold NPs occur at 520 nm, shifting to 523, 526, 527 and 523 nm after exposure to Tween 20, Tween 40, Tween 60 and Tween 80, respectively. These shifts indicate the formation of adsorbed layers around gold NPs.8,11,13 The fact that there is no broadening in the absorption spectra of gold NPs in all four kinds of surfactants indicates that the presence of surfactants effectively prevented the aggregation of gold NPs.
We then examined the ability of adsorbed surfactants to stabilize gold NPs after removing dissolved, unadsorbed surfactants from the solution. To separate the effects of unadsorbed surfactants in the solution from adsorbed surfactants, we centrifuged gold NPs to remove most of the unadsorbed surfactants. When the mixture of gold NPs and surfactants was centrifuged to gradually remove unadsorbed surfactants in the solution, aggregation occurred, indicated by red-shifts of several absorption peaks. When centrifuged once at 13000 rpm, the absorption spectrum of gold NPs modified with Tween 20 becomes broader (Fig. 1b), indicating that aggregation occurred. The other three surfactants still make gold NPs well dispersed in solutions. When centrifuged twice, the absorption spectra of gold NPs modified with all surfactants have obvious changes (Fig. 1c). This result is consistent with a previous observation that centrifugation of NPs resulted in their aggregation.6a, 8 The degree to which the four adsorbed surfactants can prevent aggregation of gold NPs, however, is different. After two cycles of centrifugation-resuspension of gold NPs treated with Tween 20, the absorption peaks occur at 633 nm as well as at 530 nm. The absorption peaks of gold NPs adsorbed with Tween 40, Tween 60 and Tween 80 occur at 528 nm, 528 nm and 525 nm, respectively. The broader spectrum compared with dispersed gold NPs, evaluated by the increase in absorbance at 600 nm, indicates a higher degree of aggregation of gold NPs (Fig. 1d).15 The data from measurements in optical absorption spectra agree with the color changes of the bulk solution. Gold NPs treated with different surfactants had different colors after two cycles of centrifugation-resuspension: blue-purple for those adsorbed with Tween 20, purple-red for those adsorbed with Tween 40 and Tween 60, and pinkish-red (the same as the original color of dispersed gold NPs) for those adsorbed with Tween 80. We conclude that the stability of gold NPs coated with surfactants increases in the following order: Tween 20 < Tween 40 < Tween 60 < Tween 80.
It is surprising that the four surfactants with similar molecular structures give rise to such different capabilities in keeping gold NPs dispersed: they all have equal numbers of hydrophilic PEG units (ESI, Fig. S1 and Table S1†), presumably primarily responsible for preventing the aggregation of gold NPs.8 The major difference lies in the number of hydrophobic methylene groups in the acetyl side chain (ESI, Fig. S1 and Table S1†), which increases in the following order: Tween 20 < Tween 40 < Tween 60 = Tween 80. Surfactants with long alkyl chains tend to adsorb more strongly and form a thicker layer on NPs, thus presenting the PEG moieties more stably on surfaces of NPs than those with short chains.16 This observation generally agrees with reports where mercaptoalkanecarboxylic acids with long alkyl chains make gold NPs more stably dispersed than short ones.6a,8
The significantly better performance of Tween 80 compared with Tween 60, although the two have the same length of alkyl chains, we believe, might be the result of the carbon–carbon double bond in the alkyl chain of Tween 80, which does not exist in any other surfactant in the Tween series we tested. We speculate that the double bond might strengthen the adsorption of Tween 80 on gold NPs via the interaction of the π orbital of the double bond with the gold surface, implied by the change of carbon–carbon double bond stretching intensity in surface-enhanced Raman spectrum when alkenes adsorbed on gold electrodes.17
