UV absorbing zwitterionic pyridinium-tetrazolate: exceptional transparency/optical nonlinearity trade-off†‡
Luca
Beverina
*a,
Alessandro
Sanguineti
a,
Glauco
Battagliarin
a,
Riccardo
Ruffo
a,
Dominique
Roberto
b,
Stefania
Righetto
b,
Raffaella
Soave
c,
Leonardo
Lo Presti
d,
Renato
Ugo
b and
Giorgio A.
Pagani
*a
aDipartimento di Scienzadei Materiali and INSTM, Università di Milano-Bicocca, via R. Cozzi 53, 20125, Milano, Italy. E-mail: luca.beverina@unimib.it
bDipartimento di Chimica Inorganica, Metallorganica e Analitica “L. Malatesta” dell’, Università degli Studi di Milano and UdR INSTM di Milano, via Venezian 21, 20133 Milano, Italy
cIstituto di Scienze e Tecnologie Molecolari (CNR-ISTM), Via Golgi 19, 20133 Milano, Italy
dDipartimento di Chimica Fisica ed Elettrochimica, Università degli Studi di Milano, Via Golgi 19, 20133 Milano, Italy
First published on 20th August 2010
Abstract
We present relevant results dealing with the transparency/optical nonlinearity trade-off in high-frequency electro-optic applications. The very simple, stable and high optical gap chromophore, the zwitterion 1-methyl-4-(tetrazol-5-ate)pyridinium, represents the best transparency/optical nonlinearity trade-off so far described in the literature. We rationalize this remarkable performance in the framework of the Bond Length Alternation theory by means of a multidisciplinary approach including: single crystal X-ray structure, Electric Field Induced Second-Harmonic Generation, solvatochromism, electrochemistry and thermal analyses.
The quest for high-performance molecule-based electro-optic (EO) materials is still a very active research field.1 Despite the two decades so far devoted to the development of impressive molecular and supramolecular materials,2 a few key issues—particularly regarding the transparency/optical nonlinearity trade-off and overall stability—still prevent this active research field to turn into a mature technology.3 The essential requirement for large bulk EO activity is the design of suitable chromophores possessing a large microscopic first hyperpolarizability (β).2 To date, the vast majority of performing EO chromophores obey a few general structural requirements: they are planar conjugated structures end capped with strong electron-donor (D) and electron-acceptor (A) residues leading to effective polarization along the π-conjugated axis.4 This design strategy, well rationalized in the framework of the Bond-Length-Alternation (BLA) theory proposed by Marder et al. in the early nineties,5 enabled over the years the production of a plethora of highly performing materials, eventually leading to the impressive bulk materials performances obtained by Jen and Dalton.2,6
Recently Marks and Facchetti succeeded in the experimental verification of the theoretical prediction of Marks and Ratner dealing with “tictoid” twisted π-electron systems.7 These derivatives have gigantic, order of magnitude enhanced, molecular hyperpolarizabilities, thus contributing to the recent interest renewal in organic nonlinear optics.1
Restricting to the definitively more common D–A strategy, it is important to note that efforts are primarily focused on the design of extensive planar π-conjugated derivatives possessing intrinsically complex structures.
Main connected drawbacks are an often challenging synthetic access and an intrinsically low optical gap, strongly eroding transparency and often stability.3 Marks et al. reported on the interesting X-shaped chromophores strategy to the transparency/nonlinearity tradeoff.3 This approach is successful, but limited by a somewhat complex chromophore structure.
In this contribution we introduce a very simple, stable and high optical gap chromophore, the pyridinium-tetrazolate 1. This molecule exploits the well documented accepting capabilities of the pyridinium ring together with the so far unexploited donating nature of the tetrazolate nitranion. The absence of a conjugated bridge ensures a higher chemical stability with respect to standard D-π-A dyes. In addition, derivative 1 synthetic access is exceedingly simple.
