Spontaneous generation and patterning of chiral polymeric surface toroids†
Gregory T.
Carroll
,
Mahthild G. M.
Jongejan
,
Dirk
Pijper
and
Ben L.
Feringa
*
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, Groningen, 9747AG, The Netherlands. E-mail: b.l.feringa@rug.nl
First published on 9th July 2010
Abstract
A chiral poly(n-hexyl isocyanate) can form ordered mesoscale toroidal structures when drop-cast on a variety of surfaces.
When a macromolecular solution is cast on a surface a complex interplay of forces can affect the morphology and pattern of the resulting film. Obtaining robust and reproducible morphologies with uncommon shapes is important not only in exploring new paradigms in the behaviour of soft materials but also in creating interfaces for applications in a variety of emerging areas including templates for ordering adsorbates and growing crystals, scaffolds for biological sensors, self-cleaning surfaces and the preparation of surfaces with tailored properties including friction, gloss and hydrophobicity.1–6 Maskless lithographic techniques not only simplify spatially controlled surface modification but can also facilitate patterning on non-planar surfaces.3 In some cases characteristic morphologies can be deposited such that long-range spatial regularity is achieved as has been reported for self-assembling systems composed of block copolymers7 or porphyrin trimers.8 Among the many shapes and patterns of mesoscale materials reported, toroidal morphologies provide a distinct and challenging shape, and have shown promise in their use as nanoreactors9 and in selective cellular translocation.10 Both theory11 and experiment12 have shown that DNA and other biological molecules can form toroidal condensates upon addition of multivalent ions. Additional methods for the assembly of biomaterials into toroids have also been reported.9,13 Self-assembly approaches to toroid formation have been extended to synthetic di-14 and triblock15 copolymers and amphiphiles.16 In some cases macromolecular toroidal structures can be directly synthesized.17 A more unusual approach is to utilise physical processes such as drying or confinement at interfaces. Porphyrins18–20 and second-generation dendrimers21 have been shown to form toroidal structures upon drying on a substrate.
Physical approaches (drying, confinement, dewetting, etc.) to producing mesoscale morphologies are interesting because their formation cannot necessarily be predicted by conventional molecular approaches to self-assembly. An interesting challenge is to gain control over physical processes that can lead to morphologies not accessible in solution. Typically, reports of toroidal structures deposited on a surface by any method show no long-range spatial patterning and sometimes a very low density. Recently, geometric confinement of liquid crystals in surface-modified microchannels allowed for toroidal defects to be ordered in 2D patterns.22 We discovered that a synthetic, chiroptical, linear homopolymer can spontaneously form a high density of toroidal structures of approximately 1–3 μm on a surface by simply drop-casting a dilute solution of the polymer (Scheme 1). In addition to extending toroidal aggregate formation to synthetic linear macromolecules with a helical backbone, we demonstrate that a large degree of macroscopic long-range order (>200 μm) over the deposition process can be obtained on various surfaces. This is an important step in showing that physical processes such as solvent drying may provide a useful assembly approach to mesoscale chemistry.