Stability of gold NPs in thiol-derivatized or unmodified oligonucleotides with the protection of nonionic surfactants
In this section, we investigate how these surfactants may stabilize gold NPs in solutions of different concentrations of DNA molecules with or without thiol-modification. We first examine the case with a low concentration of thiol-modified DNA (abbreviated as HS-DNA). When we incubated gold NPs stabilized by citrate with 10 μL of 1 μM HS-DNA (in 0.01 M PBS, pH 7.4), they aggregated, indicated by a red-shift of their absorption peak to 700 nm (Fig. 2a). When we mixed gold NPs with Tween 20, Tween 40, Tween 60 or Tween 80 before adding the solution of HS-DNA, the solution could remain pinkish-red after two cycles of centrifugation-resuspension, indicating that these surfactants can keep gold NPs modified with HS-DNA dispersed. In a high concentration of HS-DNA (30 μM), gold NPs are normally stably dispersed. Addition of nonionic surfactants does not appear to markedly affect the stability of the gold NPs for up to a few weeks (Fig. 2b). Employing a high concentration of DNA can be useful for improving the dispersity of gold NPs,18,4c but some experiments require low concentrations of DNA, where the modificiation of gold NPs with Tween might be useful. In addition, Tween is an alternative several orders of magnitude less expensive than DNA molecules. We also note that the absorption peak of Au_SDNA/Tw80 is the narrowest and conclude that Tween 80 makes gold NPs modified with HS-DNA the most dispersed (Fig. 2a and 2b).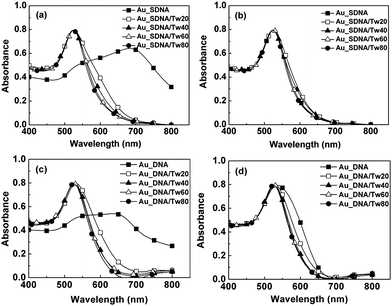 | ||
| Fig. 2 Optical absorption of Au NPs in thiol-derivatized (a, b) or unmodified (c, d) oligonucleotides. The concentrations of oligonucleotides used are 1 μM (a, c) and 30 μM (b, d) in PBS (0.01 M, pH 7.4). We incubated 600 μL of Au NPs stabilized by citrate with 10 μL of HS-DNA or DNA at 4 °C for 4 h, then removed dissolved oligonucleotides by two cycles of centrifugation at 13000 rpm for 10 min and resuspension in 0.01 M PBS (pH 7.4) and determined their optical absorption, resulting in the data of Au_SDNA and Au_DNA. The data of Au_SDNA/Tw20 (or Au_SDNA/Tw40, Au_SDNA/Tw60, Au_SDNA/Tw80) and Au_DNA/Tw20 (or Au_DNA/Tw40, Au_DNA/Tw60, Au_ DNA/Tw80) are obtained through a procedure similar to that of Au_SDNA, except that 600 μL of Tween 20 (1.84 mg mL−1, or Tween 40, Tween 60, Tween 80) was first allowed to adsorb on NPs before addition of HS-DNA or DNA. The sequences of HS-DNA and DNA are 5′ HS–C3H6 AA TTA TGG GAT CAG AGC TTA-3′ and 5′-AA TTA TGG GAT CAG AGC TTA-3′, respectively. | ||
We next examined if gold NPs can stably disperse when they are exposed to single-stranded oligonucleotides without thiol-group modification (abbreviated as DNA). Low concentrations of DNA (1 μM in 0.01 M PBS, pH 7.4) promoted the aggregation of gold NPs, similar to the addition of HS-DNA at the same concentration. When we increase the concentration of DNA to 30 μM, gold NPs aggregate somewhat during the process of centrifugation-resuspension in PBS. Addition of Tween 20, Tween 40, Tween 60 or Tween 80 can decrease the tendency of NPs to aggregate in the presence of DNA (Fig. 2c and 2d). Here we note again that adsorbed Tween 80 gives rise to the narrowest peak in the absorption spectra of gold NPs. Tween 80, therefore, consistently improves the stability of gold NPs under all conditions tested so far.
