Surface diffusion driven nanoshell formation by controlled sintering of mesoporous nanoparticle aggregates†
E. A.
Anumol
a,
B.
Viswanath
a,
P. G.
Ganesan
b,
Yunfeng
Shi
c,
Ganpati
Ramanath
c and
N.
Ravishankar
*a
aMaterials Research Centre, Indian Institute of Science, Bangalore, 560012, India. E-mail: nravi@mrc.iisc.ernet.in; Fax: +91-80-22922566; Tel: +91-80-22922566
bCenter for Future Energy Systems, Rensselaer Polytechnic Institute, Troy, NY 12180, USA
cMaterials Science and Engineering, Rensselaer Polytechnic Institute, Troy, NY 12180, USA
First published on 2nd June 2010
Abstract
We report a general method for the synthesis of hollow structures of a variety of functional inorganics by partial sintering of mesoporous nanocrystal aggregates. The formation of a thin shell initiates the transport of mass from the interior leading to growth of the shell. The principles are general and the hollow structures thus produced are attractive for many applications including catalysis, drug delivery and biosensing.
Hollow nanostructures with accessible interior volumes exhibit unique mechanical, optical and thermal properties, and find diverse applications such as solar cells and batteries, drug delivery agents, catalysts and bio-encapsulants.1 Many methods based on organic or inorganic templates, e.g., polymers, carbon, silica, or fluidic templates e.g., liquid droplets in a microemulsion or gas bubbles and template-less methods have been used to produce hollow structures of a variety of functional materials. The nanoscale Kirkendall effect has been exploited to produce hollow structures without using templates, but is restricted only to multi-component systems where diffusion rates of the components are significantly different.2–13 Hollow structure formation in TiO2via Ostwald ripening in solution phase has been reported where the presence of chemical species to cause ionic transport in the solution phase is a necessary condition.14 Here, we demonstrate a completely new method for creating large quantities of hollow structures by controlled solid-state sintering of spherical mesoporous aggregates. Since the mechanism of hollow formation relies only on surface diffusion, it is general and applicable for a variety of materials including metals and ceramics.
Sintering of a porous compact is known15,16 to be driven by surface free energy reduction, which results in mass transport from higher to lower chemical potential regions as described by the Gibbs–Thomson relationship. Typically, densification occurs by net mass flow towards the interior, or a net outward vacancy flux. Although, sintering of micron size metal and ceramic powders has been a topic of extensive research, hollow structures such as those reported here have never been reported. In case of sintering of a spherical mesoporous structure comprising nanoscale ligaments, surface diffusion dominates mass transport during the early stages of sintering. Here we show, for the first time, that sintering such a mesoporous compact leads to the formation of a hollow structure (schematic, Fig. 1a) comprised of either a single pore, or multiple isolated pores, before forming a solid particle. The hollow structure is initiated by the formation of an outer shell of sintered material that ensures that nanoscale convex particles with high chemical potential are in contact with a large concave region with a low chemical potential and thus the rate of mass transport from the interior to the shell far exceeds the rate of particle shrinking due to sintering (flux J1 ≫ J2 in Fig. 1b). The pore microstructure is determined by the relative rates of surface and grain boundary diffusivities and the sintering time; microstructures ranging from polycrystalline nanoparticles with multiple embedded pores to hollow single crystalline particles with a single hollow have been obtained. We demonstrate that the method can be exploited to produce spherical nanoporous structures of Pt, CeO2, SnO2 and TiO2 by controlled thermal treatment of pre-synthesized dried porous aggregates.14,17–19