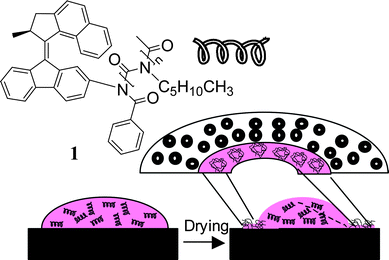 | ||
| Scheme 1 Chiral macromolecule 1 end-functionalized with an overcrowded alkene that induces a preferred helical twist of the poly(n-hexyl isocyanate) chain. Evaporation of a solution of 1 forms toroidal morphologies on a surface. | ||
Our system utilizes a poly(n-hexyl isocyanate) (PHIC) initiated by a chiral photochromic molecular motor (1) (Scheme 1).23 Poly(isocyanates) are of general interest because they provide rod-like helical macromolecular building blocks for creating novel materials.24 Interestingly, they show a cooperative response to chiral information.25 The helical sense of the side chains can be controlled through modification of the side-chains or the terminus of the polymer with chiral groups.24–29 The overcrowded alkene end-group in 1 was shown to effectively induce a preferred helical sense over the polymer backbone that could be controlled via photochemical and thermal isomerization processes. A related system was recently shown to influence the supramolecular helicity of a lyotropic liquid crystalline phase.30 The conformational changes of both the polymer and end-group associated with the rotary cycle of the molecular motor make this system an intriguing candidate to employ in micromechanical systems and soft machines.31,32 Further, the chiroptical properties of the polymer may offer interesting opportunities in constructing new photonic materials.33
In order to probe the structures that macromolecule 1 can form on surfaces we initially dissolved the polymer in toluene at a concentration of 0.01–0.05 mg ml−1. A 5 μl drop of the solution was placed on freshly cleaved muscovite mica and allowed to evaporate in air. Mica was selected because macroscale atomically flat surfaces can be obtained by simple cleavage of the top layer, facilitating investigation with atomic force microscopy (AFM) and eliminating effects of surface roughness on the morphology of the polymer deposit. Evaporation of the solvent leads to large concentric rings.34,35 However, examination of the areas between the larger macroscale deposits with AFM revealed a wide range of spatially aligned toroidal structures with a height of approximately 40–60 nm, a width of approximately 150 nm, an outer diameter of approximately 2 μm and an inner diameter ranging from 400–900 nm (Fig. 1). The inset in Fig. 1B shows a phase contrast image of a single aggregate. Interestingly, the image shows a somewhat periodic undulation in the surface composition, which was more difficult to resolve with the traditional topography image, hinting that the surface aggregates may contain orientational order induced by the packing of the helical rod-like macromolecules.
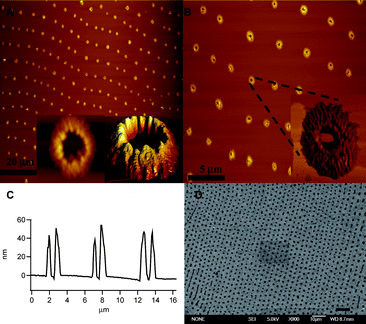 | ||
| Fig. 1 AFM images of films prepared by evaporation of a toluene solution containing 1 on mica (A, B). The inset in B is a phase contrast AFM image of an individual aggregate. The insets in A show a representative aggregate imaged at a smaller scan size (1.8 × 1.8 μm). A representative height profile is shown in C. SEM image of a film prepared on gold (D, S1†). The size of the scale bars in A, B and D are 20, 5 and 10 μm, respectively. | ||
Additionally, scanning electron microscopy (SEM) of films cast on Au and mica showed arrays of micron-sized toroidal structures covering the surface (Fig. 1D, S1†). The drying process produced a high degree of long-range order covering more than 100 microns with a periodic spacing of generally 2–6 microns. In addition to our experiments on mica, evaporation on a gold surface showed a highly ordered array of aggregated polymer. The structure of the individual aggregates on the gold substrate was less regular than on mica and may be related to different surface interactions or the surface roughness of the gold film. Additionally, deposition on quartz produced aggregated structures displaying long-range order (see S2†). The dimensions of the aggregates are similar to those observed on gold and mica, although the exact structure on quartz is less easy to deduce with optical microscopy (OM).