Stability of gold NPs in solutions of proteins with the protection of Tween 80
In this section, we report the abilities of adsorbed Tween 80 in preventing the aggregation of gold NPs stabilized by citrate, PLL-PEG and HS(CH2)11EG6OH in solutions of proteins. Many types of proteins tend to non-specifically adsorb on the solid–aqueous interfaces. This adsorption interferes with NP-based biological assays by inducing the aggregation of NPs in the presence of proteins in solutions.4d We chose several representative proteins with different physical properties (they are listed in Table 1) to carry out our study.We first evaluated how adsorbed Tween 80 could improve the stability of gold NPs stabilized by citrate. We incubated gold NPs stabilized by citrate with each protein at a concentration of 20 μg mL−1 in PBS (0.01 M, pH 7.4) at 4 °C for time-dependent measurements. We noticed that the solution of gold NPs turned from red to blue upon addition of the solution of RNase A or lysozyme and it became colorless 24 h later, accompanied by blackish blue precipitates on the bottom of the cuvettes. It kept its original red color in the solution of BSA even if incubated for over 24 h. We measured the optical absorption of gold NPs after incubation for different periods of time up to 24 h in solutions of proteins by UV-Vis spectroscopy (Fig. 3a). Generally, proteins with pI values higher than 7.4 induce more flocculation than those with pI values lower than 7.4; RNase A (pI = 9.5) induces the most flocculation amongst all the proteins tested. When gold NPs were coated with Tween 80 (unadsorbed Tween 80 was removed by centrifugation twice), most of their flocculation in solutions of proteins decreased significantly compared to the case without the presence of Tween 80 (Fig. 3b). Adsorbed Tween 80, therefore, has the ability to keep gold NPs more dispersed in solutions of proteins than adsorbed citrate alone.
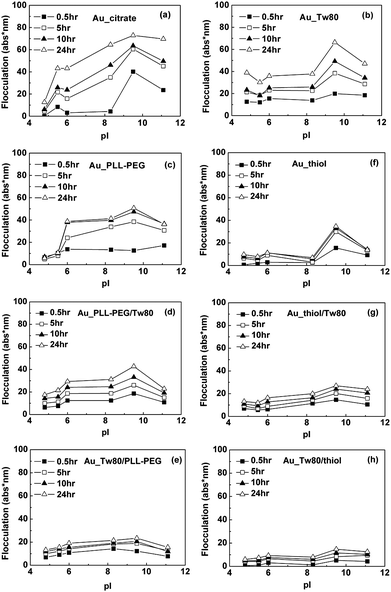 | ||
| Fig. 3 Flocculation of gold NPs stabilized by (a) citrate, (b) Tween 80, (c) PLL-PEG (1.0 mg mL−1 in PBS 0.01 M, pH 7.4), (d) co-adsorbed PLL-PEG with Tween 80, (e) first adsorbed Tween 80 then PLL-PEG, (f) HS(CH2)11EG6OH (in ethanol 0.12 mM, thiol), (g) co-adsorbed HS(CH2)11EG6OH with Tween 80, (h) first adsorbed Tween 80 then HS(CH2)11EG6OH in solutions of proteins (20 μg mL−1, in PBS 0.01 M, pH 7.4) with different pI values (Table 1) as functions of incubation time. The connecting lines are used as visual guides. Unadsorbed Tween 80 and other molecules were removed by two cycles of centrifugation at 13000 rpm for 10 min and resuspension in deionized water before incubation of gold NPs with proteins. | ||
We also wish to evaluate how effective PLL-PEG or HS(CH2)11EG6OH is in preventing the aggregation of gold NPs, since flat, bulk surfaces adsorbed with these molecules can effectively resist protein adsorption.5a, 21 Our data show that, however, these molecules cannot completely protect gold NPs from aggregation in solutions of proteins. We incubated gold NPs stabilized by citrate with PLL-PEG or HS(CH2)11EG6OH for 2 h at room temperature and separated unadsorbed molecules by two cycles of centrifugation and resuspension in deionized water. The thus-obtained gold NPs are coated with PLL-PEG or HS(CH2)11EG6OH (abbreviated as Au_PLL-PEG and Au_thiol, respectively), both of which underwent obvious aggregation after incubation with solutions of proteins (Fig. 3c and 3f). Adding Tween 80 into the mixture of gold NPs and PLL-PEG or HS(CH2)11EG6OH (incubated for 30 min, centrifuged and resuspended in deionized water for two cycles), we obtained gold NPs coadsorbed with Tween 80 and PLL-PEG or HS(CH2)11EG6OH (abbreviated as Au_PLL-PEG/Tw80 or Au_thiol/Tw80). These two kinds of NPs are more stably dispersed in most types of protein solutions than those uncoated with Tween 80 (Fig. 3d and 3g).
