Biocompatibility and drug delivery systems
Daniel S.
Kohane
*a and
Robert
Langer
b
aLaboratory for Biomaterials and Drug Delivery, Department of Anesthesiology, Division of Critical Care Medicine, Children's Hospital, Harvard Medical School, 300 Longwood Ave., Boston, MA 02115, USA. E-mail: Daniel.Kohane@childrens.harvard.edu; Fax: +(617) 730-0453; Tel: +(617) 253-6884
bKoch Institute for Integrative Cancer Technology, Massachusetts Institute of Technology, 77 Massachusetts Ave, Cambridge, MA 02139, USA
First published on 18th June 2010
Abstract
Drug delivery technology has emerged as an important focus of biotechnological research and commercial enterprise. While much attention is justifiably focused on the design and effectiveness of drug delivery devices, the nature of their interaction with surrounding tissues – their biocompatibility – is crucial. Here we discuss biocompatibility, specifically as it relates to drug delivery systems, which differ from other biomaterial-based devices by possibly containing large quantities of drugs with their own effects on tissues.
 Daniel S. Kohane | Daniel S. Kohane received his MD and his PhD degree in Physiology from Boston University. He subsequently completed residencies in Pediatrics (Children's Hospital Boston) and Anesthesiology (Massachusetts General Hospital), followed by a fellowship in Pediatric Critical Care (Children's Hospital Boston). He is currently a Senior Associate in Pediatric Critical Care at Children's Hospital Boston, where he directs the Laboratory for Biomaterials and Drug Delivery. He is an Associate Professor of Anesthesiology at Harvard Medical School. His research interests are in drug delivery and biomaterials. |
 Robert Langer | Robert Langer is an Institute Professor (there are 14 Institute Professors at MIT; being an Institute Professor is the highest honor that can be awarded to a faculty member). He has written approximately 1100 articles and has over 750 issued or pending patents. His many awards include the National Medal of Science, the Charles Stark Draper Prize, Albany Medical Center Prize and the Lemelson-MIT prize, for being “one of history's most prolific inventors in medicine.” Langer is one of the very few individuals ever elected to the Institute of Medicine, the National Academy of Engineering, and the National Academy of Sciences. |
Introduction
Over the past few decades, drug delivery systems have gone from being theoretically attractive experimental modalities to being at the cutting edge of a multi-billion dollar pharmaceutical industry, with applications in almost every field of medicine. The development of drug delivery systems entails a wide range of tasks familiar to the engineer: the development of materials suitable to the specific application (biodegradable, pH-sensitive, anionic, flexible, etc.), the attainment of a particular degree of drug loading and type of release kinetics (slow, fast, pulsatile), and proof of efficacy. In addition, it is important to demonstrate the system's safety, which encompasses at least two major entities: the safety of the systemically distributed drug, if any, and the biocompatibility of the drug delivery system. Controlling systemic drug distribution can be a relatively simple matter of engineering release kinetics so that blood levels are lower. Mitigating problems with biocompatibility can be much more difficult, involving poorly understood drug–tissue interactions and material properties.Here we discuss biocompatibility in the context of drug delivery, what it is and how to assess it, while describing contributions from both drugs and biomaterials and means of modulating them. The chemical basis of material–tissue interactions is an area where much remains to be learned, with great potential rewards.
What is biocompatibility?
