Antimicrobial biomaterials based on carbon nanotubes dispersed in poly(lactic-co-glycolic acid)†
Seyma
Aslan
,
Codruta Zoican
Loebick
,
Seoktae
Kang
,
Menachem
Elimelech
,
Lisa D.
Pfefferle
and
Paul R.
Van Tassel
*
Department of Chemical and Environmental Engineering, Yale University, New Haven, CT, USA. E-mail: Paul.VanTassel@yale.edu
First published on 2nd August 2010
Abstract
Biomaterials that inactivate microbes are needed to eliminate medical device infections. We investigate here the antimicrobial nature of single-walled carbon nanotubes (SWNTs) incorporated within the biomedical polymer poly(lactic-co-glycolic acid) (PLGA). We find Escherichia coli and Staphylococcus epidermidis viability and metabolic activity to be significantly diminished in the presence of SWNT–PLGA, and to correlate with SWNT length and concentration (<2% by weight). Up to 98% of bacteria die within one hour on SWNT–PLGA versus 15–20% on pure PLGA. Shorter SWNTs are more toxic, possibly due to increased density of open tube ends. This study demonstrates the potential usefulness of SWNT–PLGA as an antimicrobial biomaterial.
1. Introduction
Infectious disease has been the leading cause of mortality throughout human history and, despite the arrival of modern antibiotics, remains a significant public health challenge. Paradoxically, many infections are acquired within health care facilities: of the roughly 2 million nosocomial infections occurring annually within the US, nearly half are attributed to implantable devices.1 The annual death toll and cost of healthcare-associated infections are 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–90
000–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths and $17–29 billion dollars, respectively.1
000 deaths and $17–29 billion dollars, respectively.1
To address this issue, antimicrobial materials have been proposed to limit the growth of malicious microorganisms on surfaces,2 and owing to their large potential impact on public health, have been extensively studied in recent years.3–10 Early efforts focused on suppressing the initial adhesion of pathogens to the material surface. Unfortunately, even on the best performing materials, a few “pioneer” bacteria are usually able to get a foothold, multiply, and ultimately cause an infection.11 Subsequent strategies were based on the delivery of anti-infective agents from the implant surface.12–18 The active agent may be loaded into and passively diffuse through a delivery matrix, itself perhaps degrading with time, or may be part of the matrix itself.19 While effective in certain applications, these approaches have several associated concerns, such as uncontrolled release of anti-infective agent, toxicity to human cells, risk of bacterial resistance, and eventual depletion of the anti-infective agent. A recent approach, which addresses some of these concerns, involves non-leaching anti-infective agents that are permanently attached to the material surface, and are designed to kill microbes on contact.20–26 Grafted polymers containing cationic functional groups are often employed, which exert activity through attack on the bacterial membrane. While promising, grafted polymers have their drawbacks as well, including the intricate chemistry required, incompatibility with many materials, and instability in biological environments.
Recently, single walled carbon nanotubes (SWNTs)—in the form of deposited aggregates and membrane coatings—have been shown to be highly toxic toward microbes.27 Their chemical stability and ease of functionalization make SWNT attractive components toward antimicrobial biomaterials. However, pure SWNT materials/coatings are costly and offer only a limited range of material properties; they are thus unlikely to emerge as optimal antimicrobial materials. Alternatively, SWNT combined (as a minority component) with customized polymers could potentially be antimicrobial while also providing a broad range of structural, mechanical, and degradation properties.
In this study, we investigate SWNT dispersed in the common biomedical polymer poly(lactic-co-glycolic acid) (PLGA) as a potential antimicrobial biomaterial. We synthesize and isolate SWNT of diameter 0.8 to 1.2 nm, combine with PLGA in a thin film configuration, and investigate microbial toxicity in terms of nanotube length and concentration. We consider two bacteria known to contaminate biomedical implants: the Gram-negative rod Escherichia coli and the Gram-positive coccus Staphylococcus epidermidis. Our goal is to combine the antimicrobial properties of SWNT with the highly tunable structural, mechanical, and degradation properties of PLGA.