To further evaluate how effective Tween 80 is in keeping gold NPs dispersed in solutions of proteins, we incubated Tween 80 with gold NPs for 30 min before adding PLL-PEG or HS(CH2)11EG6OH into the mixture and removed unadsorbed molecules by two cycles of centrifugation-resuspension as well. To distinguish these NPs from those we used before, we abbreviate them as Au_Tw80/PLL-PEG or Au_Tw80/thiol. This procedure decreases the flocculation of modified gold NPs in solutions of all proteins we tested dramatically (Fig. 3e and 3h). To sum up, the presence of adsorbed Tween 80 can greatly improve the dispersity of gold NPs in solutions of proteins when the derivatives of PEG are used to keep gold NPs dispersed. Moreover, initial adsorption of Tween 80 on gold NPs followed by EG-thiol makes NPs most stably dispersed in solutions of proteins.
Experimental section
Materials
HAuCl4·3H2O (99.99%) was from Shangjuly Chemical Co., Ltd., China. Trisodium citrate (Na3-citrate), tannic acid, K2CO3, KCl, NaOH, Na2HPO4, KH2PO4, NaCl, boric acid (H3BO3) and absolute ethanol were from Beijing Chemical Reagents Co., China. Tween 20, Tween 40, Tween 60, Tween 80, N-hydroxycuccinimidyl ester of methoxy poly(ethylene glycol) propionic acid (Mw 5 kDa, SPA-PEG) and poly L-lysine hydrobromide (Mw 15–30 kDa, PLL·HBr) were from Sigma. Hexa(ethylene glycol)-terminated undecanylthiol (HS(CH2)11EG6OH) was from Prochimia (http://www.prochimia.com). Thiol-derivatized single-stranded oligonucleotides (HS-DNA) and unmodified single-stranded oligonucleotides (DNA) were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd (http://www.sangon.com), whose sequences are 5′ HS–C3H6 AA TTA TGG GAT CAG AGC TTA -3′ and 5′- AA TTA TGG GAT CAG AGC TTA -3′, respectively. BSA (bovine serum albumin), fibrinogen (human), γ-globulins (bovine), IgG (bovine immunoglobulin G), RNase A (bovine) and lysozyme (chicken egg white) were all from Sigma. Deionized water (electric resistivity > 18 MΩ·cm) was supplied by Milli-Q.Buffers and solutions
Phosphate-buffered saline (0.01M, pH 7.4, PBS) was prepared by addition of 8 g NaCl, 0.2 g KCl, 2.9 g Na2HPO4 and 0.2 g KH2PO4 into 1 L deionized water. Sodium borate buffer (0.05 M, pH 8.5, SBB) was prepared as the following step: we dissolved 3.09 g of H3BO3 into 500 mL of 0.1 M KCl, then added 210 mL of 0.1 M NaOH, then adjusted the total volume with water to 1 L. The solution of 1.84 mg mL−1 Tween 20 was prepared in water. Solutions of Tween 40, Tween 60 and Tween 80 were prepared at the same concentration of 1.84 mg mL−1 as Tween 20. A 0.12 mM solution of HS(CH2)11EG6OH was prepared in absolute ethanol. Both of HS-DNA and DNA were diluted to two concentrations of 1 μM and 30 μM in PBS. The concentrations of all proteins were 20 μg mL−1 in PBS. All solutions passed through 0.22 μm filters (Millipore GS).Preparation and modification of gold NPs
All glassware used in the preparation was treated with aqua regia, then washed with water copiously and dried at 110 °C. Gold NPs with mean sizes of 10 nm were prepared according to the reported method.14 Solution A comprising 1 mL of 1% (m/v) HAuCl4·3H2O and 79 mL of water and solution B comprising 4 mL of 1% (m/v) Na3-citrate, 0.1 mL of 1% (m/v) tannic acid, 0.1 mL of 25 mmol L−1 K2CO3 and 15.8 mL of water were separately heated to 60 °C, then solution B was quickly added into solution A. We kept the mixture at 60 °C until its color became deep red, when it was heated until boiling. The boiling solution became brilliantly orange-red within 5 min. After cooling to room temperature, the volume of the solution was adjusted to 100 mL with water and stored at 4 °C for use. We called them gold NPs stabilized by citrate in this paper.Equal volumes of gold NPs and an aqueous solution of 1.84 mg mL−1 Tween 20 were gently mixed and incubated for 30 min at room temperature. Excess surfactants were removed by centrifugation at 13000 rpm for 10 min (Eppendorf centrifuge 5417c, Germany) and resuspension in 0.01 M PBS (pH 7.4) for two cycles. Adsorption of gold NPs with Tween 40, Tween 60 or Tween 80 followed similar procedures to that for Tween 20.