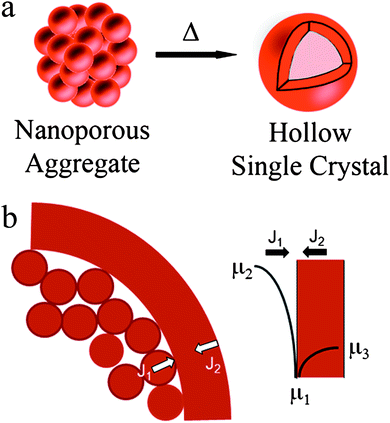 | ||
| Fig. 1 (a) Schematic illustration of the process of formation of hollow structure from nearly spherical mesoporous aggregates. (b) Schematic illustrating that rate of mass transport from the interior to the shell is much higher than rate of shrinkage. | ||
Fig. 2a–d illustrate a typical microstructure evolution sequence leading to hollow nanosphere formation from mesoporous aggregate of nanoparticles of CeO2. As-synthesized porous aggregates of CeO2 nanoparticles exhibit an equiaxed morphology. Sintering at 700 °C results in the formation of faceted holes in each cluster (Fig. 2b). At 900 °C the clusters transform to single crystalline hollow nanospheres (see Fig. 2c), confirmed by the high resolution TEM (see Fig. 2d). Energy dispersive X-ray (EDX) spectroscopy line scans (ESI†) across a trajectory indicated by dotted line (Fig. S2†, for instance) show a sharp decrease in the Ce and O intensities at the centre confirming the presence of a void in the center of the particles, consistent with the Fresnel contrast seen in Fig. 2c. The line widths of X-ray Bragg peaks corresponding to the fluorite structure of CeO2 decrease with increasing sintering temperature and time, indicative of particle coalescence and grain growth, which leads to formation of single crystal spherical nanoshells. However, the cluster size remains essentially unchanged at ∼60 nm upon sintering (ESI†), indicating that the CeO2 nanoparticles in the clusters coarsen while retaining the memory of the initial cluster diameter. Sintering at higher temperature and/or longer times leads to the formation of fully sintered particles with diameter that are 20–30% smaller than the starting cluster size due to pore annihilation. These results clearly indicate that initially the mass transport rate from the interior to the shell is much faster than the sintering rate that leads to particle shrinkage. Upon continued exposure to high temperatures and times, the shells are compacted into dense particles through sintering. The specific surface area obtained from BET method shows a drastic decrease with increase in annealing temperature as shown in Fig. S6 (ESI†) as a result of closure of the pores and increase in particle size at high temperatures.
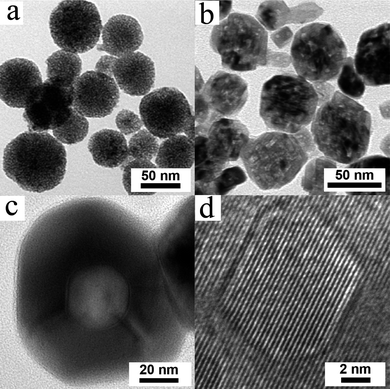 | ||
| Fig. 2 Stages of formation of hollow single crystals of CeO2 at different sintering temperatures (a) mesoporous structure at 250 °C, (b) faceted structure with multiple faceted pores at 700 °C, (c) hollow single crystal at 900 °C. (d) High resolution image from a hollow particle showing the single crystal nature. | ||
Similar experiments starting with nanoporous aggregates of Pt, SnO2 and TiO2 also lead to the formation of hollow structures of the respective material with essentially identical microstructure evolution sequence as in the case of CeO2 described above (see Fig. 3 and ESI†). In all the cases investigated, the hollow nanoshells are polycrystalline with a grain size much larger than the starting nanoparticle size, and the spherical nanoshell diameter is similar to that of the porous clusters (ESI†).
 | ||
| Fig. 3 Nanoporous clusters of (a) Pt, (b) SnO2 and (c) TiO2 and the resulting hollow structures of (d) Pt, (e) SnO2 and (f) TiO2 formed by annealing. In all these cases, the resulting hollow structure is polycrystalline. | ||
We carried out molecular dynamics (MD) simulations on a model 2-D Lennard Jones system (see ESI†) to understand mechanistic features of spherical nanoshell formation from nanoparticle aggregates. In particular, we studied the sintering behavior of a porous aggregate of nanoparticles with a sintered outer shell to determine if this leads to the formation of a hollow nanoshell.