2. Experimental
2.1. SWNT synthesis
SWNTs are synthesized via CO decomposition at 1073 K and 6 atm using small pore (C10) Co-MCM-41 catalyst. The catalyst is synthesized by the isomorphous substitution of silicon atoms with cobalt. The total metal loading is 3%. The MCM-41 is templated with a 10-carbon atom alkyl chain length surfactant yielding an average pore diameter of 2.5 nm, as determined by nitrogen physisorption measurements. Details of the experimental procedure are provided elsewhere.28The resulting samples, containing carbon nanotubes, silica, and metal particles, are purified using reflux. A NaOH solution is used to eliminate the silica, and an HCl treatment is employed to remove the metal. The residual cobalt content in the carbon nanotubes samples is under 0.7%.29 The resultant purified SWNTs are several micrometres long. SWNTs are shortened by grinding with cyclodextrin.30
2.2. SWNT characterization
SWNTs are dispersed in ethanol and dried on Holey Carbon Film, 200 mesh copper (Electron Microscope Science). Transmission electron microscopy (TEM) (Tecnai F20 Microscope, Phillips) images (Fig. 1) confirm the presence of SWNT of length ca. 300 nm and diameter from 0.8 to 1.2 nm. SWNT and PLGA are dispersed together in chloroform at 0.1% w/v and dried on Holey Carbon Film prior to TEM imaging. Raman spectroscopy results are obtained with a Laser Raman Spectrophotometer (NSR-3100, Jasco). Full characterization (multi-wavelength Raman, fluorescence spectroscopy, and transmission electron microscopy) of SWNT is provided elsewhere.31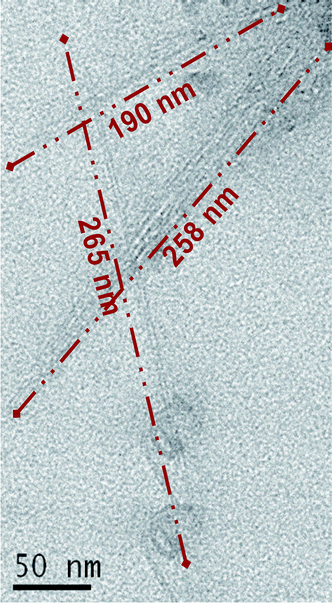 | ||
| Fig. 1 TEM image of SWNT following cyclodextrin-mediated soft cutting. The lengths of three representative tubes are shown. The average SWNT length is ca. 300 nm. | ||
2.3. Film fabrication
Microscope cover glasses serve as the substrate to the SWNT–PLGA film. Surface cleaning is carried out by exposure to UV light for 5 minutes, followed by washing with 2% Hellmanex® and deionized water.SWNTs are dispersed in chloroform in a probe sonicator for 30 minutes, at which time 0.1% w/v poly(DL-lactic-co-glycolic acid) (PLGA 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, Mw 90
15, Mw 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–126
000–126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) is added and sonication continues for another 30 minutes. SWNT is present at 1/7000 (low), 1/700 (medium), and 1/70 (high) concentrations (w SWNT/w PLGA). Short and long SWNT films are prepared identically, with the exception of the cyclodextrin grinding step. Raman spectroscopy shows the time of sonication to have no impact on SWNT length (see ESI†).
000) is added and sonication continues for another 30 minutes. SWNT is present at 1/7000 (low), 1/700 (medium), and 1/70 (high) concentrations (w SWNT/w PLGA). Short and long SWNT films are prepared identically, with the exception of the cyclodextrin grinding step. Raman spectroscopy shows the time of sonication to have no impact on SWNT length (see ESI†).
For dip coating, cleaned substrates are immersed into PLGA–SWNT solutions for 30 seconds. Substrates are removed at ca. 1 cm s−1 and allowed to dry. For spin coating, a single drop of PLGA–SWNT solution is placed on a cleaned glass substrate and spun (Specialty Coating Systems P6708D) at 100 rpm for 30 s, 200 rpm for 50 s, and 300 rpm for 40 s (with 1 s ramp time between segments).
2.4. Film characterization
Film thickness of spin-coated films is found to be 70 ± 20 nm via a PhE-101 discrete wavelength ellipsometer (Angstrom Advanced Inc.), where a 70° angle and the Cauchy Model are employed. A contact angle goniometer (VCA-2500 from AST Products, Inc.) is used to obtain (deionized) water contact angles. To study film morphology, scanning electron microscopy (XL30 microscope, FEI, USA) images are obtained. Samples are coated with gold for 30 s under 30 mA.2.5. Antimicrobial assays
Bacterial viability is quantified via LIVE/DEAD® Cell Viability Assay. Films are incubated for one hour with bacteria at a concentration of 1 × 107 cells mL−1 in HEPES buffer (pH 7.4) at 37 °C. Films are then exposed to propidium iodide (Invitrogen, LIVE/DEAD® BacLight™ Bacterial Viability Kit) for 15 minutes to stain the dead cells with red fluorescence (owing to compromised membrane permeability). To stain the live cells with green fluorescence, films are incubated with SYTO®-9 nucleic acid stain (Invitrogen, LIVE/DEAD® BacLight™ Bacterial Viability Kit) for 5 min. Live and dead cells are enumerated through fluorescence microscopy (Olympus BX41 Model U-LH100HG, Japan). Statistical analysis is performed by employing single factor analysis of variance (ANOVA) in Excel Statistic 2003 Program, with three samples for each assay.To quantify metabolic (respiratory) activity, bacteria are treated with 5-cyano-2,3-ditolyl-tetrazolium chloride (CTC) (Sigma-Aldrich, USA). Films are incubated with bacteria for 30 min at 37 °C. Following removal of growth medium, M63 is added and another 30 min is allowed for incubation (at the same temperature). CTC (6 mM) is then added for one hour. In order to stain the remaining inactive cells, DAPI is added for 5 min. The percentage of active living cells is calculated by dividing the cells stained with red (CTC) by the total number of cells stained with blue (DAPI).
