Special positive birefringence properties of mesogen-jacketed liquid crystalline polymer films for optical compensators
Si
Chen
ab,
Lan-Ying
Zhang
a,
Xiao-Lin
Guan
a,
Xing-He
Fan
*a,
Zhihao
Shen
*a,
Xiao-Fang
Chen
a and
Qi-Feng
Zhou
*a
aBeijing National Laboratory for Molecular Sciences, Key Laboratory of Polymer Chemistry and Physics of Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, China. E-mail: fanxh@pku.edu.cn; zshen@pku.edu.cn; qfzhou@pku.edu.cn
bCollege of Chemical Engineering and Materials, Zhejiang University of Technology, Hangzhou, 310014, China
First published on 8th February 2010
Abstract
In this communication, we have described a simple way to get high positive birefringence polymer films. Solution cast films without any post-processing could show positive birefringence values of up to 0.0350. Such films are composed of several kinds of mesogen-jacketed liquid crystalline polymers (MJLCPs) and have potential applications in optical compensators.
Liquid crystal displays (LCDs) play very important roles in our modern life. From the earliest twisted nematic (TN) LCDs to the recent in-plane switching (IPS) LCDs, people have never stopped pursuing better display effects, including larger viewing angles, clearer contrast, better color rendition, achromatic dark states and so on. These improved display effects could be simply obtained by compensating for the phase retardation which occurs inside the liquid crystal cell. It has been understood that an optically uniaxial negative or positive birefringence compensator is necessary to improve the viewing angles of LCDs.1,2 In principle, the compensator's birefringence magnitude in the films is chosen so that the phase shift of the light that occurs due to the birefringence of the liquid crystal molecules inside the liquid crystal cell is negated by that of the compensator. Theoretically, the total net phase shift becomes zero.2 So a liquid crystal cell with negative birefringence needs a positive birefringence compensator and vice versa.
A liquid crystal display device generally comprises a liquid crystal cell sandwiched between a first polarizer and a second polarizer, in which the liquid crystal cell has a liquid crystal layer containing a rod-shaped liquid crystal compound between a pair of substrates.3 We consider the liquid crystal display layer or the compensator as a kind of film. The film's birefringence can be expressed as a function of the birefringence and order parameter of a uniaxial liquid crystal unit: Δn = Δnm × Sm−f. Δnm is the birefringence of a uniaxial unit, which is positive for rod-shaped liquid crystal compounds and negative for discotic-shaped compounds. Sm−f is the order parameter of a uniaxial unit with respect to the film’s normal direction, which is negative for the traditional TN LCDs and positive for the IPS LCDs.4–9 So the birefringence of the TN LCDs' display layer is positive and the compensator should have a negative value. In other case, the birefringence of the IPS LCDs' display layer is negative, and the compensator should have a positive value.2,11
Polymers have recently emerged as promising optical materials because of their unique optical properties, processability, and low cost for device integration.10 An ideal compensator should be a flexible thin film with high birefringence which is easy to apply to a substrate. So polymers with high birefringence are the first choice for preparing compensators. But current commercial positive materials require expensive post-synthesis processes such as locking liquid crystal molecules into a perpendicular alignment by photopolymerization,12,13 being performed by an applied electric field,14 magnetic field impression,14 processing of rubbing,15 stretching,16 and light irradiation.17
Mesogen-jacketed liquid crystalline polymers (MJLCPs) are a kind of polymers whose mesogenic units are attached laterally to the main chain without or with only short spacers.18–20 Because of the spatial requirement of the bulky and rigid mesogenic units, the main chain of the polymer has to take the extended conformation and the “jacket” is formed by the mesogenic units around the backbone, which makes MJLCPs have many special properties.21 This phenomenon is described as the “jacketed effect”, which plays a very important role. Firstly, monomers' side chains could be designed according to the requirement of certain properties, so special functional groups could be introduced into the side chains. These side chains could be connected to the main chain either in an unbalanced arrangement22 or from the position adjacent to the gravity centers.23 Secondly, such particularly designed monomers could be polymerized by controllable methods (such as atom transfer radical polymerization, ATRP) and high molecular weight, narrow polydispersity polymers could be obtained.22 The MJLCPs' backbones are somewhat rigid, and their diameter is influenced by the length of mesogens and alkoxy tails of the monomers. Thirdly, the MJLCPs could dissolve in normal organic solvents and the films cast from MJLCP solutions show large positive birefringence values, without being subjected to any of the processes mentioned above. However, the MJLCPs' positive birefringence properties have received little attention.