The definition of biocompatibility (and biomaterials) has been discussed extensively in the literature1,2 and in editorial comments on manuscripts. In brief, it is an expression of the benignity of the relation between a material and its biological environment. Some would extend that definition to include adequate functionality in a given biological context.3 It is important to be aware of the following relevant considerations.First, it is highly anatomically dependent. For example, biodegradable polymeric microspheres composed of the α-hydroxy acid poly(lactic-co-glycolic) acid (PLGA) cause a well-characterized tissue reaction,4 which is generally – but quite subjectively – referred to as mild (more on this below). Such particles injected in the loose connective tissue surrounding nerves (Fig. 1) cause quite vigorous inflammation, with numerous neutrophils and macrophages (“acute inflammation”). In one to two weeks, the types of inflammatory cell change to include macrophages and lymphocytes. If particles are large, they induce formation of giant foreign body cells. Particles gradually become ensconced in a fibrous capsule containing fibroblasts and perforated by blood vessels. This inflammation can persist at the nerve for days, weeks, or months, depending on the formulation, but generally resolves completely when the particles are resorbed, leaving little or no trace.5 The same inflammatory pattern – which is quite generic in most environments – is seen when particles are injected into the peritoneal cavity, the area within the abdomen and pelvis that contains the intestines and other organs. There, however, the outcome is quite different: PLGA microparticles generally cause peritoneal adhesions6 – which are not at all benign. Similarly, in orthopedic use the combination of local inflammation7 and perhaps acidosis from the degradation products of PLGA can eventually cause bone destruction with associated drainage of inflammation through skin defects.8 Materials can also be unsuitable even if generally biocompatible in the sense of having a benign tissue reaction. For example, an inert but opaque material might be useful in, say bone, but would be inappropriate in the visual axis of the eye.
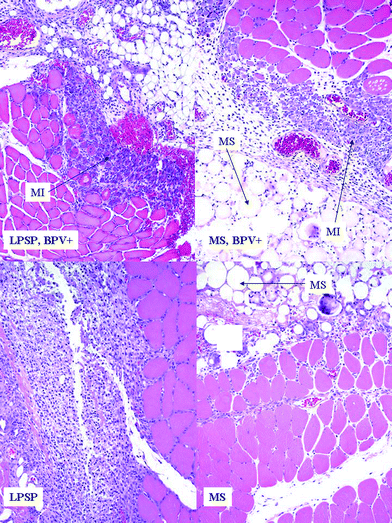 | ||
| Fig. 1 Inflammatory response and reaction of muscle 4 days after injection. Reaction to PLGA microspheres (MS) and lipid-protein-sugar particles (LPSPs) with bupivacaine (BPV+) showed inflammation and muscle injury (MI), whereas particles without bupivacaine showed no muscle fiber injury, and less inflammation adjacent to the pocket of particles. Original magnification is 10× in all frames. Individual LPSPs cannot easily be discerned in these views. (Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium, J. Biomed. Mater. Res., Part A, 2002, 59(3), 450–459. Copyright 2002 Wiley Periodicals, Inc., A Wiley Company; reprinted with permission of John Wiley & Sons, Inc.) | ||
Second, our understanding of whether or not a material is biocompatible is limited by our understanding of biological processes and their consequences. For example, we do not know whether there are untoward effects (e.g. neuronal changes in the central nervous system) of having an inflammatory mass from microparticles remain adjacent to a nerve for weeks to months, even if it eventually resolves completely.5,9 Our assessment is also limited by the sensitivity of our ability to detect problems (see below).
Third, whether or not a material is biocompatible does not simply depend on the intrinsic properties of the material itself – or at any rate of the composition of matter. For example, although PLGA microparticles cause peritoneal adhesions, PLGAnanoparticles generally do not.6 The difference between the two formulations is that the microparticles remain in the peritoneal cavity for weeks, while the nanoparticles leave within days and end up in the reticuloendothelial system (the network of cells, free and in organs throughout the body, that carry out immunological functions such as ingesting bacteria), particularly the liver and spleen. Duration of tissue exposure to the materials has a pronounced effect on biocompatibility.
Fourth, biocompatibility is somewhat subjective. The tissue reaction seen in Fig. 1 is quite vigorous, and in some cases the same processes can lead to nontrivial adverse events, as with the orthopedic devices mentioned above. On the other hand, the inflammation generally disappears completely over time, and the surrounding tissues do not show much evidence of injury. It may be that, as with many other pharmaceutical issues, biocompatibility is a relative matter – another variable for which there is a risk–benefit ratio. The risk is the local irritation, the benefit is the sustained release of a drug.