3. Results
3.1. SWNT characteristics
In Fig. 1, we show a TEM image of pure SWNT after cyclodextrin-mediated soft cutting. Prior to cutting, nanotube length is ca. 3–5 µm (see ESI†), and following cutting, the measured diameter is 300 ± 100 nm. The role of the cyclodextrin is to exfoliate thin “ropes” of SWNT, making them more susceptible to local conformational strains. This leads to the “cutting” of nanotube ropes, most probably at defect sites.30In Fig. 2, we show a TEM image of SWNT–PLGA, demonstrating SWNT to be better dispersed in the presence compared to the absence of the polymer matrix (see Fig. 1). (The holes are likely due to disruption of the thin carbon film of the TEM grid.)
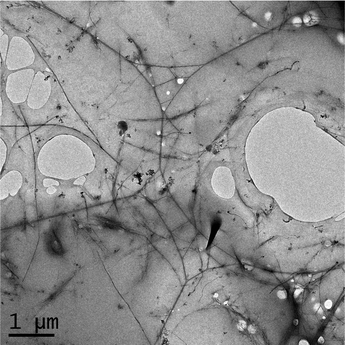 | ||
| Fig. 2 TEM image of cyclodextrin shortened SWNT in PLGA at a weight ratio of 1%. | ||
In Fig. 3, we show Raman spectra, at excitation wavelength 785 nm, of SWNT–PLGA composite films. All films containing SWNT exhibit G-bands around 1590 cm−1, confirming the presence of SWNT. Also evident are peaks corresponding to the radial breathing mode around 300 cm−1, suggesting at least some of the nanotubes not to be fully embedded within the polymer.
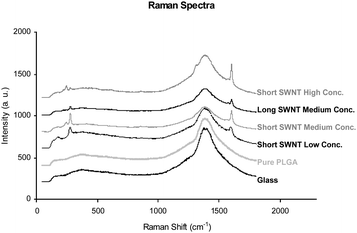 | ||
| Fig. 3 Raman spectra at excitation wavelength 785 nm of the glass substrate, a pure PLGA film, and SWNT–PLGA films of low concentration (1/7000 w/w) short (ca. 300 nm) SWNT, medium concentration (1/700) short SWNT, medium concentration long (>3 µm) SWNT, and high concentration (1/70) short SWNT. | ||
3.2. Film characteristics
In Table 1, we show the advancing and receding water contact angles of films formed by dip- and spin-coating methods. We find hysteresis—that is, the difference between advancing and receding values—to be significantly lower in SWNT-containing films formed via spin-coating. Since contact angle hysteresis generally correlates with surface heterogeneity,32 we employ only spin-coated films for the remainder of this work.| Dip coating | Spin coating | Hysteresis | |||||
|---|---|---|---|---|---|---|---|
| Advancing angle | Receding angle | Advancing angle | Receding angle | Dip coating | Spin coating | ||
| Pure PLGA | 61 ± 1 | 40 ± 3 | 90 ± 13 | 60 ± 17 | 21 ± 2 | 30 ± 21 | |
| Short SWNT | Low conc. | 76 ± 6 | 60 ± 10 | 82 ± 6 | 77 ± 6 | 16 ± 12 | 5 ± 8 |
| Med conc. | 90 ± 12 | 35 ± 2 | 72 ± 7 | 60 ± 11 | 55 ± 12 | 12 ± 13 | |
| High conc. | 72 ± 2 | 37 ± 2 | 82 ± 6 | 65 ± 5 | 35 ± 3 | 17 ± 8 | |
| Long SWNT | Med conc. | 88 ± 6 | 40 ± 10 | 88 ± 5 | 69 ± 6 | 48 ± 12 | 19 ± 8 |
In Fig. 4, we show SEM images of SWNT–PLGA films. Many of these images show evidence of cracks, and it appears that long SWNTs are associated with a smaller number of cracks. (Pure PLGA has the highest number of cracks.) We hypothesize that long SWNT, of length 3–5 µm, distributes the strain during drying, thereby suppressing crack formation. For short SWNT, of length ca. 300 nm, material texture is roughly the same as in the SWNT-free material. In previous studies, carbon nanotubes have been shown to improve fracture toughness, wear resistance, fatigue suppression, and crack bridging.33–36 Zhang et al. showed that the rate at which cracks grow can be decreased by reducing nanotube diameter, increasing nanotube length, and improving nanotube dispersion.35 Consistent with the literature, our SEM images reveal crack-bridging for long SWNT samples.