We incubated 600 μL of Au NPs stabilized by citrate with 10 μL of HS-DNA (at a concentration of 1 μM or 30 μM in 0.01M PBS, pH 7.4) at 4 °C for 4 h then removed dissolved HS-DNA by two cycles of centrifugation at 13000 rpm for 10 min and resuspension in PBS (0.01M, pH 7.4). We abbreviate these products as Au_SDNA. The product of Au_SDNA/Tw20 (or Au_SDNA/Tw40, Au_SDNA/Tw60, Au_SDNA/Tw80) was obtained through a similar procedure to that of Au_SDNA except that 600 μL of Tween 20 (1.84 mg mL−1, or Tween 40, Tween 60, Tween 80) was added into the mixture of Au NPs and HS-DNA before separation of them. The products of Au_DNA and others with nonionic surfactants were obtained by the procedure similar to that of Au_SDNA.
The synthesis of PLL-PEG followed a procedure reported in the literature.21 We added 216 mg of SPA-PEG into the solution of 84 mg of PLL·HBr in 1.05 mL of 0.05 M SBB and incubated the mixture for 6 h at room temperature, then dialyzed it (molecule weight cutoff size 14 kDa) for 24 h, first with PBS (0.01M, pH 7.4), then with deionized water. The product was lyophilized and stored at −20 °C for use. We incubated equal volumes of gold NPs and the solution of 1 mg mL−1 PLL-PEG in PBS (0.01 M, pH 7.4) for 2 h at room temperature and separated them by two cycles of centrifugation at 13000 rpm for 10 min and resuspension in PBS. We obtained gold NPs modified with PLL-PEG and Tween 80 by addition of Tween 80 into the mixture of NPs and PLL-PEG and separation of them. Alternatively, we first adsorbed Tween 80 on gold NPs and then incubate them with PLL-PEG.
We obtained gold NPs modified with HS(CH2)11EG6OH by incubation of equal volumes of gold NPs and 0.12 mM ethanol solution of HS(CH2)11EG6OH for 2 h at room temperature and separated them by two cycles of centrifugation at 13000 rpm for 10 min and resuspension in deionized water. We obtained gold NPs coadsorbed with HS(CH2)11EG6OH and Tween 80 with the same procedure as gold NPs adsorbed with PLL-PEG and Tween 80.
Determination of the flocculation of gold NPs
Optical absorption data were acquired at room temperature with a Varian Cary 100 UV-Vis spectrophotometer using 1 cm path length and 100 μL capacity black-body quartz cuvettes. We determined the flocculation of gold NPs coated with each surfactant through different cycles of centrifugation-resuspension in aqueous solutions. We incubated 200 μL of gold NPs with 200 μL of the 20 μg mL−1 solution of each protein for 0.5 h, 5 h, 10 h and 24 h at 4 °C for determination by UV-Vis.Conclusions
Tween 80 can help solve two problems: the aggregation of gold NPs during the process of centrifugation and the inability of gold NPs to remain dispersed in biological media. It makes gold NPs stably dispersed in aqueous solutions or solutions containing nucleic acids, even if those NPs underwent multiple centrifugations, better than Tween 20, Tween 40 and Tween 60. Adsorbed Tween 80 alone, therefore, can be used as a stabilizing agent for gold NPs undergoing surface modification through multiple rounds of centrifugation-resuspension. It also dramatically improves the stability of gold NPs already stabilized by citrate, PLL-PEG or HS(CH2)11EG6OH in solutions of proteins of different sizes, isoelectric points and shapes. Because Tween 80 can consistently decrease the tendency of gold NPs to aggregate in solutions containing biological molecules when it is used to coat gold NPs, we believe that this commonly found benchtop reagent can find wider uses for bioanalysis.Acknowledgements
We thank Ms. Yan Cheng for initial experiments. Funding for this work was provided by Chinese Academy of Sciences (KJCX2-YW-M15), the National Science Foundation of China (90813032, 20890020) and the Ministry of Science and Technology (2006CB705600, 2009CB930001, 2008ZX1001-1010).References
- (a) N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547 CrossRef CAS; (b) C. Kim, P. Ghosh and V. M. Rotello, Nanoscale, 2009, 1, 61 RSC.