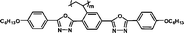
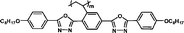
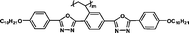
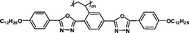
We have synthesized high Δnm liquid crystal monomers. As expected, polymers should also exhibit high positive Δn values over 0.030. To obtain higher positive Δn, heterocyclic groups were introduced into the MJLCPs' side chains. In this communication, we describe a simple way to produce high positive birefringence films for optical compensators with several kinds of MJLCPs, and evaluate the positive Δn properties of heterocyclic-group-based compounds in order to investigate the effect of these heterorings on the optical properties and try to find the determinant factors of polymer films' birefringence values.
The example monomers and polymers were synthesized according to the literature.22–24P–Ct could develop into a hexatic columnar nematic (ΦHN) phase,23PCt could develop into a hexatic columnar (ΦH) phase,24 and all other polymers could develop into a well-defined smectic A phase22,23 when heated to certain temperature. All the liquid crystalline phases were unchanged even when cooled to room temperature.
Monomers' birefringence values (Δnm) were evaluated as extrapolated values from mixtures containing 10 wt% of each test compound in SLC069015 (supplied by Shijiazhuang Yongsheng Huastsing Liquid Crystal Co. Ltd, China).25,26 An Atago abbe refractometer NAR-4T (for high refractive index measurement) was used to test Δnm at 20 °C with a sodium light source (λ = 589 nm). The monomers' birefringence values (Δnm) are listed in Table 1.
| Sample | Molecular structure | n 1 mix | n 2 mix | Δnm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a n 1 mix , n2mix and Δnm values were extrapolated values from the mixture of the liquid crystal monomer (10 wt%) and SLC069015 (90 wt%) from an equation: Δnmix = (1−C)Δnh' + C×Δnm, where Δnmix = n2mix−n1mix, Δnh' = 0.2140 according to the known host compound SLC069015, C is the concentration (in wt%) of the monomer in the host compound. b Obtained from ref. 26. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4C2Vm |
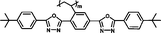
|
1.52 | 1.70 | 0.30a | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
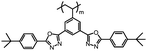
| MC6 |
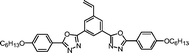
|
1.51 | 1.73 | 0.18a | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
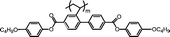
| M-OC12 |
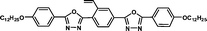
|
1.52 | 1.73 | 0.18a | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
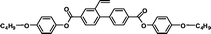
| M-OC6 |
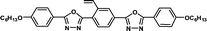
|
1.51 | 1.73 | 0.20b | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
It is well known that high Δnm value can be achieved by increasing the molecular conjugation length. From the typical results listed in Table 1, we found that although the structures of the monomers did not contain CN, NCS, alkyne, and other highly conjugated groups, they still had comparatively high birefringence values. The presence of 1,3,4-oxadiazole destroyed the linear conjugation. Symmetry lowering also plays an important role in increasing the anisotropy of liquid crystals.27 Meanwhile, a longer alkoxy tail has negative effect for Δnm values.28 Therefore, the Δnm value of 4C2Vm is higher than M–OC6 and M–OC12, and the Δnm value of M–OC12 is lower than M–OC6.
Polymer samples were dissolved in suitable solvents. And then the obtained solution was cast onto a piece of cleaned glass with the size of 1 × 1.5 inches. The thickness of the polymer film was controlled in the range of 15–20 μm by adjusting the content of solid in the solution. After the solvent evaporated, the polymer film was peeled off the glass to obtain a piece of free-standing film. Birefringence value (Δn) of the free-standing polymer films was measured by a prism coupler (Model 2010), from Metricon Corp. at 633 nm.4 For each film sample, at least three different points were tested to obtain the average birefringence value. Polymer films' birefringence values (Δn) are listed in Table 2.
From Table 2 we noticed that MJLCPs' solution casting films show compelling positive birefringence values. In the P–OCn (n = 6, 8, 10, and 12) and P–Ct series, the polymer films' birefringence values (Δn) increase with the increase of the alkoxy tail length at first, and reach a maximum value when n = 8. Then Δn decreases with the alkoxy tail length. This is because of the side chains' stereo-hindrance effect and longer alkoxy tail reducing the molecular polarizability.