It will be apparent from the preceding that whether a material is considered biocompatible depends on time, context, and a subjective appreciation. Biocompatibility can refer to very local tissue phenomena, or to events that affect the entire organism, depending on the formulation, its specific method of use, and the speaker. Consequently, the point is frequently made that referring to a material as being “biocompatible” in an absolute sense – as is done in many publications – is somewhat misleading.
Assessment of biocompatibility
Generally, the evaluation of a formulation's biocompatibility progresses through in vitro and in vivo phases. In many cases, the types of experiments involved are quite similar. Nonetheless, the specifics of the evaluation depend on the nature of the problems that can be anticipated given the compounds involved. For example in drug delivery systems containing the polyene macrolide amphotericin B, formulations are commonly evaluated for their potential for destroying red blood cells (“hemocompatibility”).10,11In vitro studies provide a rough assessment of the ability of relevant cell types to survive in the presence of a material. This can be done with any of a number of tests such as the MTT assay (or variants thereof, which quantitate the net production of a dye by functioning mitochondria), measures of DNA synthesis and cell proliferation, and dye-based cell membrane integrity tests.12 If necessary, subsequent tests can address the mechanism of toxicity, e.g. by looking at markers of cell death13 or tissue injury.14 It may be important to test the effects of both direct contact with cells and indirect exposure to diffusible components, such as residual solvents or monomers, breakdown products, drugs, acid, etc. Two chambered systems, where the material is in a continuous aqueous phase with the cells under study, but is physically separated from them, are commonly used to study indirect effects (e.g. from diffusible components). Carcinogenesis and mutagenesis are also important considerations. There are standardized assays for these, but they are usually studied relatively late, prior to clinical studies.
In vivo studies are essential for an understanding of biocompatibility. Even if we assume that the cell-based models used in vitro accurately reflect their counterparts inside the body, they do not take the rest of the body into account. A material may not be directly cytotoxic, but may yet induce a reaction that is destructive. For example, a UV-cross-linkable chitosan that caused mild to moderate depression in cell viability in cell culture caused exuberant peritoneal adhesions when placed in the peritoneal cavity.15 In contrast, concentrations of the drugbupivacaine that killed 100% of muscle cells in culture16 caused little or no muscle injury when injected in vivo17 – perhaps because of interstitial fluid turnover. Particularly in drug delivery, the material may not cause any tissue injury at all (and perhaps be biocompatible in that sense) but kill the animal nonetheless, either from drug release14 or from some unforeseen problem such as intravascular coagulation,18 embolic events,19 chelation of ions vital to homeostasis, etc. As a result of these factors, and as demonstrated above, in vitro findings frequently lack predictive value re. in vivo outcomes. This problem is the reason that in vitro–in vivo correlation (often abbreviated to IVIVC) is so frequently discussed in drug delivery and biomaterial circles.
In vivo studies of biocompatibility can vary considerably depending on the context. A common assay for injected or implanted materials is hematoxylin–eosin (H&E) stained paraffin-embedded sections. These are generally excellent for looking at gross tissue morphology and tissue reaction – including inflammation. There is a range of specialized stains, as well as immunohistochemical methods, for detecting specific biological changes. It is important to know the limitations of the techniques used. For example, H&E sections are relatively insensitive for detecting nerve injury. Epon-embedded sections or electron microscopy may allow detection of more subtle signs of tissue injury. A review of histopathology as it relates to drug delivery is beyond the scope of this minireview (but see, for example, ref. 4).
Both in vitro and in vivo experiments are amenable to analysis with specific molecular probes, such as antibody-based assays of release of pro-inflammatory molecules15 or looking for changes in the expression of genes known to be associated with tissue injury.14 Toxicogenomic approaches,20 which have been used to accelerate drug discovery, may prove useful as well.
Biomaterials are not necessarily inert
Relatively recently, there has been considerable interest in developing materials that respond to their environment.21–24 Concurrently, there has been a growing realization among investigators that even putatively inert biomaterials, such as are used as barrier devices or as drug delivery systems, are not mere “innocent bystanders” in their environments. For example, the cross-linked chitosan referred to above may have caused peritoneal adhesions by causing inflammation by inducing nearby cells to release TNF-α and MIP-2 (a murine analog of the chemoattractantIL-8).15 Conversely, the preventive effects of cross-linked hyaluronic acid gels on adhesion formation may have been because they induce release of tissue plasminogen activator,25 which has been shown to mitigate adhesion formation.26Microparticulate formulations are also not inert. One obvious way in which they affect their environment is the inflammatory reaction they can cause, as a simple consequence of the fact that they are very much what innate immunity was designed to destroy. As will be seen below, they can affect tissue reaction to drugs. The biological consequences of nanoparticles are not well understood, but are potentially significant, given their enhanced capability to enter cells compared to larger particles – hence the burgeoning field of nanotoxicology.
The drug in drug delivery
It is important to remember that the drug itself can have important effects on the biocompatibility of a drug delivery system, particularly for formulations that involve a stationary depot. This is illustrated very well in the case of the sustained release of local anesthetics. A wide range of drug delivery systems, including polymeric particles,5,9 spray-dried lipid-protein-sugar particles,5,27,28 liposomes,14 cross-linked hyaluronic acid gels,29 and rheological blends of polysaccharides30 have been employed to provide sustained release of bupivacaine. In the absence of drug, these formulations cause varying degrees of inflammation, but minimal or no actual tissue injury. However, when loaded with bupivacaine, all caused varying degrees of muscle injury.Release of drugs from many drug delivery systems can be characterized by rapid initial release (“burst release”), followed by a more prolonged phase with slower release. Apart from being wasteful of drug payload, burst release is also potentially harmful. The burst release from PLGA microspheres of the very hydrophilic ultrapotent local anesthetic tetrodotoxin caused severe systemic toxicity (poisoning);9 if that could be survived, prolonged release occurred safely, and helped provide prolonged duration local anesthesia. The magnitude of burst release also affected local tissue injury, with higher release rates of bupivacaine causing more muscle injury.16 Burst release and its consequences can be mitigated by a variety of means. For example, changing the delivery vehicle from polymeric microparticles9 to liposomes14 almost completely negated systemic toxicity from burst release of hydrophilic local anesthetics. Within each type of drug delivery device, there are numerous parameters that can alter release kinetics, such as the type of excipient used,14,31polymer concentration or molecular weight, drugconjugation to the vehicle,32 altering the cross-linking density of the vehicle,33 and many others. Release kinetics are markedly affected by device size, because of the differences in surface area to volume ratios.34 Consequently, macroscopic devices such as drug-eluting contact lenses may show very slow drug release over very extended time frames.35 However, such are generally not injectable. The effects of burst release can also be attenuated by pharmacological means. Incorporating the vasoconstrictor epinephrine into the carrier fluid for tetrodotoxin-containing PLGA particles reduced systemic toxicity,9 presumably by slowing diffusion of the toxin from the site of injection into the systemic circulation (the bloodstream).36
The sustained release that follows the burst can also cause tissue injury. For example, we have found that concentrations of bupivacaine that were minimally toxic in vitro when exposure was short (hours) became very toxic when the duration of exposure was prolonged (days to weeks).16 That observation raised the possibility that local tissue toxicity could be an inevitable consequence of sustained release of local anesthetics.
There may be unanticipated interactions between the drug delivery system and drug, affecting tissue reaction. Co-injection of blank (no drug) particles greatly increased muscle injury from bupivacainein vivo, even though they had no effect on it in vitro.16 We do not know the mechanism by which this happens – perhaps by inducing inflammation, or by affecting local pH, or by slowing the diffusion of drug away from the site of injection. It also bears mentioning that the drug delivery system itself can account entirely for problems with biocompatibility. Tetrodotoxin itself causes no tissue injury, but combinations of that compound with chemical permeation enhancers – which prolong the duration of nerve blockade – can result in quite severe tissue reaction.37
These considerations are not of purely academic interest, as can be seen by the fate of a long-acting sustained-release formulation for bupivacaine.38 This product was slated for commercialization, but it was found during preclinical animal studies and human clinical trials that the formulation caused local tissue injury, including to nerves, and the Investigational New Drug application (IND #53,441) was withdrawn.
One difficulty in anticipating problems of this sort is that most conventional pharmacology – including the literature and curricula – focuses on therapeutic effects at roughly therapeutic concentrations over clinically relevant time frames. Even the toxicological literature mostly addresses the toxic effects seen within that parameter space. In contrast, here we are concerned with the extremely high local concentrations that may occur in the immediate vicinity of depot formulations, and which may persist over extended periods. Local anesthetic-induced myotoxicity is an excellent example of this. First, its local toxicity would not be predicted from its principal intended means of action (sodium channel blockade). Second, although muscle injury is described in the local anesthetic literature, it is rarely an issue in general clinical practice. In fact, local anesthetics are often injected into “trigger points” in muscle in some pain syndromes. Nonetheless, as we have seen, the combination of high concentrations, long durations of exposure, and the presence of a drug delivery system can result in tissue injury.
It is therefore important to be alert to the potential for toxicity from drugs in delivery systems. Carefully designed and executed testing is likely to discover problems during the preclinical period. Hospital pharmacies can be excellent resources in identifying potential problem compounds, due to their experience with the consequences of inadvertent leakage of intravenous drugs from blood vessels. Naturally, one should be particularly suspicious of drugs with extremes of pH, hydrophobicity, osmolarity; compounds with known cytotoxic effects including solvents and surfactants; and reactive moieties. Vasoconstriction can also be harmful if sustained, or if along the course of an end-artery (an artery without collateral flow such as to the finger, eyes, and penis), causing ischemia. These concerns also apply to carriers for the drug delivery system, drug breakdown products, drug preservatives if any, and other putatively inert excipients.
Mitigating tissue reaction directly
Biocompatibility can be improved by modulating tissue reaction by pharmacological and other means. Incorporation of anti-inflammatory compounds can reduce inflammation in and around a device. For example, incorporation of the glucocorticoid receptor agonist budesonide in polysaccharide-based hydrogels greatly reduced the penetration of inflammatory cells,39 as did conjugation of dexamethasone (another glucocorticoidagonist) to the backbone of the hydrogel.32 Other drugs, although not specifically anti-inflammatory, can have similar effects. Incorporation of tissue plasminogen activator (tPA) into hydrogels can affect tissue reaction by altering the balance between clot formation and clot destruction (fibrinolysis), thus preventing peritoneal adhesions.33 Biomaterials can be combined to affect tissue reaction. As noted above, PLGA particles cause adhesions when they remain in the peritoneal cavity. However, they do not when contained within an in situ cross-linking hydrogel that prevents direct particle contact with surrounding tissues.40There are many other approaches to mitigating tissue reaction, all of which could affect biocompatibility, such as altering surface micro- or nanostructure,41 and surface modification. One surface modification that has an important impact on drug delivery is PEGylation – the decoration of particle surfaces with poly(ethylene glycol) – which reduces particle interaction with the reticuloendothelial system and therefore increases circulation time. Its effect on tissue reaction is less well understood.
The chemistry of biocompatibility
Biomaterials have often originated in materials used in other industries42 that had desirable mechanical properties but were not specifically designed to interact with surrounding tissues or with blood. Efforts to develop more biocompatible materials are hampered by deficits in our understanding of material–tissue interactions, and consequently of which chemical interactions should be studied. The problem is exacerbated by the fact that, as we have seen, biocompatibility is often not a simple interaction between one material and one cell type, but may involve degradation products, separate effects from drug and carrier, and numerous cell types that present themselves at different times. Even questions regarding physicochemical variables that would seem relatively straightforward to address, such as – for example – whether it is better for a material to be hydrophilic or hydrophobic in preventing peritoneal adhesions, do not have clear-cut answers.43Assuming that a well-defined system can be identified, it may be possible to achieve insights by the careful study of structure–activity relationships, perhaps followed by rational design of better molecules, in a manner analogous to some approaches taken with gene therapy and intracellulardrug delivery.44 Alternatively, high throughput screening methods can rapidly identify materials that can have desirable properties in terms of cell survival, proliferation, and differentiation, and perhaps help identify relevant chemical structural motifs.45 Similar considerations apply to the drugs that are delivered.
The dearth of specific chemical knowledge in this field underscores the fact that this is an area of research that is ripe for serious consideration by scientists interested in the interaction of materials and biology. The fruit of such endeavors would be immensely invaluable. One obvious benefit of such knowledge is that it would allow the design of better drug delivery vehicles. Another, that should not be underestimated, is that it could allow the development of in vitro screening systems to assess biocompatibility. Such systems are among the most desirable objectives of biocompatibility research, as they would reduce the need for time consuming, expensive, statistically messy, and ethically charged animal experimentation.
Acknowledgements
This work was supported by GM073626 (to DSK) from NIGMS (National Institute of General Medical Sciences) and EB006365 (to RL) from NIBIB (National Institute of Biomedical Imaging and Bioengineering).References
- D. F. Williams, On the mechanisms of biocompatibility, Biomaterials, 2008, 29, 2941–2953 CrossRef CAS.
- D. F. Williams, On the nature of biomaterials, Biomaterials, 2009, 30, 5897–5909 CrossRef CAS.
- C. J. Kirkpatrick, F. Bittinger, M. Wagner, H. Kohler, T. G. van Kooten, C. L. Klein and M. Otto, Current trends in biocompatibility testing, Proc. Inst. Mech. Eng., Part H, 1998, 212, 75–84 Search PubMed.
- J. M. Anderson, In vivo biocompatibility of implantable delivery systems and biomaterials, Eur. J. Pharm. Biopharm., 1994, 40, 1–8 CAS.
- D. S. Kohane, M. Lipp, R. C. Kinney, D. C. Anthony, D. N. Louis, N. Lotan and R. Langer, Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium, J. Biomed. Mater. Res., Part A, 2002, 59, 450–459 Search PubMed.
- D. S. Kohane, J. Y. Tse, Y. Yeo, R. Padera, M. Shubina and R. Langer, Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum, J. Biomed. Mater. Res., Part A, 2006, 77a, 351–361 CrossRef CAS.
- M. van der Elst, C. P. A. T. Klein, J. M. de Blieck-Hogervorst, P. Patka and H. J. T. M. Haarman, Bone tissue response to biodegradable polymers used for intramedullary fracture fixation: A long-term in vivo study in sheep femora, Biomaterials, 1999, 20, 121–128 CrossRef CAS.
- O. Bostman and H. Pihlajamaki, Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review, Biomaterials, 2000, 21, 2615–2621 CrossRef CAS.
- D. S. Kohane, S. E. Smith, D. N. Louis, G. Colombo, P. Ghoroghchian, N. G. Hunfeld, C. B. Berde and R. Langer, Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres, Pain, 2003, 104, 415–421 CrossRef CAS.
- A. Zumbuehl, L. Ferreira, D. Kuhn, A. Asthashkina, L. Long, Y. Yeo, T. Iaconis, M. Ghannoum, G. R. Fink, R. Langer and D. S. Kohane, Antifungal hydrogels, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12994–12998 CrossRef.
- S. P. Hudson, R. Langer, G. R. Fink and D. S. Kohane, Injectable in situ cross-linking hydrogels for local antifungal therapy, Biomaterials, 2010, 31, 1444–1452 CrossRef CAS.
- T. F. Slater, B. Sawyer and U. Straeuli, Studies on succinate-tetrazolium reductase systems. III. Points of coupling of four different tetrazolium salts, Biochim. Biophys. Acta, 1963, 77, 383–393 CrossRef CAS.
- W. N. Haining, D. G. Anderson, S. R. Little, M. S. von Bergwelt-Baildon, A. A. Cardoso, P. Alves, K. Kosmatopoulos, L. M. Nadler, R. Langer and D. S. Kohane, pH-triggered microparticles for peptide vaccination, J. Immunol., 2004, 173, 2578–2585 CAS.
- H. Epstein-Barash, I. Shichor, A. H. Kwon, S. Hall, M. W. Lawlor, R. Langer and D. S. Kohane, Prolonged duration local anesthesia with minimal toxicity, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 7125–7130 CrossRef CAS.
- Y. Yeo, J. A. Burdick, C. B. Highley, R. Marini, R. Langer and D. S. Kohane, Peritoneal application of chitosan and UV-cross-linkable chitosan, J. Biomed. Mater. Res., Part A, 2006, 78a, 668–675 CrossRef CAS.
- R. Padera, E. Bellas, J. Y. Tse, D. D. Hao and D. S. Kohane, Local myotoxicity from sustained release of bupivacaine from microparticles, Anesthesiology, 2008, 108, 921–928 CrossRef CAS.
- R. Padera, J. Tse, E. Bellas and D. S. Kohane, Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity, Muscle Nerve, 2006, 34, 747–753 CrossRef CAS.
- S. P. Hudson, R. F. Padera, R. Langer and D. S. Kohane, Biocompatibility of mesoporous silicates, Biomaterials, 2008, 29, 4045–4055 CrossRef CAS.
- D. S. Kohane, N. Plesnila, S. S. Thomas, D. Le, R. Langer and M. A. Moskowitz, Lipid-sugar particles for intracranial drug delivery: safety and biocompatibility, Brain Res., 2002, 946, 206–213 CrossRef CAS.
- M. R. Fielden and K. L. Kolaja, The state-of-the-art in predictive toxicogenomics, Curr. Opin. Drug Discov. Dev., 2006, 9, 84–91 Search PubMed.
- D. A. Barrera, E. Zylstra, P. T. Lansbury Jr. and R. Langer, Synthesis and RGD peptide modification of a new biodegradable copolymer: poly(lactic-acid-co-lysine), J. Am. Chem. Soc., 1993, 115, 11010–11011 CrossRef CAS.
- S. Zhang, Fabrication of novel biomaterials through molecular self-assembly, Nat. Biotechnol., 2003, 21, 1171–1178 CrossRef CAS.
- G. A. Silva, C. Czeisler, K. L. Niece, E. Beniash, A. A. Harrington, J. A. Kessler and S. I. Stupp, Selective differentiation of neural progenitor cells by high-epitope density nanofibers, Science, 2004, 303, 1352–1355 CrossRef CAS.
- M. P. Lutolf, G. P. Raeber, A. H. Zisch, N. Tirelli and J. A. Hubbell, Cell-responsive synthetic hydrogels, Adv. Mater., 2003, 15, 888–892 CrossRef CAS.
- Y. Yeo, C. B. Highley, E. Bellas, T. Ito, R. Marini, R. Langer and D. S. Kohane, In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model, Biomaterials, 2006, 27, 4698–4705 CrossRef CAS.
- H. Orita, M. Fukasawa and W. Girgis, Inhibition of postsurgical adhesions in a standardized rabbit model: intraperitoneal treatment with tissue plasminogen activator, Int. J. Fertil., 1991, 36, 172–177 Search PubMed.
- G. Colombo, R. Langer and D. S. Kohane, Effect of excipient composition on the biocompatibility of bupivacaine-containing microparticles at the sciatic nerve, J. Biomed. Mater. Res., 2004, 68a, 651–659 Search PubMed.
- G. Colombo, R. Padera, R. Langer and D. S. Kohane, Prolonged duration local anesthesia with lipid-protein-sugar particles containing bupivacaine and dexamethasone, J. Biomed. Mater. Res., Part A, 2005, 75a, 458–464 CrossRef CAS.
- X. Jia, G. Colombo, R. Padera, R. Langer and D. S. Kohane, Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid, Biomaterials, 2004, 25, 4797–4804 CrossRef CAS.
- T. Hoare, E. Bellas, D. Zurakowski and D. S. Kohane, Rheological blends for drug delivery. II: Prolongation of nerve blockade, biocompatibility, and in vitro–in vivo correlations, J. Biomed. Mater. Res., Part A, 2010, 92a, 586–595 CAS.
- D. S. Kohane, M. Lipp, R. C. Kinney, N. Lotan and R. Langer, Sciatic nerve blockade with lipid-protein-sugar particles containing bupivacaine, Pharm. Res., 2000, 17, 1243–1249 CrossRef CAS.
- T. Ito, I. P. Fraser, Y. Yeo, C. B. Highley, E. Bellas and D. S. Kohane, Anti-inflammatory function of an in situ cross-linkable conjugate hydrogel of hyaluronic acid and dexamethasone, Biomaterials, 2007, 28, 1778–1786 CrossRef CAS.
- Y. Yeo, E. Bellas, C. B. Highley, R. Langer and D. S. Kohane, Peritoneal adhesion prevention with an in situ cross-linkable hyaluronan gel containing tissue-type plasminogen activator in a rabbit repeated-injury model, Biomaterials, 2007, 28, 3704–3713 CrossRef CAS.
- D. S. Kohane, Microparticles and nanoparticles for drug delivery, Biotechnol. Bioeng., 2007, 96, 203–209 CrossRef CAS.
- J. B. Ciolino, T. R. Hoare, N. G. Iwata, I. Behlau, C. H. Dohlman, R. Langer and D. S. Kohane, A drug-eluting contact lens, Invest. Ophthalmol. Visual Sci., 2009, 50, 3346–3352 CrossRef.
- D. S. Kohane, J. Yieh, N. T. Lu, R. Langer, G. R. Strichartz and C. B. Berde, A re-examination of tetrodotoxin for prolonged duration local anesthesia, Anesthesiology, 1998, 89, 119–131 CrossRef CAS.
- E. J. Simons, E. Bellas, M. W. Lawlor and D. S. Kohane, Effect of chemical permeation enhancers on nerve blockade, Mol. Pharmaceutics, 2009, 6, 265–273 CrossRef CAS.
- J. Castillo, J. Curley, J. Hotz, M. Uezono, J. Tigner, M. Chasin, R. Wilder, R. Langer and C. Berde, Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres, Anesthesiology, 1996, 85, 1157–1166 CrossRef CAS.
- Y. Yeo, M. Adil, E. Bellas, A. Astashkhina, N. Chaudary and D. S. Kohane, Prevention of peritoneal adhesions with an in situ cross-linkable hyaluronan hydrogel delivering budesonide, J. Controlled Release, 2007, 120, 178–185 CrossRef CAS.
- Y. Yeo, T. Ito, E. Bellas, C. B. Highley, R. Marini and D. S. Kohane, In situ cross-linkable hyaluronan hydrogels containing polymeric nanoparticles for preventing post-surgical adhesions, Ann. Surg., 2007, 245, 819–824 CrossRef.
- M. Goldberg, R. Langer and X. Jia, Nanostructured materials for applications in drug delivery and tissue engineering, J. Biomater. Sci., Polym. Ed., 2007, 18, 241–268 CrossRef CAS.
- N. A. Peppas and R. Langer, New challenges in biomaterials, Science, 1994, 263, 1715–1720 CrossRef CAS.
- Y. Yeo and D. S. Kohane, Polymers in the prevention of peritoneal adhesions, Eur. J. Pharm. Biopharm., 2008, 68, 57–66 CrossRef CAS.
- S. R. Little and D. S. Kohane, Polymers for intracellular delivery of nucleic acids, J. Mater. Chem., 2008, 18, 832–841 RSC.
- D. G. Anderson, S. Levenberg and R. Langer, Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells, Nat. Biotechnol., 2004, 22, 863–866 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