- (a) R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078 CrossRef CAS; (b) K. N. T. Thanh and Z. Rosenzweig, Anal. Chem., 2002, 74, 1624 CrossRef CAS.
- (a) G. F. Paciotti, L. Myer, D. Weinreich, D. Goia, N. Pavel, R. E. McLaughlin and L. Tamarkin, Drug Delivery, 2004, 11, 169 CrossRef CAS; (b) E. C. Dreaden, S. C. Mwakwari, Q. H. Sodji, A. K. Oyelere and M. A. El-Sayed, Bioconjugate Chem., 2009, 20, 2247 CrossRef CAS; (c) J. M. Tam, J. O. Tam, A. Murthy, D. R. Ingram, L. L. Ma, K. Travis, K. P. Johnston and K. V. Sokolov, ACS Nano, 2010, 4, 2178 CrossRef CAS; (d) D. A. Giljohann, D. S. Seferos, A. E. Prigodich, P. C. Patel and C. A. Mirkin, J. Am. Chem. Soc., 2009, 131, 2072 CrossRef CAS.
- (a) L. R. Hirsch, J. B. Jackson, A. Lee, N. J. Halas and J. L. West, Anal. Chem., 2003, 75, 2377 CrossRef CAS; (b) M. R. Ivanov, H. R. Bednar and A. J. Haes, ACS Nano, 2009, 3, 386 CrossRef CAS; (c) W. Zhao, L. Lin and I.-M. Hsing, Bioconjugate Chem., 2009, 20, 1218 CrossRef CAS; (d) H. Jans, X. Liu, L. Austin, G. Maes and Q. Huo, Anal. Chem., 2009, 81, 9425 CrossRef CAS.
- (a) L. M. Feller, S. Cerritelli, M. Textor, J. A. Hubbell and S. G. P. Tosatti, Macromolecules, 2005, 38(25), 10503 CrossRef CAS; (b) S. Saxer, C. Portmann, S. Tosatti, K. Gademann, S. Zürcher and M. Textor, Macromolecules, 2010, 43, 1050 CrossRef CAS; (c) B. Malisova, S. Tosatti, M. Textor, K. Gademann and S. Zürcher, Langmuir, 2010, 26(6), 4018 CrossRef CAS; (d) Y. Li, B. Yuan, H. Ji, D. Han, S. Q. Chen, F. Tian and X. Jiang, Angew. Chem., Int. Ed., 2007, 46(7), 1094 CrossRef CAS; (e) D. Liu, Y. Xie, H. Shao and X. Jiang, Angew. Chem., Int. Ed., 2009, 48(24), 4406 CrossRef CAS; (f) Z. Chen, Y. Li, W. Liu, D. Zhang, Y. Zhao, B. Yuan and X. Jiang, Angew. Chem., Int. Ed., 2009, 48(44), 8303 CrossRef CAS.
- (a) C. S. Weisbecker, M. V. Merritt and G. M. Whitesides, Langmuir, 1996, 12, 3763 CrossRef CAS; (b) W. P. Wuelfing, S. M. Gross, D. T. Miles and R. W. Murray, J. Am. Chem. Soc., 1998, 120, 12696 CrossRef CAS; (c) H. Otsuka, Y. Akiyama, Y. Nagasaki and K. Kataoka, J. Am. Chem. Soc., 2001, 123(34), 8226 CrossRef CAS; (d) M. Zheng, F. Davidson and X. Huang, J. Am. Chem. Soc., 2003, 125, 7790 CrossRef; (e) T. Sakai and P. Alexandridis, Langmuir, 2004, 20(20), 8426 CrossRef CAS; (f) T. Sakai and P. Alexandridis, Langmuir, 2005, 21(17), 8019 CrossRef CAS; (g) M. Zheng, Z. Li and X. Huang, Langmuir, 2004, 20, 4226 CrossRef CAS; (h) Y. Zhou, S. Wang, K. Zhang and X. Jiang, Angew. Chem., Int. Ed., 2008, 47(39), 7454 CrossRef CAS.