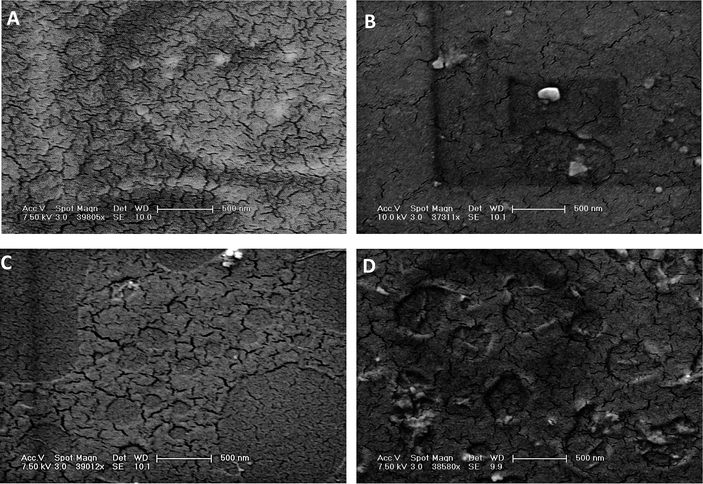 | ||
| Fig. 4 SEM images of SWNT–PLGA films. (A) Pure PLGA, (B) long (>3 µm) SWNT, medium (1/700 w/w) concentration, (C) short (ca. 300 nm) SWNT, medium concentration, and (D) short SWNT, high (1/70) concentration. SEM scale bar is 500 nm. | ||
3.3. Cell viability
In Fig. 5, we show the percentage of E. coli inactivated following one hour incubation, for various SWNT/PLGA films. Glass cover slips and pure PLGA films are fairly non-toxic, exhibiting inactivation rates of less than 20%. In contrast, we observe inactivation rates to exceed 80% in the presence of SWNT. Short SWNTs are associated with the highest rates of inactivation, possibly owing to the higher density of open tube ends (at a given SWNT concentration). We also observe cell inactivation to increase with SWNT concentration. In fact, the lowest concentration employed exhibits roughly the same level of toxicity as the SWNT-free (control) systems, suggesting a lower threshold for SWNT toxicity. The inactivation observed for short SWNT at medium and high concentrations, and for long SWNT at medium concentration, is statistically significant to p < 0.01.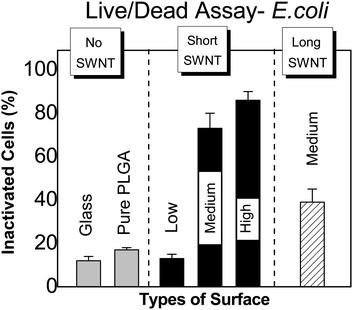 | ||
| Fig. 5 Percent inactivated E. coli on SWNT–PLGA films, as determined by LIVE/DEAD® Assay, for various SWNT lengths and concentrations (as defined in the caption to Fig. 3). | ||
In Fig. 6, we show the percentage of S. epidermidis inactivated following one hour incubation, for various SWNT/PLGA films. Consistent with the E. coli results, we observe a very high percentage of bacterial inactivation at high SWNT concentration (98%, p < 0.01), and a lower, statistically insignificant value at low SWNT concentration.