In order to test the effect of different solvents we prepared solutions in chloroform, a solvent that forms a well-defined drop on mica that does not readily spread, unlike toluene which almost completely spreads. The AFM images obtained after evaporation of a solution of 1 in chloroform (Fig. 2) show again many toroidal structures and long-range order, although deviations in the image presented are clearly evident. The lesser degree of spatial order in the pattern compared to films cast from toluene is probably a result of the faster evaporation. The dimensions of the structures are similar to the surface toroids formed from an evaporated toluene solution and are quite regular with heights typically ranging from 10–20 nm and outer and inner diameter widths of approximately 1.5–2.5 μm and 500 nm, respectively, although in some areas smaller rings can be found (outer diameter approximately 500 nm). The toroidal structures often contain a noticeable rim of material within the ring, occasionally straddling the length of the cavity. In some areas the rings of polymer fuse into one another whereas in other cases the joined ridges appear to have drained to the perimeter, forming a larger cavity. In addition to chloroform and toluene, deposition of a solution from THF showed aggregates with a toroidal morphology (Fig. 3). The dimensions of the aggregates are similar to the samples prepared from toluene and CHCl3, although we also found regions where the size distribution gave average diameters of approximately 500 nm. The observation of similar patterns from various solvents and on substrates differing in both chemical composition and surface roughness suggests a general tendency of 1 to form films composed of toroidal aggregates upon evaporation of a good solvent for the polymer when the concentration is in the range employed.
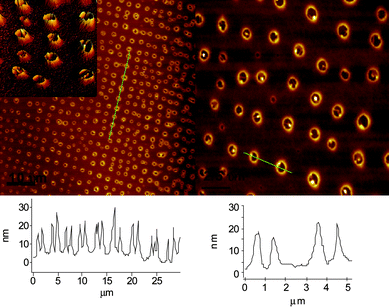 | ||
| Fig. 2 AFM topography images of films of 1 prepared by evaporation from chloroform on mica reveal a toroidal morphology. | ||
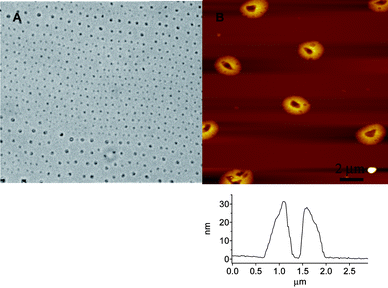 | ||
| Fig. 3 OM (A) and AFM topography (B) images of films of 1 prepared by evaporation from THF on mica reveal a toroidal morphology. | ||
As mentioned above, 1 is chiral with a bias towards a particular helicity of the polymer. The CD spectrum of 1 in chloroform is shown in S3.† Surfaces containing the toroidal morphologies shown above do not contain enough material for CD spectroscopy analysis. However, by preparing films of 1 by spin-coating, the surface concentration could be adjusted in order to obtain a CD spectrum of 1 in the solid state. Although such films are structurally different, they at least show that 1 displays an optical response in the solid state (S3†). Spin-coating PHIC without the overcrowded alkene end-group or the overcrowded alkene without PHIC produces no signal (S3†), demonstrating that the end-group influences the helicity of the polymer in the film. The solution and surface spectra appear quite different (S3†) and may result from differences in the environment, perturbations in the polymer structure upon adsorption to a surface and aggregation or birefringent optical effects. The signal is generally unaffected by irradiation, demonstrating that the conformation is stable towards any changes in the structure of the end-group at 20 °C.‡
Pattern formation by droplet evaporation in the form of large concentric rings on the order of the size of the drying droplet upon accumulation of material at the perimeter of the fluid is a known phenomenon.34,36 These large broad deposits have been attributed to pinning of the contact line of the droplet followed by a flow of solvent to the contact line to replenish evaporated fluid. The solute is then delivered to the contact line where it accumulates. In the present work the toroidal structures are sub-patterns found between the pinned contact lines. The regularity of the patterns in the present work is probably related to the above mechanism in which a flow is directed towards the perimeter of the droplet, delivering polymer which is then trapped within the confines of a thin film with a large length but small width. The length scale of the drying process deposits the polymer in laterally ordered rows. The seemingly lower degree of order found for the CHCl3 solution indicates that the process is sensitive to the rate of evaporation. A faster evaporation results in a qualitatively less even periodicity.