- The chemical names of Tween 20, Tween 40, Tween 60 and Tween 80 are polyoxyethylene(20) sorbitan monolaurate, polyoxyethylene(20) sorbitan monopalmitate, polyoxyethylene(20) sorbitan monostearate, polyoxyethylene(20) sorbitan monooleate, respectively..
- K. Aslan and V. H. Pérez-Luna, Langmuir, 2002, 18, 6059 CrossRef CAS.
- E. D. Goddard and K. P. Ananthapadmanabhan, Interactions of surfactants with polymers and proteins, CRC press Inc, New York, 1993, 295–360 Search PubMed; G. Guillon, C. Roy and S. Jard, Eur. J. Biochem., 1978, 92, 341 Search PubMed.
- Flocculation parameters were defined as the integral of the absorbance between 600 and 800 nm with the optical absorption spectra normalized to the optical absorption peak of gold NPs stabilized by citrate, see ref. 8 and 21..
- (a) P. Mulvaney, Langmuir, 1996, 12, 788 CrossRef CAS; (b) W. H. Ni, H. J. Chen, J. Su, Z. H. Sun, J. F. Wang and H. K. Wu, J. Am. Chem. Soc., 2010, 132, 4806 CrossRef CAS.
- G. Mie, Ann. Phys., 1908, 25, 377 CAS.
- (a) R. H. Magruder III, L. Yang, R. F. HaglundJr, C. W. White, L. Yang, R. Dorsinville and R. R. Alfano, Appl. Phys. Lett., 1993, 62, 1730 CrossRef; (b) P. K. Jain, W. Qian and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 136 CrossRef CAS.
- J. W. Slot and H. J. Geuze, Eur. J. Cell biol., 1985, 38, 87 CAS.
- K. Aslan, C. C. Luhrs and V. H. Pérez-Luna, J. Phys. Chem. B, 2004, 108, 15631 CrossRef CAS.
- T. Sato and R. Ruch, Stabilization of Colloidal Dispersions by Polymer Adsorption, Marcel Dekker, New York, 1980 Search PubMed.
- M. L. Patterson and M. J. Weaver, J. Phys. Chem., 1985, 89, 5046 CrossRef CAS.
- (a) M. Cárdenas, J. Barauskas, K. Schillén, J. L. Brennan, M. Brust and T. Nylander, Langmuir, 2006, 22, 3294 CrossRef CAS; (b) H. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036 CrossRef CAS; (c) K. Akamatsu, M. Kimura, Y. Shibata, S. Nakano, D. Miyoshi, H. Nawafune and N. Sugimoto, Nano Lett., 2006, 6, 491 CrossRef CAS.
- G. B. Sigal, M. Mrksich and G. M. Whitesides, J. Am. Chem. Soc., 1998, 120, 3464 CrossRef CAS.
- A. G. Amit, R. A. Mariuzza, S. E. Phillips and R. J. Poljak, Science, 1986, 233, 747 CAS.
- (a) P. Harder, M. Grunze, R. Dahint, G. M. Whitesides and P. E. Laibinis, J. Phys. Chem. B, 1998, 102(2), 426 CrossRef; (b) S. Herrwerth, W. Eck, S. Reinhardt and M. Grunze, J. Am. Chem. Soc., 2003, 125, 9359 CrossRef CAS; (c) G. L. Kenausis, J. Voros, D. L. Elbert, N. P. Huang, R. Hofer, L. Ruiz, M. Textor, J. A. Hubbell and N. D. Spencer, J. Phys. Chem. B, 2000, 104, 3298 CrossRef CAS; (d) N.-P. Huang, R. Michel, J. Voros, M. Textor, R. Hofer, A. Rossi, D. L. Elbert, J. A. Hubbell and N. D. Spencer, Langmuir, 2001, 17(2), 489 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical structures and physicochemical properties of nonionic surfactants of Tween series, transmission electron microscopy of gold NPs stabilized by citrate and size distribution. See DOI: 10.1039/c0nr00309c |
| This journal is © The Royal Society of Chemistry 2010 |