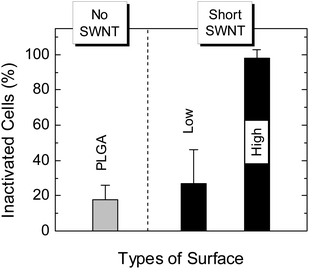 | ||
| Fig. 6 Percent inactivated S. epidermidis on SWNT–PLGA films, as determined by LIVE/DEAD® Assay, for short SWNT at high and low concentrations (as defined in the caption to Fig. 3). | ||
3.4. Cell metabolic activity
In Fig. 7, we show the rate of E. coli metabolic activity on SWNT–PLGA films containing short SWNT of differing concentration. We find the metabolic activity of bacteria on films of high SWNT concentration to be significantly lower than on films of low concentration (p < 0.03).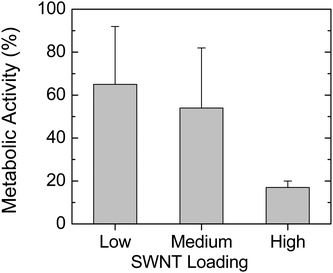 | ||
| Fig. 7 Metabolic activity rate of SWNT–PLGA samples with E. coliversus short SWNT concentration (as defined in the caption to Fig. 3). | ||
4. Discussion
Infectious disease has been the leading cause of mortality throughout human history. Materials capable of inactivating microbial species offer obvious benefits. Antimicrobial materials exert their influence in three ways—resisting microbial adhesion, leaching of biocidal agents, and killing on contact—as reviewed recently by Lichter et al.37 Clearly, contact killing is the most appealing, but many of the strategies developed to date involve quite elaborate chemistry/processing, and leave open questions concerning long-term compatibility with the biological environment.We propose here single walled carbon nanotubes (SWNTs) as potential building blocks for contact killing antimicrobial materials. SWNT has recently been shown to inactivate bacteria,27 and offers the benefits of being highly stable and easy to functionalize. We investigate here the antimicrobial nature of SWNT, within a narrow diameter range (0.8 to 1.2 nm), when combined with the commonly used biomaterial poly(lactic-co-glycolic acid) (PLGA). We find this composite material—here cast as a thin film—to be highly toxic to two common biomedical implant pathogens: E. coli and S. epidermidis. The effect is strongly influenced by SWNT length and concentration: short (ca. 300 nm) nanotubes at a concentration of 1/70 (w/w) yield the highest degree of bacterial inactivation. We note the highest rates of bacterial inactivation observed here compare very favorably to those observed above for other antimicrobial materials.
Several recent studies attest to the antimicrobial nature of carbon nanotubes.27,38–47 Narayan et al. showed nanotube films, formed via high temperature laser ablation of graphite on silicon, to completely inhibit bacterial colony formation.38,39 However, the nanotube structure was not well controlled and the process may not be amenable to many biomedical materials. Using the same well-characterized, narrow diameter distribution SWNT employed here, in the form of aggregated deposits and as coatings on a poly(vinylidene fluoride) membrane, Kang et al. found high levels of cytotoxicity to E. coli.27 They also determined SWNT to be significantly more cytotoxic than larger diameter multi-walled carbon nanotubes.40 Kim et al. used photothermal carbon nanotube therapy to both detect and inactivate pathogens by irradiated carbon nanotubes.41 They found the highest rate of inactivation with near-infrared irradiated carbon nanotubes in the form of clusters (100% inactivation) and a lower rate with shortened, dispersed nanotubes (80% inactivation). Of note is the somewhat larger diameter SWNT (1.7 nm) compared to that used here. Gu et al. reported galactose modified SWNT to result in up to a 60% reduction in colony forming units of E. coli suspensions.46,47 Brady-Estevez et al. designed SWNT containing filters for removal of bacteria from water, and showed 79% inactivation and 94% loss of metabolic activity.42 Nepal et al. considered the anti-microbial activity of SWNT together with the enzyme lysozyme, as dispersions and as layered films.43 These SWNT–lysozyme systems were effective against the Gram-positive bacterium Micrococcus lysodeikticus, but the antimicrobial activity was attributed to enzymatic lysis of the cell membrane and disappeared when the enzyme was replaced by DNA. Simmons et al. have shown SWNT combined with the polymer poly(vinylpyrrolidone-iodine) to be anti-microbial and a promising material for wound healing applications.44 They employed purified SWNT with notably small ash (3.7% wt) and iron (1.7% wt) content.44 Finally, Liu et al. have shown surfactant stabilized individual nanotubes suspended in solution to be highly toxic to microbes via a physical puncture mechanism.45 These authors used the same catalyst as employed here, Co-MCM-41, and obtained a narrow diameter distributed, purified SWNT with antimicrobial activity independent of metal residues (present up to 1 µg mL−1).45 To generalize these results, single walled carbon nanotubes tend to be more effective than multi-walled carbon nanotubes, with presentation mode and concentration being important variables.