It is reasonable to assume that sintering of nanoparticles commences on the periphery of the porous aggregate due to the higher degree of freedom for rearrangement by diffusive processes. Annealing leads to the formation of cluster with multiple pores pinned to grain boundaries (see Fig. 4). Our simulations show that the initially large number of random particle orientations decrease upon annealing due to grain growth as well as pore consolidation, with negligible change to the overall cluster size. This result indicates that mass transport from the interior to the shell is faster than the rate of shrinkage of the particles, as inferred from our experimental results. The final nanoshell microstructure shown in Fig. 4 (simulation time t4) corresponds to a cluster with multiple pores pinned to the grain boundaries in the cluster. Whilst the lack of connectivity of pores in the 2-D model system of the simulations imposes a topological limitation to preclude the capturing of the formation of single hollow, the key feature of enhanced mass transport from the interior to the shell is evident.
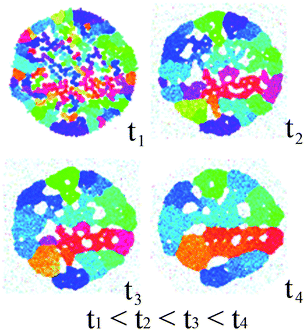 | ||
| Fig. 4 Annealing simulation of nanoparticle aggregates with a preformed outer shell using molecular dynamics. The radius of the outer shell is about 60 nm. The grains are colored according to their crystalline orientation so that the difference in hue value is proportional to the degree of misalignment. The simulation duration is about 0.1 µs. | ||
In summary, we have demonstrated a new, versatile method for forming spherically shaped hollow nanoshells from nanoporous aggregates of nanoparticles for metallic and oxide systems. These could be applicable for many potential applications ranging from catalysis to drug delivery.
Notes and references
- E. B. David, Angew. Chem., Int. Ed., 1999, 38, 2870–2872 CrossRef CAS.
- Y. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711–714 CrossRef CAS.
- K. N. Tu and U. Gosele, Appl. Phys. Lett., 2005, 86, 093111–093113 CrossRef.
- Q. Zhang, W. Wang, J. Goebl and Y. Yin, Nano Today, 2009, 4, 494–507 CrossRef CAS.
- H. C. Zeng, J. Mater. Chem., 2006, 16, 649–662 RSC.
- F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111–1114 CrossRef CAS.
- Y. Zhang, E.-W. Shi and Z.-Z. Chen, J. Cryst. Growth, 2008, 310, 2928–2933 CrossRef CAS.
- H. Zeng, W. Cai, P. Liu, X. Xu, H. Zhou, C. Klingshirn and H. Kalt, ACS Nano, 2008, 2, 1661–1670 CrossRef CAS.
- H.-P. Liang, L.-J. Wan, C.-L. Bai and L. Jiang, J. Phys. Chem. B, 2005, 109, 7795–7800 CrossRef CAS.
- S. Han, B. Jang, T. Kim, S. M. Oh and T. Hyeon, Adv. Funct. Mater., 2005, 15, 1845–1850 CrossRef CAS.
- M.-M. Titirici, M. Antonietti and A. Thomas, Chem. Mater., 2006, 18, 3808–3812 CrossRef CAS.
- J.-H. J. Chia-Jung Chung, J. Am. Ceram. Soc., 2008, 91, 3074–3077 CrossRef.
- J. Li and H. C. Zeng, J. Am. Chem. Soc., 2007, 129, 15839–15847 CrossRef CAS.
- H. G. Yang and H. C. Zeng, J. Phys. Chem. B, 2004, 108, 3492–3495 CrossRef CAS.
- J. W. Christian, The Theory of Transformations in Metals and Alloys, Pergamon, 2002 Search PubMed.
- D. A. Porter and K. Easterling, Phase Transformations in Metals and Alloys, Chapman and Hall, 1992 Search PubMed.
- B. Viswanath, S. Patra, N. Munichandraiah and N. Ravishankar, Langmuir, 2009, 25, 3115–3121 CrossRef CAS.
- X. Liang, X. Wang, Y. Zhuang, B. Xu, S. Kuang and Y. Li, J. Am. Chem. Soc., 2008, 130, 2736–2737 CrossRef CAS.
- S. Y. Ho, A. S. W. Wong and G. W. Ho, Cryst. Growth Des., 2009, 9, 732–736 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, MD simulation details, XRD, size distribution. See DOI: 10.1039/c0nr00228c |
| This journal is © The Royal Society of Chemistry 2010 |