Compared with the traditional orientate method for an orienting film being performed by the process of rubbing, stretching, applying an electric field, magnetic field impression, light irradiation and photopolymerization, MJLCP solution cast films without post-processing could also show high positive Δn. This special property is probably related to the “jacketed effect” in MJLCPs. And also MJLCPs films are stable with light, heat, and oxygen, so they are ideal candidates for optical compensators.
But the situation also becomes more complicated when it comes to polymer films. The birefringence (Δnm) of the liquid crystal units that constructpolymers is one of the influencing factors. The order parameter (Sm−p) of the liquid crystal units with respect to the polymer segment direction (unit within segment), and the order parameter (Sp−f) of the segment with respect to the film's normal direction should also be considered. In MJLCPs, Sm−pis negative when the liquid crystal rod units are perpendicular to the segment direction. The more orderly the liquid crystal rod units formed, the larger Sm−p absolute value is. The same applies to Sp−f, which is always negative or zero for a solution cast polymer film and requires a sufficiently large segment size or a sufficiently long persistent length for a large absolute value.4
From Table 1 and Table 2 we also find an interesting phenomena that the monomers' Δnm of 4C2Vm is much higher than M–OC6 and M–OC12, but the polymer films' Δn is exactly the opposite. Considering the three influencing factors mentioned above, we speculate the order parameters result in such significant difference. Compare with the liquid crystal side chains in 4C2Vp, the liquid crystal side chains in P–OC6 and P–OC12 are much more like “rigid rod”, and the total interaction between the rods is much higher than in the 4C2Vp. This must make contributions to a higher absolute value of Sm−p. Meanwhile, P–OC6 and P–OC12 must have higher Sp−f from the solution casting process but we could not find the exact reason now. But for the identical system, Δnof P–OC6 and P–OC12 have the similar tendency with Δnm of the monomers. This tells us that besides the “jacketed effect”, the structure of the monomers is also very important for Δn.
To sum it up, a polymer film's birefringence value (Δn) could be expressed as: Δn = Δnm × Sm−p × Sp−f.4 High values of Δn could be realized when the absolute values of all the three influencing factors were high. From Table 1 we could see that structure is the primary influencing factor for monomers to achieve high Δnm values, while from Table 2 we found that for polymer films, situation was more complicated. Considering Sm−p, as already mentioned above, the more orderly the liquid crystalline rod units formed, the larger Sm−p's absolute value is. So smectic phases, especially SA phase are recommended to achieve high Sm−p values. But as the value of Sp−f depends on the solution casting process, the exact way to achieve high Sp−f values is not clear to us now. Anyhow, liquid crystalline behaviors, structures of mesogens and structures of polymers are all determinant for Δn.
Different to conventional side-on side chain liquid crystalline polymers, MJLCPs' backbones are somewhat rigid and have some special characteristics.29 MJLCPs could develop into well defined liquid crystalline phases when heated to a certain temperature and the liquid crystalline phase remains unchanged even cooled to room temperature. And some MJLCP chains could orientate by themselves, developing diffraction arcs in the results of 2D-WAXD experiments after heating and annealing at a certain temperature without alignment.30 This somewhat resembles the polymer films reported here which are capable of forming anisotropically aligned films from solution casting without being subject to any further treatment. Nearly all the MJLCPs we have synthesized before could form positive birefringence films by solution casting, but now we have found out that the exact values of Δn correlate with the specific structures of the polymers. Follow-on research work is in progress and we hope to find the relationship between the birefringence value and the structures of MJLCPs. Meanwhile, considering that MJLCPs could be designed to have different liquid crystalline phases, such as the ΦHN phase and the Sm phase, and such liquid crystalline phases could remain unchanged when cooled to room temperature, we hope to further design controllable MJLCPs whose side-chain mesogens have better order properties in liquid crystalline phase and realize the application of MJLCPs in optical compensation films for IPS LCDs.
More detailed work is still ongoing to reveal the effect of side-chain mesogens on the birefringence value of MJLCPs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos.: 20634010, 20874002, 20874003 and 20974002). The authors also express thanks to Prof. Zhang, D. for help in the characterization of polymer films' birefringence values.Notes and references
- F. M. Li, F. W. Harris and S. Z. D. Cheng, Polymer, 1996, 37, 5321 CrossRef CAS.
- S. Z. D. Cheng, F. M. Li, E. P. Savitski and F. W. Harris, Trends Polym. Sci., 1997, 5, 51 CAS.
- Y. Li, I. Nagata and T. Hosokawa, US Patent, US 20080090027–A1, 2008.