In any eventual biomaterial application, the question of carbon nanotube toxicity to human cells must be addressed. The literature is somewhat contradictory on this point, with some studies showing toxicity48–50 and others various levels of compatibility.51–55 The discrepancy may be due to differences in tube diameter, length, and purity (i.e. metal content). Fadel et al. have investigated human T cells on the same SWNT as utilized in this study, and showed good compatibility without any evidence of toxicity.53
How do SWNTs exert their antimicrobial activity? Two possible mechanisms include mechanical disruption, where nanotubes act to physically pierce or otherwise perturb the bacterial membrane, and oxidative stress, where the high reductive potential of nanoscale carbon acts to directly or indirectly (e.g., through formation of radical species) damage the cell membrane. The earlier studies of Kang et al.27,40 suggest both mechanisms may be present. Electron micrographs provided visual evidence of compromised cell membranes, and DNA and RNA assays revealed the presence of cytoplasmic nucleic acids in the culture medium. DNA micro-array analysis showed genes known to be upregulated during mechanical disruption and oxidative stress to be upregulated in bacteria exposed to SWNT, suggesting both effects to be involved in membrane compromise and subsequent inactivation.40 Since we employ essentially the same SWNT as Kang et al., both mechanical disruption and oxidative stress likely contribute to the mechanism of antimicrobial activity observed here.
We observe a lower threshold to SWNT anti-microbial activity: systems of concentration less than 1/700 (w/w) perform at about the same level as pure polymer. Some of the studies referenced above report a concentration effect as well. In addition, we find, at a given weight fraction, shorter SWNT to offer increased cytotoxicity compared to longer SWNT. Since shorter tubes imply a higher density of tube ends (at a given SWNT weight fraction), and since tube ends are likely to be important in a mechanical disruption mechanism, our results suggest the number of tube ends to be an important governing variable. However, further studies are needed, keeping tube end density constant while varying tube length, in order to more firmly establish this hypothesis.
We observe SWNT–PLGA to exert anti-microbial activity against Gram-positive (S. epidermidis) and Gram-negative (E. coli) bacteria. Gram-negative bacteria display an outer lipopolysaccharide and protein membrane, and so might be expected to be more robust in the presence of anti-microbial agents acting via membrane disruption. While we observe slightly higher inactivation rates for the Gram-positive species employed here, our results certainly do not suggest that the outer membrane can protect Gram-negative bacteria from the influence of SWNT.
Acknowledgements
We thank Nan Li, Salim Derrouiche, Xiaoming Wang, Dr Sangchoon Jeon, Antony Joseph, and Nebi Demirsoy for their assistance in this study. We thank the National Science Foundation for financial support through CBET-0756323.Notes and references
- W. R. Jarvis, in 6th International Conference of the Hospital-Infection-Society, ed. S. J. Dancer, W. B. Saunders Co. Ltd, Amsterdam, Netherlands, 2007, pp. 3–9 Search PubMed.
- A. J. Kugel, L. E. Jarabek, J. W. Daniels, L. J. V. Wal, S. M. Ebert, M. J. Jepperson, S. J. Stafslien, R. J. Pieper, D. C. Webster, J. Bahr and B. J. Chisholm, J. Coat. Technol. Res., 2009, 6, 107–121 Search PubMed.
- W. J. Ye, M. F. Leung, J. Xin, T. L. Kwong, D. K. L. Lee and P. Li, Polymer, 2005, 46, 10538–10543 CrossRef CAS.
- W. K. Leung, A. P. S. Lau and K. L. Yeung, J. Appl. Microbiol., 2009, 106, 1463–1472 CrossRef CAS.
- B. Gottenbos, H. C. van der Mei, F. Klatter, D. W. Grijpma, J. Feijen, P. Nieuwenhuis and H. J. Busscher, Biomaterials, 2003, 24, 2707–2710 CrossRef CAS.
- M. C. McBride, R. K. Malcolm, A. D. Woolfson and S. P. Gorman, Biomaterials, 2009, 30, 6739–6747 CrossRef CAS.
- R. X. Chen, N. Cole, M. D. P. Willcox, J. Park, R. Rasul, E. Carter and N. Kumar, Biofouling, 2009, 25, 517–524 CrossRef CAS.
- G. Guerrero, J. Amalric, P. H. Mutin, A. Sotto and J. P. Lavigne, Pathol. Biol., 2009, 57, 36–43 CrossRef CAS.
- P. A. Norowski and J. D. Bumgardner, J. Biomed. Mater. Res., Part B, 2009, 88, 530–543 CrossRef.
- O. D. Schneider, S. Loher, T. J. Brunner, P. Schmidlin and W. J. Stark, J. Mater. Chem., 2008, 18, 2679–2684 RSC.
- A. G. Gristina, Science, 1987, 237, 1588–1595 CrossRef CAS.
- R. K. Malcolm, S. D. McCullagh, A. D. Woolfson, S. P. Gorman, D. S. Jones and J. Cuddy, J. Controlled Release, 2004, 97, 313–320 CrossRef.
- A. Piozzi, I. Francolini, L. Occhiaperti, R. Di Rosa, V. Ruggeri and G. Donelli, J. Chemother. (Firenze, Italy), 2004, 16, 446–452 Search PubMed.
- H. Gollwitzer, K. Ibrahim, H. Meyer, W. Mittelmeier, R. Busch and A. Stemberger, J. Antimicrob. Chemother., 2003, 51, 585–591 CrossRef CAS.
- P. Basak, B. Adhikari, I. Banerjee and T. K. Maiti, J. Mater. Sci.: Mater. Med., 2009, 20, 213–221 CrossRef.
- M. Liong, B. France, K. A. Bradley and J. I. Zink, Adv. Mater., 2009, 21, 1684–1689 CrossRef CAS.
- N. Stobie, B. Duffy, S. J. Hinder, P. McHale and D. E. McCormack, Colloids Surf., B, 2009, 72, 62–67 CrossRef CAS.
- V. Ruggeri, I. Francolini, G. Donelli and A. Piozzi, J. Biomed. Mater. Res., Part A, 2007, 81a, 287–298 CrossRef CAS.
- G. L. Y. Woo, M. L. Yang, H. Q. Yin, F. Jaffer, M. W. Mittleman and J. P. Santerre, J. Biomed. Mater. Res., 2002, 59, 35–45 CrossRef CAS.
- J. C. Tiller, C. J. Liao, K. Lewis and A. M. Klibanov, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 5981–5985 CrossRef CAS.
- B. Gottenbos, H. C. van der Mei, F. Klatter, P. Nieuwenhuis and H. J. Busscher, Biomaterials, 2002, 23, 1417–1423 CrossRef CAS.
- S. B. Lee, R. R. Koepsel, S. W. Morley, K. Matyjaszewski, Y. J. Sun and A. J. Russell, Biomacromolecules, 2004, 5, 877–882 CrossRef CAS.
- Z. Li, D. Lee, X. X. Sheng, R. E. Cohen and M. F. Rubner, Langmuir, 2006, 22, 9820–9823 CrossRef CAS.
- J. A. Lichter and M. F. Rubner, Langmuir, 2009, 25, 7686–7694 CrossRef CAS.
- S. J. Grunzinger, P. Kurt, K. M. Brunson, L. Wood, D. E. Ohman and K. J. Wynne, Polymer, 2007, 48, 4653–4662 CrossRef CAS.
- N. Cole, E. B. H. Hume, A. K. Vijay, P. Sankaridurg, N. Kumar and M. D. P. Willcox, Invest. Ophthalmol. Visual Sci., 2010, 51, 390–395 CrossRef.
- S. Kang, M. Pinault, L. D. Pfefferle and M. Elimelech, Langmuir, 2007, 23, 8670–8673 CrossRef CAS.
- S. Lim, N. Li, F. Fang, M. Pinault, C. Zoican, C. Wang, T. Fadel, L. D. Pfefferle and G. L. Haller, J. Phys. Chem. C, 2008, 112, 12442–12454 CrossRef CAS.
- Y. Chen, L. Wei, B. Wang, S. Y. Lim, D. Ciuparu, M. Zheng, J. Chen, C. Zoican, Y. H. Yang, G. L. Haller and L. D. Pfefferle, ACS Nano, 2007, 1, 327–336 CrossRef CAS.
- J. Chen, M. J. Dyer and M. F. Yu, J. Am. Chem. Soc., 2001, 123, 6201–6202 CrossRef CAS.
- N. Li, X. M. Wang, F. Ren, G. L. Haller and L. D. Pfefferle, J. Phys. Chem. C, 2009, 113, 10070–10078 CrossRef CAS.
- L. Muthuselvi and A. Dhathathreyan, Pramana, 2006, 66, 563–574 CrossRef CAS.
- A. K. Keshri, J. Huang, V. Singh, W. B. Choi, S. Seal and A. Agarwal, Carbon, 2010, 48, 431–442 CrossRef CAS.
- K. Balani, T. Zhang, A. Karakoti, W. Z. Li, S. Seal and A. Agarwal, Acta Mater., 2008, 56, 571–579 CrossRef CAS.
- W. Zhang, R. C. Picu and N. Koratkar, Nanotechnology, 2008, 19, 5.
- S. Pasupuleti, R. Peddetti, S. Santhanarn, K. P. Jen, Z. N. Wing, M. Hecht and J. P. Halloran, Mater. Sci. Eng., A, 2008, 491, 224–229 CrossRef.
- J. A. Lichter, K. J. Van Vliet and M. F. Rubner, Macromolecules, 2009, 42, 8573–8586 CrossRef CAS.
- R. J. Narayan, in Materials and Devices for Smart Systems, ed. Y. Furuya, E. Quandt, Q. Zhang, K. Inoue and M. Shahinpoor, Materials Research Society, Warrendale, 2004, pp. 299–304 Search PubMed.
- R. J. Narayan, C. J. Berry and R. L. Brigmon, Mater. Sci. Eng., B, 2005, 123, 123–129 CrossRef.
- S. Kang, M. Herzberg, D. F. Rodrigues and M. Elimelech, Langmuir, 2008, 24, 6409–6413 CrossRef CAS.
- J. W. Kim, E. V. Shashkov, E. I. Galanzha, N. Kotagiri and V. P. Zharov, Lasers Surg. Med., 2007, 39, 622–634 CrossRef.
- A. S. Brady-Estevez, S. Kang and M. Elimelech, Small, 2008, 4, 481–484 CrossRef CAS.
- D. Nepal, S. Balasubramanian, A. L. Simonian and V. A. Davis, Nano Lett., 2008, 8, 1896–1901 CrossRef CAS.
- T. J. Simmons, S. H. Lee, T. J. Park, D. P. Hashim, P. M. Ajayan and R. J. Linhardt, Carbon, 2009, 47, 1561–1564 CrossRef CAS.
- S. B. Liu, L. Wei, L. Hao, N. Fang, M. W. Chang, R. Xu, Y. H. Yang and Y. Chen, ACS Nano, 2009, 3, 3891–3902 CrossRef CAS.
- L. R. Gu, T. Elkin, X. P. Jiang, H. P. Li, Y. Lin, L. W. Qu, T. R. J. Tzeng, R. Joseph and Y. P. Sun, Chem. Commun., 2005, 874–876 RSC.
- L. R. Gu, P. J. G. Luo, H. F. Wang, M. J. Meziani, Y. Lin, L. M. Veca, L. Cao, F. S. Lu, X. Wang, R. A. Quinn, W. Wang, P. Y. Zhang, S. Lacher and Y. P. Sun, Biomacromolecules, 2008, 9, 2408–2418 CrossRef CAS.
- M. Bottini, S. Bruckner, K. Nika, N. Bottini, S. Bellucci, A. Magrini, A. Bergamaschi and T. Mustelin, Toxicol. Lett., 2006, 160, 121–126 CrossRef CAS.
- S. K. Manna, S. Sarkar, J. Barr, K. Wise, E. V. Barrera, O. Jejelowo, A. C. Rice-Ficht and G. T. Ramesh, Nano Lett., 2005, 5, 1676–1684 CrossRef CAS.
- A. A. Shvedova, V. Castranova, E. R. Kisin, D. Schwegler-Berry, A. R. Murray, V. Z. Gandelsman, A. Maynard and P. Baron, J. Toxicol. Environ. Health, Part A, 2003, 66, 1909–1926 Search PubMed.
- X. Chen, U. C. Tam, J. L. Czlapinski, G. S. Lee, D. Rabuka, A. Zettl and C. R. Bertozzi, J. Am. Chem. Soc., 2006, 128, 6292–6293 CrossRef CAS.
- S. Koyama, Y. A. Kim, T. Hayashi, K. Takeuchi, C. Fujii, N. Kuroiwa, H. Koyama, T. Tsukahara and M. Endo, Carbon, 2009, 47, 1365–1372 CrossRef CAS.
- T. R. Fadel, E. R. Steenblock, E. Stern, N. Li, X. Wang, G. L. Haller, L. D. Pfefferle and T. M. Fahmy, Nano Lett., 2008, 8, 2070–2076 CrossRef CAS.
- A. O. Lobo, M. A. F. Corat, E. F. Antunes, M. B. S. Palma, C. Pacheco-Soares, E. E. Garcia and E. J. Corat, Carbon, 2010, 48, 245–254 CrossRef CAS.
- L. F. Dong, C. M. Witkowski, M. M. Craig, M. M. Greenwade and K. L. Joseph, Nanoscale Res. Lett., 2009, 4, 1517–1523 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Raman spectra before and after SWNT cutting via cyclodextrins, and sample images from viability and metabolic activity assays are included. See DOI: 10.1039/c0nr00329h |
| This journal is © The Royal Society of Chemistry 2010 |