The mechanism behind toroid formation is less clear. A number of explanations can be considered. As a drop of solvent containing 1 evaporates, polymer accumulates at the perimeter. The formation of toroids either occurs in solution, in the confined “wet” thin film, or evolves as the thin film dries. Although studies regarding PHIC aggregation have been described,37 we are unaware of any reports of PHIC forming toroidal structures in solution. Given that the solvents used in our work are good solvents for PHIC, one would expect the polymers to swell and make more contact with the solvent than itself in dilute solution. However, as the solvent evaporates and the polymer becomes more concentrated near the solid–liquid–vapor interface, the resulting conditions may induce the aggregation/gelation behaviour of the polymer.
A second consideration is the formation of ring morphologies induced by nucleation at defects or impurities. For example, the attachment of particles to bubbles has been described38 and implicated in the formation of ring structures during solvent evaporation of porphyrin solutions.18,19 Additionally, water droplets have been used as templates to assemble poly(fluorene) macromolecules into rings when drop-cast from chloroform solutions saturated with H2O.39 In the present system, the polymer may collect around microbubbles as the solvent dries. The drying of the droplet would then leave a residue of polymer encapsulating air which would ultimately collapse. However, we did not observe the morphologies with AFM when PHIC without the motor was drop-cast, indicating that the presence of nucleation sites cannot be the only requirement for toroid formation. We wish to point out that this does not necessarily mean that the induced chirality of the polymer is the key structural feature for obtaining toroids as other properties of the polymer could be involved, such as the molecular weight, polydispersity or surface energy of the end-group. Additionally, one may consider that as the droplet dries, a metastable thin film is produced which undergoes morphological changes as a result of dewetting, the first stage of which is the formation of holes in the film followed by expansion of the holes which is accompanied by the growth of a rim.40,41 However, in a typical polymer dewetting mechanism the final morphology is either a droplet of polymer with a finite contact angle or an undulating pattern for very thin films.42 The Tg value of 1, which is −23.6 °C, (measured by differential scanning calorimetry), seems low enough to allow for further mobility of the polymer chains at room temperature, however we did not observe the rings to decay into droplets and observed their presence even one year after formation, indicating high stability. We cannot completely exclude a dewetting mechanism. Tg for thin films can increase or decrease with film thickness, so the value of a bulk sample measured by DSC does not necessarily reflect the value in the films.43 Additionally, various effects can lead to inhibition of dewetting or partial dewetting morphologies.44,45
We have shown that evaporation of a solution of 1 in organic solvent can produce a film displaying highly stable ordered toroidal morphologies on a variety of surfaces and from a variety of organic solvents. The system shows how simple drop-casting of materials can be used to pattern surfaces with interesting morphologies. Non-conventional approaches, such as solvent drying, to produce mesostructured and patterned surfaces can complement more traditional self-assembly approaches to create patterns that display order on different length scales. For example, we expect that the patterns in our system could be further modified and controlled by drop-casting on surfaces that have been chemically and morphologically structured through self-assembly and lithographic methods.
Acknowledgements
This research was financially supported by the Netherlands Organization for Scientific Research (NWO-CW, G.T.C. and D.P; Spinoza grant 438031 to B.L.F) and NanoNed (grants 467711, 467712 M.J., G.T.C., B.L.F.).References
- F. Garbassi, M. Morra and E. Occhiello, Polymer Surfaces: From Physics to Technology, Updated Edition, John Wiley & Sons, Inc., New York, 1998 Search PubMed.
- E. J. Park, G. T. Carroll, N. J. Turro and J. T. Koberstein, Soft Matter, 2009, 5, 36–50 RSC.
- S. Granick, S. K. Kumar, E. J. Amis, M. Antonietti, A. C. Balazs, A. K. Chakraborty, G. S. Grest, C. Hawker, P. Janmey, E. J. Kramer, R. Nuzzo, T. P. Russell and C. R. Safinya, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 2755–2793 CrossRef CAS.
- J. R. Lancaster, J. Jehani, G. T. Carroll, Y. Chen, N. J. Turro and J. T. Koberstein, Chem. Mater., 2008, 20, 6583–6585 CrossRef CAS.
- G. T. Carroll, D. N. Wang, N. J. Turro and J. T. Koberstein, Glycoconjugate J., 2008, 25, 5–10 CrossRef.
- K. Lee, F. Pan, G. T. Carroll, N. J. Turro and J. T. Koberstein, Langmuir, 2004, 20, 1812–1818 CrossRef CAS.
- S. Park, D. H. Lee, J. Xu, B. Kim, S. W. Hong, U. Jeong, T. Xu and T. P. Russell, Science, 2009, 323, 1030–1033 CrossRef CAS.
- R. van Hameren, P. Schon, A. M. van Buul, J. Hoogboom, S. V. Lazarenko, J. W. Gerritsen, H. Engelkamp, P. C. M. Christianen, H. A. Heus, J. C. Maan, T. Rasing, S. Speller, A. E. Rowan, J. Elemans and R. J. M. Nolte, Science, 2006, 314, 1433–1436 CrossRef CAS.
- R. Djalali, J. Samson and H. Matsui, J. Am. Chem. Soc., 2004, 126, 7935–7939 CrossRef CAS.
- L. Alexander, K. Dhaliwal, J. Simpson and M. Bradley, Chem. Commun., 2008, 3507–3509 RSC.
- I. C. B. Miller, M. Keentok, G. G. Pereira and D. R. M. Williams, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 71, 031802 CrossRef CAS.
- M. X. Tang, W. J. Li and F. C. Szoka, J. Gene Med., 2005, 7, 334–342 CrossRef CAS.
- S. S. Daube, T. Arad and R. Bar-Ziv, Nano Lett., 2007, 7, 638–641 CrossRef CAS.
- H. Huang, B. Chung, J. Jung, H.-W. Park and T. Chang, Angew. Chem., Int. Ed., 2009, 48, 4594–4597 CrossRef CAS , S4594/4591–S4594/4597.
- D. J. Pochan, Z. Chen, H. Cui, K. Hales, K. Qi and K. L. Wooley, Science, 2004, 306, 94–97 CrossRef CAS.
- E. Lee, Y. H. Jeong, J. K. Kim and M. Lee, Macromolecules, 2007, 40, 8355–8360 CrossRef CAS.
- A. J. Boydston, T. W. Holcombe, D. A. Unruh, J. M. J. Frechet and R. H. Grubbs, J. Am. Chem. Soc., 2009, 131, 5388–5389 CrossRef CAS.
- A. P. H. J. Schenning, F. B. G. Benneker, H. P. M. Geurts, X. Y. Liu and R. J. M. Nolte, J. Am. Chem. Soc., 1996, 118, 8549–8552 CrossRef CAS.
- J. Hofkens, L. Latterini, P. Vanoppen, H. Faes, K. Jeuris, S. De Feyter, J. Kerimo, P. F. Barbara, F. C. De Schryver, A. E. Rowan and R. J. M. Nolte, J. Phys. Chem. B, 1997, 101, 10588–10598 CrossRef CAS.
- L. Latterini, R. Blossey, J. Hofkens, P. Vanoppen, F. C. De Schryver, A. E. Rowan and R. J. M. Nolte, Langmuir, 1999, 15, 3582–3588 CrossRef CAS.
- S. Masuo, H. Yoshikawa, T. Asahi, H. Masuhara, T. Sato, D.-L. Jiang and T. Aida, J. Phys. Chem. B, 2001, 105, 2885–2889 CrossRef CAS.
- M. C. Choi, T. Pfohl, Z. Y. Wen, Y. L. Li, M. W. Kim, J. N. Israelachvili and C. R. Safinya, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17340–17344 CrossRef CAS.
- D. Pijper and B. L. Feringa, Angew. Chem., Int. Ed., 2007, 46, 3693–3696 CrossRef.
- M. M. Green, J.-W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. B. Selinger and J. V. Selinger, Angew. Chem., Int. Ed., 1999, 38, 3139–3154 CAS.
- M. M. Green, N. C. Peterson, T. Sato, A. Teramoto, R. Cook and S. Lifson, Science, 1995, 268, 1860–1866 CrossRef CAS.
- M. M. Green, J.-W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. B. Selinger and J. V. Selinger, Angew. Chem., Int. Ed., 1999, 38, 3139–3154 CAS.
- M. M. Green, C. Andreola, B. Munoz, M. P. Reidy and K. Zero, J. Am. Chem. Soc., 1988, 110, 4063–4065 CrossRef CAS.
- D. Pijper and B. L. Feringa, Soft Matter, 2008, 4, 1349–1372 RSC.
- K. Maeda and E. Yashima, Supramolecular Chirality, Springer-Verlag, Berlin, 2006, pp. 47–88 Search PubMed.
- D. Pijper, M. G. M. Jongejan, A. Meetsma and B. L. Feringa, J. Am. Chem. Soc., 2008, 130, 4541–4552 CrossRef CAS.
- P. Calvert, Adv. Mater., 2009, 21, 743–756 CrossRef CAS.
- G. London, G. T. Carroll, T. F. Landaluce, M. M. Pollard, P. Rudolf and B. L. Feringa, Chem. Commun., 2009, 1712–1714 RSC.
- A. S. Schwanecke, V. A. Fedotov, V. V. Khardikov, S. L. Prosvirnin, Y. Chen and N. I. Zheludev, Nano Lett., 2008, 8, 2940–2943 CrossRef CAS.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Nature, 1997, 389, 827–829 CrossRef CAS.
- Z. Lin and S. Granick, J. Am. Chem. Soc., 2005, 127, 2816–2817 CrossRef CAS.
- R. D. Deegan, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 61, 475–485 CrossRef CAS.
- M. Kanao, Y. Matsuda and T. Sato, Macromolecules, 2003, 36, 2093–2102 CrossRef CAS.
- O. I. Vinogradova, Colloids Surf., A, 1994, 82, 247–254 CrossRef CAS.
- Y. Liu, T. Murao, Y. Nakano, M. Naito and M. Fujiki, Soft Matter, 2008, 4, 2396–2401 RSC.
- P. Mueller-Buschbaum, J. Phys.: Condens. Matter, 2003, 15, R1549–R1582 CrossRef.
- G. Reiter, Phys. Rev. Lett., 1992, 68, 75–78 CrossRef CAS.
- R. Xie, A. Karim, J. F. Douglas, C. C. Han and R. A. Weiss, Phys. Rev. Lett., 1998, 81, 1251–1254 CrossRef CAS.
- J. L. Keddie, R. A. L. Jones and R. A. Cory, Faraday Discuss., 1994, 98, 219–230 RSC.
- G. T. Carroll, M. E. Sojka, X. G. Lei, N. J. Turro and J. T. Koberstein, Langmuir, 2006, 22, 7748–7754 CrossRef CAS.
- C. Yuan, M. Ouyang and J. T. Koberstein, Macromolecules, 1999, 32, 2329–2333 CrossRef CAS.
- B. T. Muellers, J. W. Park, M. S. Brookhart and M. M. Green, Macromolecules, 2001, 34, 572–581 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Sample preparation, instrumental methods, SEM images and CD spectra. See DOI: 10.1039/c0sc00159g |
| ‡ In some cases we observed an attenuation of the signal after irradiation with UV light followed by a restoration upon remaining in the dark, however these changes appeared to be very sensitive to the ambient temperature and difficult to reproduce. The optical activity of solid state poly(isocyanate) films has previously been studied by Green and co-workers and shown to be temperature dependent.46 |
| This journal is © The Royal Society of Chemistry 2010 |