- F. W. Harris, D. Zhang, X. L. Zheng, T. C. Germroth, T. Kuo, J. K. Jing and B. M. King, Int. Patent, WO 2008121583–A1, 2008.
- M. Z. Jiao, S. Gauza, Y. Li, J. Yan, S. T. Wu and T. Chiba, Appl. Phys. Lett., 2009, 94, 101107 CrossRef.
- H. Kawamoto, Proc. IEEE, 2002, 90, 460 CrossRef CAS.
- H. Ikeno and H. Nagai, US Patent, US 2009051861–A1, 2009.
- E. Uchida and N. Kawatsuki, Macromolecules, 2006, 39, 9357 CrossRef CAS.
- N. Kawatsuki, K. Goto, T. Kawakami and T. Yamamoto, Macromolecules, 2002, 35, 706 CrossRef CAS.
- C. Badarau and Z. W. Wang, Macromolecules, 2004, 37, 147 CrossRef CAS.
- M. Lavrentovich, T. Sergan and J. Kelly, Liq. Cryst., 2003, 30, 851 CrossRef CAS.
- D. B. Taber, L. G. Hale, B. K. Winker, W. J. Gunning, M. C. Skarohid, J. D. Sampica and T. A. Seder, Proc. Soc. Photo-Opt. Instrum. Eng., 1998, 3363, 162.
- N. Kawatsuki, T. Kawakami and T. Yamamoto, Adv. Mater., 2001, 13, 1337 CrossRef CAS.
- A. Fukunaga, Y. Sato and K. Shimoda, Japan Patent, JP 2009025789–A, 2009.
- C. L. Kuo, T. Miyashita, M. Suzuki and T. Uchida, Appl. Phys. Lett., 1996, 68, 1461 CrossRef CAS.
- S. Lee, R. Khosravani, J. Peng, V. Grubsky, D. S. Starodubov, A. E. Willner and J. Feinberg, IEEE Photonics Technol. Lett., 1999, 11, 1277 CrossRef.
- K. Yoshiara, S. Matsumoto, M. Takabayashi, Y. Shimakura and T. Sugihara, Rev. Laser Eng., 2005, 33, 433 Search PubMed.
- Q. F. Zhou, H. M. Li and X. D. Feng, Macromolecules, 1987, 20, 233 CrossRef CAS.
- Q. F. Zhou, X. L. Zhu and Z. Q. Wen, Macromolecules, 1989, 22, 49.
- X. Zhu, Q. Yang and Q. F. Zhou, Chin. J. Polym. Sci., 1992, 10, 187 CAS.
- Y. X. Liu, D. Zhang, X. H. Wan and Q. F. Zhou, Chin. J. Polym. Sci., 1998, 16, 283 CAS.
- S. Chen, L. C. Gao, X. D. Zhao, X. F. Chen, X. H. Fan, P. Y. Xie and Q. F. Zhou, Macromolecules, 2007, 40, 5718 CrossRef CAS.
- C. P. Chai, X. Q. Zhu, P. Wang, M. Q. Ren, X. F. Chen, Y. D. Xu, X. H. Fan, C. Ye, E. Q. Chen and Q. F. Zhou, Macromolecules, 2007, 40, 9361 CrossRef CAS.
- Y. D. Xu, Q. Yang, Z. Shen, X. F. Chen, X. H. Fan and Q. F. Zhou, Macromolecules, 2009, 42, 2542 CrossRef CAS.
- C. Sekine, K. Iwakura, N. Konya, M. Minami and K. Fujisawa, Liq. Cryst., 2001, 28, 1375 CrossRef CAS.
- X. L. Guan, L. Y. Zhang, Z. L. Zhang, Z. H. Shen, X. F. Chen, X. H. Fan and Q. F. Zhou, Tetrahedron, 2009, 65, 3728 CrossRef CAS.
- H. F. Hsu, W. C. Lin, Y. H. Lai and A. Y. Lin, Liq. Cryst., 2003, 30, 939 CrossRef CAS.
- C. Sekine, K. Iwakara, N. Konya, M. Minai and K. Fujisawa, Liq. Cryst., 2001, 28, 1375 CrossRef CAS.
- Y. F. Zhao, X. H. Fan, X. H. Wan, X. F. Chen, Y. Yi, L. S. Wang, X. Dong and Q. F. Zhou, Macromolecules, 2006, 39, 948 CrossRef CAS.
- S. Chen, L. Y. Zhang, L. C. Gao, X. F. Chen, X. H. Fan, Z. H. Shen and Q. F. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 505 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |