Homoleptic copper(I) phenylselenolate polymer as a single-source precursor for Cu2Se nanocrystals. Structure, photoluminescence and application in field-effect transistor†
Kam-Hung Lowa, Cheng-Hui Lib, Vellaisamy A. L. Royc, Stephen Sin-Yin Chuia, Sharon Lai-Fung Chana and Chi-Ming Che*ab
aInstitute of Molecular Functional Materials, Department of Chemistry and HKU-CAS Joint Laboratory on New Materials, The University of Hong Kong, Pokfulam Road, Hong Kong SAR. E-mail: cmche@hku.hk; Fax: +852 2857 1586; Tel: +852 2859 2154
bCoordination Chemistry Institute and the State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, People's Republic of China
cDepartment of Physics and Materials Science, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong SAR
First published on 29th June 2010
Abstract
We present the one-dimensional polymeric structures of [Cu(SePh)]∞1 and [Cu(SeMe)]∞2 that were solved by using powder X-ray diffraction data. Using a field-effect transistor (FET) set-up, the hole mobility of complex 1 was found to be 4 × 10−2 cm2 V−1 s−1, comparable to that of regioregular poly(3-hexylthiophene) (P3HT). Phase-pure orthorhombic and cubic Cu2Se were synthesized from 1 as a single precursor under vacuum and inert atmosphere, respectively.
Self-assembled coordination polymers (SACP) utilizing hydrogen bonding, π–π stacking interaction, van der Waals interactions, and metal ion-ligand coordination are polymeric materials that could be architecturally controlled and with applications in materials science. Interest in functional SACP have increased in recent years and novel applications of SACP have emerged in physisorption,1 chemisorption,2 gas storage,3 and catalysis.4 To broaden the application horizon of SACP, we are exploring the innovative uses of SACP in organic electronics, which are presently dominated by molecular and polymeric organic materials. We envisage that homoleptic [Cu(ER)]∞ (E = S, Se) adopt a polymeric one-dimensional chain structure with spatially pre-organized Cu and E atoms, which could be useful precursors for the synthesis of copper(I) chalcogenides (Cu2E) with an ordered structure. Among the copper chalcogenides, binary Cu2Se is used for the synthesis of the widely acclaimed photovoltaic material, copper indium diselenide (CuInSe2),5 and has therefore attracted our attention. Despite copper(I) organoselenolates being well documented in the literature,6 structural reports on homoleptic copper(I) organoselenolates are sparse. This may be attributed to the low solubility of [Cu(SeR)]∞ in common solvents rendering it difficult to obtain quality crystals for X-ray diffraction study. Herein is described that [Cu(SeR)]∞ (R = Ph 1, Me 2, Ph-p-tBu 3) polymers have chain-like structures, as solved by using powder X-ray diffraction (XRD) data, and could be used as ‘single source precursors’ for the synthesis of phase-pure and homogeneously dispersed Cu2Se nanocrystals under mild conditions. In general, binary copper(I) selenide is prepared by mechanical alloying,5d electrodeposition,5e hydro/solvo-thermal syntheses,5f,gγ-irradiation,5h microwave-assisted heating5i or sonochemical methods.5j,k These methods mostly result in products of several polymorphic phases, as well as requiring special instrumentation, and in some cases high temperature or pressure. In this work, the synthetic method developed for Cu2Se nanocrystals is simple and inexpensive, involving no organic solvent and additive, and affords not only the common cubic7 crystal phase of Cu2Se but also a less common orthorhombic8 crystal phase of Cu2Se nanocrystals, both of which were obtained in high yields and purity. The charge-transporting properties of nanorods of [Cu(SePh)]∞ polymer have been investigated using an organic field effect transistor set-up, revealing that electrically stable semi-conducting polymeric organometallic materials with structurally defined architecture can be readily prepared by one-pot self-assembly reaction.
A solid sample of [Cu(SePh)]∞1 was prepared by refluxing a methanolic suspension of Cu2O with benzeneselenol, generated in situ from an acidified solution upon reduction of diphenyl diselenide in an alkaline solution.9‡ [Cu(SeMe)]∞2 and [Cu(SePh-p-tBu)]∞3 were prepared by similar methods with dimethyl diselenide and di(4-tert-butylphenyl) diselenide as starting materials, respectively. The [Cu(SeR)]∞ (R = Ph 1, Me 2, Ph-p-tBu 3) formulation is consistent with results of elemental analyses. All of the powder XRD patterns revealed that the as-obtained solid samples of 1, 2 and 3 were polycrystalline with no unreacted Cu2O. The powder X-ray diffraction pattern of 1 showed diffraction peaks with 2θ values of 7.347, 10.382, 14.659 and 16.396°, that match with the characteristic ratios of q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √2q
√2q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2q
2q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √5q where q = sin(2θ), or equivalent to indexed reflections [110], [200], [220] and [310], indicative of a primitive tetragonal crystal lattice. Fig. 1a and b depict the chain-like structure of [Cu(SePh)]∞ viewed along the [100] and [001] directions of the crystal structure, respectively, with the profile of the final cycle of Rietveld refinement of 1 shown in Fig. 1c. Each Cu atom adopts a distorted trigonal-planar geometry coordinated with three Se atoms of the phenylselenolate ligands. The distortion from a trigonal-planar geometry is caused by weak Cu⋯Se interactions (2.93(2) Å). The Cu–Se distances of 2.40(3)–2.41(1) Å are comparable to those of [Cu(2-Se-NC5H4)4] (2.360(1)–2.392(1) Å),6a [Me4N]2[Cu4(SePh)6] (2.371(1)–2.434(1) Å),10 [Cu16Se4(SePh)8(μ-1,4-Ph2PC6H4PPh2)4] (2.365(3)–2.700(3) Å)6c and [Cu73Se35(SePh)3(PiPr3)21] (2.354(8)–2.407(9) Å).11 The Se–Cu–Se angles of 94.87–125.43° are comparable to those of transition metal complexes with organoselenolate ligands.12 The chain structure of 1 reveals Cu⋯Cu distances of 2.49(2), 2.70(1), 2.93(2) Å, that could allow the presence of weak cuprophilic interactions similar to those present in the tetrametallic cluster of copper(I) pyridineselenolate.6a Intra-chain π⋯π stacking distances of 3.145–3.197 Å are found in 1, similar to that of [Cu(SPh)]∞.13 The structure of 2 (Fig. 1e and f) was also solved by using powder diffraction data (see ESI†). Despite this complex crystallizing in the orthorhombic space group of Pbam, it adopts a polymeric chain-like structure with Cu–Se and Cu⋯Cu distances of 2.36(3)–2.52(7) and 2.77(5)–2.89(6) Å, respectively, similar to that of 1. Replacement of the phenyl substituent in 1 by the 4-tert-butylphenyl group led to the formation of nano-sized rod-like crystals of 3. Indexing its powder XRD pattern gave a monoclinic cell (a = 19.875(10) Å, b = 6.856(4) Å, c = 16.258(6) Å, β = 111.65(4)°, V = 2058.8 Å3), that might accommodate two formula mass units of [Cu(SePh-p-tBu)] in the asymmetric unit. TEM images revealed that a solid sample of 1 contained phase-pure nanorods with width 34.7 ± 15.1 nm and length 624 ± 451 nm with an aspect ratio of 18
√5q where q = sin(2θ), or equivalent to indexed reflections [110], [200], [220] and [310], indicative of a primitive tetragonal crystal lattice. Fig. 1a and b depict the chain-like structure of [Cu(SePh)]∞ viewed along the [100] and [001] directions of the crystal structure, respectively, with the profile of the final cycle of Rietveld refinement of 1 shown in Fig. 1c. Each Cu atom adopts a distorted trigonal-planar geometry coordinated with three Se atoms of the phenylselenolate ligands. The distortion from a trigonal-planar geometry is caused by weak Cu⋯Se interactions (2.93(2) Å). The Cu–Se distances of 2.40(3)–2.41(1) Å are comparable to those of [Cu(2-Se-NC5H4)4] (2.360(1)–2.392(1) Å),6a [Me4N]2[Cu4(SePh)6] (2.371(1)–2.434(1) Å),10 [Cu16Se4(SePh)8(μ-1,4-Ph2PC6H4PPh2)4] (2.365(3)–2.700(3) Å)6c and [Cu73Se35(SePh)3(PiPr3)21] (2.354(8)–2.407(9) Å).11 The Se–Cu–Se angles of 94.87–125.43° are comparable to those of transition metal complexes with organoselenolate ligands.12 The chain structure of 1 reveals Cu⋯Cu distances of 2.49(2), 2.70(1), 2.93(2) Å, that could allow the presence of weak cuprophilic interactions similar to those present in the tetrametallic cluster of copper(I) pyridineselenolate.6a Intra-chain π⋯π stacking distances of 3.145–3.197 Å are found in 1, similar to that of [Cu(SPh)]∞.13 The structure of 2 (Fig. 1e and f) was also solved by using powder diffraction data (see ESI†). Despite this complex crystallizing in the orthorhombic space group of Pbam, it adopts a polymeric chain-like structure with Cu–Se and Cu⋯Cu distances of 2.36(3)–2.52(7) and 2.77(5)–2.89(6) Å, respectively, similar to that of 1. Replacement of the phenyl substituent in 1 by the 4-tert-butylphenyl group led to the formation of nano-sized rod-like crystals of 3. Indexing its powder XRD pattern gave a monoclinic cell (a = 19.875(10) Å, b = 6.856(4) Å, c = 16.258(6) Å, β = 111.65(4)°, V = 2058.8 Å3), that might accommodate two formula mass units of [Cu(SePh-p-tBu)] in the asymmetric unit. TEM images revealed that a solid sample of 1 contained phase-pure nanorods with width 34.7 ± 15.1 nm and length 624 ± 451 nm with an aspect ratio of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1d). Subsequent SAED study on individual nanocrystals of 1 revealed a d-spacing value of 2.05 Å, that is consistent with the [002] reflection derived from the X-ray powder diffraction data. Complex 2 crystallized as highly bundled microrods with width 73.8 ± 28.2 nm and length 2013 ± 1128 nm with an aspect ratio of 27
1 (Fig. 1d). Subsequent SAED study on individual nanocrystals of 1 revealed a d-spacing value of 2.05 Å, that is consistent with the [002] reflection derived from the X-ray powder diffraction data. Complex 2 crystallized as highly bundled microrods with width 73.8 ± 28.2 nm and length 2013 ± 1128 nm with an aspect ratio of 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Complex 3 crystallized as phase-pure microrods with width 157 ± 49 nm and length 5733 ± 2420 nm with an aspect ratio of 36
1. Complex 3 crystallized as phase-pure microrods with width 157 ± 49 nm and length 5733 ± 2420 nm with an aspect ratio of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (see Fig. S1–3, ESI†).
1 (see Fig. S1–3, ESI†).
![Perspective drawings of the 1-D structure of [Cu(SePh)]∞1 viewed along (a) the [100] direction and (b) the [001] direction. (c) Observed (red), calculated (green), and difference (magenta) profiles for the final cycle of Rietveld refinement of 1. Tick marks indicate peak positions of calculated Bragg reflections. (d) TEM images of 1 with magnification of 9900. The SAED pattern of an individual nanorod is shown as an inset. Perspective drawings of the 1-D structure of [Cu(SeMe)]∞2 viewed along (e) the [100] direction and (f) the [001] direction.](/image/article/2010/SC/c0sc00212g/c0sc00212g-f1.gif) | ||
| Fig. 1 Perspective drawings of the 1-D structure of [Cu(SePh)]∞1 viewed along (a) the [100] direction and (b) the [001] direction. (c) Observed (red), calculated (green), and difference (magenta) profiles for the final cycle of Rietveld refinement of 1. Tick marks indicate peak positions of calculated Bragg reflections. (d) TEM images of 1 with magnification of 9900. The SAED pattern of an individual nanorod is shown as an inset. Perspective drawings of the 1-D structure of [Cu(SeMe)]∞2 viewed along (e) the [100] direction and (f) the [001] direction. | ||
Results of thermogravimetric analysis (TGA) revealed low-temperature transformation reactions for complexes 1, 2 and 3 (decomposition temperature, Td = 165, 164 and 179 °C, respectively) with Td values lower than that of [Cu(SPh)]∞ (220 °C). Heating a solid sample of 1 under vacuum (4 × 10−4 Torr) at 180 °C for 2 h afforded phase-pure orthorhombic Cu2Se nanocrystals, the powder XRD pattern of which matches the JCPDS card 00-037-1187 (Fig. 2a). When the heating was conducted under nitrogen or argon at 180 °C for 2 h, black cubic Cu2Se crystals with a powder XRD pattern which matches that of JCPDS card 03-065-2982 (Fig. 2b) were obtained. When the thermolysis was conducted in air at 180 °C red Cu2O was obtained, as evidenced by matching its XRD pattern with JCPDS card 01-077-0199. Energy-dispersive X-ray spectroscopy (EDS) analysis and inductively coupled plasma mass spectrometry (ICP-MS) revealed that both the orthorhombic and cubic Cu2Se crystals have the same composition of copper and selenium with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio being observed.14 Thermolyses of 2 and 3 under vacuum at 180 °C also gave Cu2Se as confirmed by the results of XRD (Fig. 2c and d, respectively) and EDS. In the case of 2, a mixture of the orthorhombic and cubic phase of Cu2Se was observed together with Cu3Se2 (umangite) as a minor phase. The mean crystallite sizes of Cu2Se prepared from the thermolysis of 1 under vacuum, of 1 under Ar, of 2 under vacuum, and of 3 under vacuum were found to be 207.8, 227.3, 176.5 and 182.0 nm, respectively, by using the Scherrer formula.15
1 ratio being observed.14 Thermolyses of 2 and 3 under vacuum at 180 °C also gave Cu2Se as confirmed by the results of XRD (Fig. 2c and d, respectively) and EDS. In the case of 2, a mixture of the orthorhombic and cubic phase of Cu2Se was observed together with Cu3Se2 (umangite) as a minor phase. The mean crystallite sizes of Cu2Se prepared from the thermolysis of 1 under vacuum, of 1 under Ar, of 2 under vacuum, and of 3 under vacuum were found to be 207.8, 227.3, 176.5 and 182.0 nm, respectively, by using the Scherrer formula.15
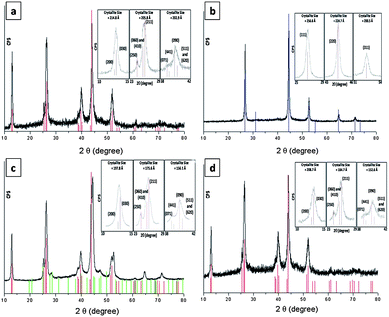 | ||
| Fig. 2 PXRD patterns of solid samples upon thermolysis of (a) 1 under vacuum, (b) 1 under Ar, (c) 2 under vacuum and (d) 3 under vacuum. Color sticks are authentic diffractions of 00-037-1187 (red), 03-065-2982 (blue) and 00-47-1745 (green) as taken from the JCPDS database. Selected major diffractions were modeled by pseudo-Voigt functions for crystallite size calculations (inset). | ||
The conditions at which 1 is thermolyzed are crucial to the phase-purity, particle size and morphology of the as-formed Cu2Se solid. TEM images of Cu2Se nanocrystals from thermolysis of 1 under vacuum, N2, Ar or air at 180 °C for 2 h are given in Fig. S4, ESI†). Cu2Se nanoparticles with number average diameter (dnumber) 33.0 ± 13.0 nm were formed when 1 was heated at 180 °C in N2 for 2 h. Under Ar flow with the same temperature and thermolysis time, Cu2Se nanocrystals with similar sizes (dnumber = 38.2 ± 12.0 nm) but irregular-shaped morphology were obtained, and at 350 °C under Ar, larger Cu2Se aggregates (dnumber = 57.0 ± 15.8 nm) were formed. On the contrary, thermolysis of Cu-containing polymer chains such as [Cu2(acetate)4(L)]∞ (L = 2,5-disubstituted 1,3,4-oxadiazole),16 [Cu(L)(dmb)][BF4]∞ (L = diphosphine ligand; dmb = 1,8-diisocyano-p-menthane)17 and [Cu(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)]∞18 under N2 atmosphere has been reported to give Cu, Cu2O or CuO as the major solid residue. The differences in measured sizes between PXRD and TEM are reasoned by the fact that the size obtained by using X-ray methods is the effective length, measured in the direction of the diffraction vector, along which diffraction is coherent.15b Since the Cu2Se particles are made up of several crystallites with different domains, the particle sizes as obtained from TEM are consistently larger than determined from PXRD. The yellow vapor evolved during thermolysis of 1 at 180 °C in N2 was collected using a cold-finger apparatus. The major organic product was Ph2Se mixed with a minor amount of Ph2Se2 (<10%). Based on the results of PXRD, NMR and GC-MS analyses (Fig. S8, ESI†), we propose the thermolysis reaction of complex 1 is as follows:
CPh)]∞18 under N2 atmosphere has been reported to give Cu, Cu2O or CuO as the major solid residue. The differences in measured sizes between PXRD and TEM are reasoned by the fact that the size obtained by using X-ray methods is the effective length, measured in the direction of the diffraction vector, along which diffraction is coherent.15b Since the Cu2Se particles are made up of several crystallites with different domains, the particle sizes as obtained from TEM are consistently larger than determined from PXRD. The yellow vapor evolved during thermolysis of 1 at 180 °C in N2 was collected using a cold-finger apparatus. The major organic product was Ph2Se mixed with a minor amount of Ph2Se2 (<10%). Based on the results of PXRD, NMR and GC-MS analyses (Fig. S8, ESI†), we propose the thermolysis reaction of complex 1 is as follows:
| 2[Cu(SePh)] → [Cu2Se] + Ph2Se↑ | (1) |
The optical band gap of 1 in the solid state was found to be 2.23 eV by diffuse reflectance measurement from a plot of {hν![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln[(Rmax−Rmin)/(R−Rmin)]}2 against hν and extrapolating the linear region of the curve to the x-axis.19 At room temperature, polymer 1 is weakly emissive (λmax = 715 nm) in the solid state, but displays an intense emission with λmax = 707 nm at 77 K. At both temperatures, the emission λmax of 1 is slightly red-shifted from that of [Cu(SPh)]∞ (λmax = 650 and 640 nm at room temperature and 77 K, respectively, Fig. S9, ESI†). We tentatively assign the emission to a triplet excited state having mixed metal-centered 3d→(4s, 4p) and ligand to metal Se → Cu(I) character.20 Since both 1 and [Cu(SPh)]∞ polymers13 display similar chain-like structures with comparable intra-chain π⋯π distances, replacement of the heteroatoms (S → Se) of the 1-D polymer lowers the HOMO–LUMO energy gap of the ligand-to-metal charge-transfer transition, leading to a red-shift in emission energy from 2.59 to 2.23 eV (see Fig. S9, ESI†).13
ln[(Rmax−Rmin)/(R−Rmin)]}2 against hν and extrapolating the linear region of the curve to the x-axis.19 At room temperature, polymer 1 is weakly emissive (λmax = 715 nm) in the solid state, but displays an intense emission with λmax = 707 nm at 77 K. At both temperatures, the emission λmax of 1 is slightly red-shifted from that of [Cu(SPh)]∞ (λmax = 650 and 640 nm at room temperature and 77 K, respectively, Fig. S9, ESI†). We tentatively assign the emission to a triplet excited state having mixed metal-centered 3d→(4s, 4p) and ligand to metal Se → Cu(I) character.20 Since both 1 and [Cu(SPh)]∞ polymers13 display similar chain-like structures with comparable intra-chain π⋯π distances, replacement of the heteroatoms (S → Se) of the 1-D polymer lowers the HOMO–LUMO energy gap of the ligand-to-metal charge-transfer transition, leading to a red-shift in emission energy from 2.59 to 2.23 eV (see Fig. S9, ESI†).13
A bottom contact field-effect transistor (FET) using nanorods of 1 as an active layer has been fabricated (Fig. 3). The drain-source current (IDS) increases negatively with the negative gate voltages (VG), indicative of a p-type field effect transistor behaviour. The charge mobility (μ) of the device calculated at the linear regime where VDS≪VG is 4 × 10−2 cm2 V−1 s−1, which is comparable with FET devices fabricated with the well-studied regioregular P3HT (μhole = 1 × 10−1 cm2 V−1 s−1).21 In addition, we have performed transient measurements to study the electrical stability of nanorods of 1. In literature, the channel current of field effect transistor devices with organic semiconductors as active layers were reported to show significant decay upon device bias.22a In this work, the devices based on the nanorods of 1 showed no decay in the channel current upon prolonged device operation for 260 min or more, revealing the electrical stability of the polymer 1. On the contrary, for a FET device made with poly[2-methoxy-5-(2′-ethylhexyloxy)-1,4-phenylene vinylene] (MEH-PPV) as the active layer, IDS was reduced by more than 30% in 90 min (Fig. 3). This is, to the best of our knowledge, the first ever reported electrically stable and semiconducting organometallic polymer.22 We have also performed electrical measurements on orthorhombic and cubic Cu2Se nanocrystals using the FET set-up. For cubic Cu2Se, a huge channel current, up to 10 mA for 40 mV at the drain, with no field effect, was observed. This behavior is attributed to the ionic conducting nature of cubic Cu2Se.23 On the other hand, the orthorhombic Cu2Se obtained from the same complex 1 exhibited semi-conducting properties but with a low field effect mobility (< 10 −6) (see Fig. S10 and S11, ESI†).24
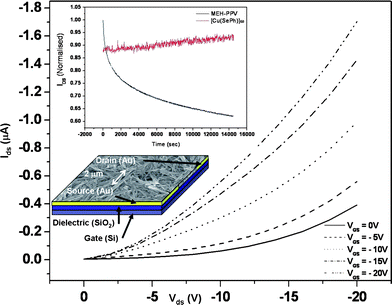 | ||
| Fig. 3 Output characteristics of the FET device constructed from 1 as the active layer. The inset shows the transient measurements of the devices made from nanorods 1 (red) and MEH-PPV (black) as active layer with gate and drain voltages fixed at −5 V. An SEM image and the schematic structure of the FET device are also shown. | ||
In summary, the 1-D structure of complex 1 has been revealed by using powder X-ray diffraction data. The 1-D chain polymer with pre-organized Cu and Se atoms can be used for the synthesis of phase-pure orthorhombic or cubic phases of Cu2Se nanocrystals under mild conditions. The well-defined array of Cu and Se atoms and low thermal stability of this polymer are critical for the successful preparation of Cu2Se nanoparticles of high purity under mild conditions. Selective synthesis of different crystal phases of the as-formed Cu2Se nanoparticles in this work reveals the usefulness of crystalline metal–organic polymers as single-source precursors for the synthesis of nanostructured metal selenides.
Acknowledgements
This work was supported by a grant from the University Grants Committee of the Hong Kong Special Administrative Region, China (Project No. [AoE/P-03/08]).Notes and references
- D. Tanaka, K. Nakagawa, M. Higuchi, S. Horike, Y. Kubota, T. C. Kobayashi, M. Takata and S. Kitagawa, Angew. Chem., Int. Ed., 2008, 47, 3914 CrossRef CAS
.
-
(a) P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Férey, P. Coureur and R. Gref, Nat. Mater., 2009, 1, 1
; (b) Q. Li, W. Zhang, O.Š. Miljanić, C.-H. Sue, Y.-L. Zhao, L. Liu, C. B. Knobler, J. F. Stoddart and O. M. Yaghi, Science, 2009, 325, 855 CrossRef CAS
.
-
(a) T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973 CrossRef CAS
; (b) N. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127 CrossRef CAS
.
- B. Kesanli and W. Lin, Coord. Chem. Rev., 2003, 246, 305 CrossRef CAS
.
-
(a) B. Tell, P. M. Bridenbaugh and H. M. Kasper, J. Appl. Phys., 1976, 47, 619 CrossRef CAS
; (b) W. S. Chen, J. M. Stewart and R. A. Mickelsen, Appl. Phys. Lett., 1985, 46, 1095 CrossRef CAS
; (c) S. Niki, P. J. Fons, A. Yamada, Y. Lacroix, H. Shibata, H. Oyanagi, M. Nishitani, T. Negami and T. Wada, Appl. Phys. Lett., 1999, 74, 1630 CrossRef CAS
; (d) T. Ohtani, M. Motoki, K. Koh and K. Ohshima, Mater. Res. Bull., 1995, 30, 1495 CrossRef CAS
; (e) R. N. Bhattacharya, A. M. Fernandez, M. A. Contreras, J. Keane, A. L. Tennant, K. Ramanathan, J. R. Tuttle, R. N. Noufi and A. M. Hermann, J. Electrochem. Soc., 1996, 143, 854 CAS
; (f) W. Wang, Y. Geng, P. Yan, F. Liu, Y. Xie and Y. Qian, J. Am. Chem. Soc., 1999, 121, 4062 CrossRef CAS
; (g) W. Wang, P. Yan, F. Liu, Y. Xie, Y. Geng and Y. Qian, J. Mater. Chem., 1998, 8, 2321 RSC
; (h) Z. Qiao, Y. Xie, J. Xu, X. Liu, Y. Zhu and Y. Qian, Can. J. Chem., 2000, 78, 1143 CrossRef CAS
; (i) J. Zhu, O. Palchik, S. Chen and A. Gedanken, J. Phys. Chem. B, 2000, 104, 7344 CrossRef CAS
; (j) Y. Xie, X. Zheng, X. Jiang, J. Lu and L. Zhu, Inorg. Chem., 2002, 41, 387 CrossRef CAS
; (k) S. Xu, H. Wang, J.-J. Zhu and H.-Y. Chen, J. Cryst. Growth, 2002, 234, 263 CrossRef CAS
.
-
(a) Y. Cheng, T. J. Emge and J. G. Brennan, Inorg. Chem., 1996, 35, 7339 CrossRef CAS
(this is the first publication involving structurally characterized molecular homoleptic copper selenolate); (b) M. Bettenhausen, A. Eichhöfer, D. Fenske and M. Semmelmann, Z. Anorg. Allg. Chem., 1999, 625, 593 CrossRef CAS
; (c) S. Dehnen, A. Eichhöfer and D. Fenske, Eur. J. Inorg. Chem., 2002, 279 CrossRef
; (d) M. Semmelmann, D. Fenske and J. F. Corrigan, J. Chem. Soc., Dalton Trans., 1998, 2541 RSC
.
- A. Tonejc, J. Mater. Sci., 1980, 15, 3090 CrossRef CAS
.
- O. C. Kopp and O. B. Cavin, J. Cryst. Growth, 1984, 67, 391 CrossRef CAS
.
- T. G. Back, S. Collins, M. V. Krishna and K. W. Law, J. Org. Chem., 1987, 52, 4258 CrossRef CAS
.
- X. Jin, K. Tang, Y. Long and Y. Tang, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 1799 CrossRef
.
- N. Zhu and D. Fenske, J. Chem. Soc., Dalton Trans., 1999, 1067 RSC
.
- S. G. Murray and F. R. Hartley, Chem. Rev., 1981, 81, 365 CrossRef CAS
.
- C.-M. Che, C.-H. Li, S. S.-Y. Chui, V. A. L. Roy and K.-H. Low, Chem.–Eur. J., 2008, 14, 2965 CrossRef CAS
.
- Orthorhombic Cu2Se: 62.61 wt% Cu, 37.39 wt% Se; cubic Cu2Se: 62.53 wt% Cu, 37.47 wt% Se. These values correspond to a Cu
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se ratio of 2.07–2.08
Se ratio of 2.07–2.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Inductively coupled plasma mass spectrometry (ICP-MS) analysis showed that a 5.39 mg sample of the orthorhombic Cu2Se nanocrystals contained 3.34 mg of copper; 0.6% more than the theoretical value of 61.68% (3.32 mg) for a formula of Cu2Se.
1. Inductively coupled plasma mass spectrometry (ICP-MS) analysis showed that a 5.39 mg sample of the orthorhombic Cu2Se nanocrystals contained 3.34 mg of copper; 0.6% more than the theoretical value of 61.68% (3.32 mg) for a formula of Cu2Se. -
(a) P. Scherrer, Nachr. Ges. Wiss. Göttingen, 1918, 26, 98 Search PubMed
; (b) J. I. Langford and A. J. C. Wilson, J. Appl. Crystallogr., 1978, 11, 102 CrossRef CAS
. The pseudo-Voigt function fitting was performed with the Bruker DIFFRACplus EVA program.
- Y.-B. Dong, J.-P. Ma, M. D. Smith, R.-Q. Huang, B. Tang, D. Chen and H.-C. zur Loye, Solid State Sci., 2002, 4, 1313 CrossRef CAS
.
- E. Fournier, F. Lebrun, M. Drouin, A. Decken and P. D. Harvey, Inorg. Chem., 2004, 43, 3127 CrossRef CAS
.
- S. S. Y. Chui, M. F. Y. Ng and C.-M. Che, Chem.–Eur. J., 2005, 11, 1739 CrossRef CAS
.
- V. Kumar, S. K. Sharma, T. P. Sharma and V. Singh, Opt. Mater., 1999, 12, 115 CrossRef CAS
.
- V. W.-W. Yam, K. K.-W. Lo and K.-K. Cheung, Inorg. Chem., 1996, 35, 3459 CrossRef CAS
.
- B. Ong, Y. Wu, L. Jiang, P. Liu and K. Murti, Synth. Met., 2004, 142, 49 CrossRef CAS
.
-
(a) V. Podzorov, E. Menard, A. Borissov, V. Kiryukhin, J. A. Rogers and M. E. Gershenson, Phys. Rev. Lett., 2004, 93, 086602 CrossRef CAS
; (b) V. A. L. Roy, Y.-G. Zhi, Z.-X. Xu, S.-C. Yu, P. W. H. Chan and C.-M. Che, Adv. Mater., 2005, 17, 1258 CrossRef CAS
; (c) P. Stallinga, H. L. Gomes, F. Biscarini, M. Murgia and D. M. de Leeuw, J. Appl. Phys., 2004, 96, 5277 CrossRef CAS
; (d) H. L. Gomes, P. Stallinga, F. Dinelli, M. Murgia, F. Biscarini, D. M. de Leeuw, M. Muccini and K. Müllen, Polym. Adv. Technol., 2005, 16, 227 CrossRef CAS
.
- M. B. Solomon, in Physics of Superionic Conductors, Springer-Verlag, Berlin, Germany 1979, p. 175 Search PubMed
.
-
(a) R. S. Mane, S. P. Kajve, C. D. Lokhande and S.-H. Han, Vacuum, 2006, 80, 631 CrossRef CAS
; (b) S. K. Haram and K. S. V. Santhanam, J. Electroanal. Chem., 1995, 396, 63 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of instrumentation, structure refinements, TEM images, FET device fabrication and charge mobility measurements. CCDC reference number 655620 (1). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00212g |
‡ Synthesis of [Cu(SePh)]∞1: Diphenyl diselenide (PhSeSePh, 0.718 g, 2.00 mmol) was added to a water–methanol mixture (60 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) of NaOH (0.092 g, 2.3 mmol) and NaBH4 (0.087 g, 2.3 mmol). The resulting suspension was heated until a clear solution was obtained. Upon cooling to room temperature, the reaction mixture was acidified to pH ∼ 5–6 using concentrated hydrochloric acid (12 M). Cu2O (0.289 g, 2.00 mmol) was added to the stirred reaction mixture, which was then heated to ca. 60 °C for 72 h. The resulting yellow suspension was filtered and the solid was washed with water, methanol, acetone, dichloromethane and diethyl ether. Yield: 82% (0.72 g). When [Cu(MeCN)4]PF6 was used, the same reaction product [Cu(SePh)]∞ but of poor crystallinity was formed. 1H NMR data for the yellow vapor evolved during thermolysis of [Cu(SePh)]∞ at 200 °C (CDCl3): δ 7.24–7.37 (m, 10H, PhSePh), 7.46–7.52 (m, 6 meta- and para-H, PhSeSePh), 7.61–7.65 (m, 4 ortho-H, PhSeSePh). Anal. (%). Calc. for C6H5CuSe (Mr 219.6): C 32.80, H 2.28. Found: C 32.69, H 2.34. 1 v/v) of NaOH (0.092 g, 2.3 mmol) and NaBH4 (0.087 g, 2.3 mmol). The resulting suspension was heated until a clear solution was obtained. Upon cooling to room temperature, the reaction mixture was acidified to pH ∼ 5–6 using concentrated hydrochloric acid (12 M). Cu2O (0.289 g, 2.00 mmol) was added to the stirred reaction mixture, which was then heated to ca. 60 °C for 72 h. The resulting yellow suspension was filtered and the solid was washed with water, methanol, acetone, dichloromethane and diethyl ether. Yield: 82% (0.72 g). When [Cu(MeCN)4]PF6 was used, the same reaction product [Cu(SePh)]∞ but of poor crystallinity was formed. 1H NMR data for the yellow vapor evolved during thermolysis of [Cu(SePh)]∞ at 200 °C (CDCl3): δ 7.24–7.37 (m, 10H, PhSePh), 7.46–7.52 (m, 6 meta- and para-H, PhSeSePh), 7.61–7.65 (m, 4 ortho-H, PhSeSePh). Anal. (%). Calc. for C6H5CuSe (Mr 219.6): C 32.80, H 2.28. Found: C 32.69, H 2.34. |
| This journal is © The Royal Society of Chemistry 2010 |