We optimized the original procedure (Scheme 1, route a)9 by reacting the potassium salt of 2 with CH3I in MeOH at 130 °C under microwave irradiation in a pressurized vessel (Scheme 1, route b). The product precipitates already as analytical sample.
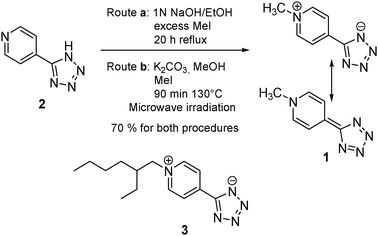 | ||
| Scheme 1 Synthesis of zwitterions 1 and 3. | ||
We show derivative 1 in both the allowed canonical representations: the zwitterionic (top) and the quinoidal (bottom). A slight modification of the above procedure enabled the synthesis of the ethylhexyl-functionalized derivative 3 we exploited for optical characterization (see ESI‡).
The top part of Fig. 1 shows the linear absorption spectra of 3 in selected solvents. Coherently with its very small conjugation length, the chromophore possesses a high optical gap. The absorption band cut-off barely reaches 400 nm. In contrast with what is commonly observed, this high optical gap is characterized by a strongly solvatochromic behavior.10 Interestingly, the inset of Fig. 1 shows that 3 solvatochromism is not monotonous, with a transition between a positive and a negative regime for ET(30)8 values around 38 kcal mol−1.
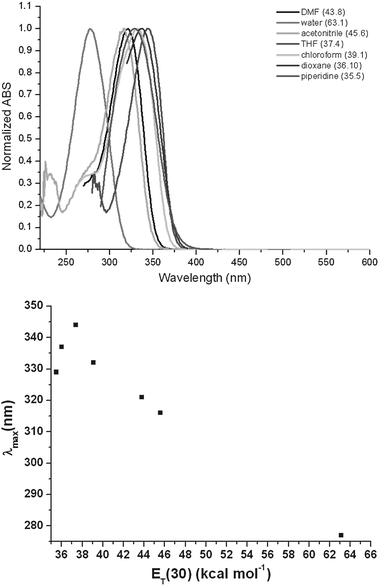 | ||
| Fig. 1 Top: derivative 3UV-Vis absorption spectra in selected solvents. Bottom: correlation between λmax and ET(30) empirical parameter8 (reported in brackets in the top figure) for the employed solvents. | ||
This result can be rationalized assuming a dominant zwitterionic ground state in polar solvents and a quinoidal structure in very low ET(30) ones, passing through a cyanine-like form for ET(30) values close to that of THF. According to the BLA model, highest negative β values are expected for molecules possessing an electronic structure intermediate between a localized charge distribution (zwitterionic limit) and the delocalized cyanine limit. Common DA molecules can only span limited portions of the BLA plot, and although changes in the sign of the first hyperpolarizabilities as a consequence of solvent effects are known, they are usually limited to small deviations around the cyanine limit.11 The solvatochromic results we observed for 3 suggested the possibility to access the whole BLA plot thus reaching the highest hyperpolarizability limit in solvents possessing an ET(30) value intermediate between that of THF (cyanine limit) and water (zwitterionic limit). Indeed, derivative 3 displays the surprisingly large μβ1.907 value of −2010 × 10−48 esu as determined by the EFISH technique in DMF working with a non-resonant incident wavelength of 1.907 μm.
Although not particularly large in general, this value becomes really surprising once referred to the very large optical gap of 3. Its value is in fact considerably larger than that of the commonly accepted benchmark for SHG capabilities, 4-(N,N-dimethylamino)-4′-nitrostilbene (DANS), for which μβ1.907 = 446–580 × 10−48 esu and λmax = 420–450 nm.12 Moreover both 1 and 3 show excellent photochemical and chemical stabilities along with remarkable thermal stability (see ESI‡ for DSC-TGA traces). In this respect, the use of a nitranionic donating group was intentionally aimed at the stabilization towards ambient oxidation, the main drawback of the carbanion-based zwitterionic derivatives some of us described in the past.11
The crystal structure of 1 is key to understand the observed remarkable solvatochromism and nonlinear optical performances. We have successfully grown very large X-ray quality single crystals of 1 through the controlled cooling from 130° C to room temperature of an oversaturated, pressurized methanol solution of 1. The molecular structure features an almost complete co-planarity between the tetrazole and pyridine rings, the torsion angle between the two being 3.5° only (Fig. 2a). The solid state packing diagram (Fig. 2b) shows crystallization in antiparallel pairs within a monoclinic C2/c space group. The inter-ring distance C1–C2 (1.455(2) Å) is significantly shorter than that observed in typical biaryls (∼1.487 Å),13 an evident sign of a very strong conjugation between Donating and Accepting rings.14 Nonetheless, the atomic distances of the tetrazolate portion are very similar to those reported for a series of tetrazolate salts.15 In short, the crystal structure shows that the D and A portions of 1 are very strongly conjugated. The charge-separated structure is stabilized by the cofacial intramolecular pyridinium-tetrazolate interaction. In solution the contribution of the zwitterionic structure depends upon the specific solvent Reichardt parameter.8 In particular in DMF and CH3CN, the structure still behaves as a zwitterion as it is also confirmed by cyclic voltammetry. The CV plot of 1CH3CN solution (Fig. 3) shows in fact one strong irreversible reduction peak at −1.34 eV vs. Fc/Fc+ coherently with the reduction of a pyridinium salt having an electron donating substituent (no additional electrochemical process was observed at more positive potentials).16
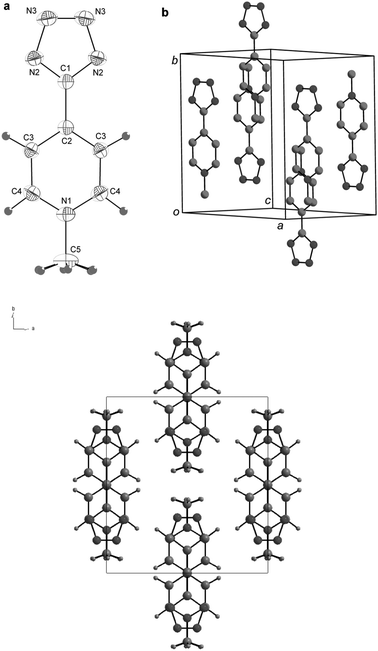 | ||
| Fig. 2 X-Ray single crystal structure of derivative 1 (298 K). (a) ORTEP plot with atom numbering scheme; (b) packing diagram. | ||
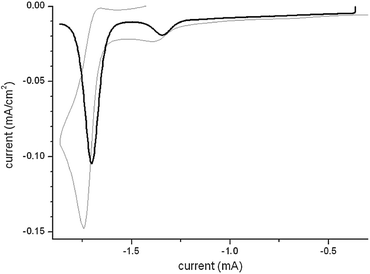 | ||
| Fig. 3 Cyclic Voltammetry (gray) and Differential Pulse Voltammetry (black) curves for a 0.1 M solution of tetrabutylammonium p-toluenesulfonate in CH3CN of derivative 1. | ||
We speculate that the particularly large βμ value we measured is a consequence of the capability to effectively reach the optimum BLA value in a structure that, thanks to the very efficient DA conjugation evidenced by the X-ray structure, enables the complete span of the BLA plot.
In summary, we designed and easily synthesized a very simple zwitterionic, nitranion end capped dipolar chromophore of low molecular weight. The derivative possesses large Second Harmonic Generation capabilities (μβ1.907 = −2010 × 10−48 esu, DMF) together with unprecedented high optical gap enabling an excellent nonlinearity-transparency trade-off. By a careful choice of the donor, we also provided the zwitterions with high thermal, chemical and photochemical stabilities. Derivative 1 crystallizes in a centrosymmetric fashion, thus excluding the use of crystals as bulk NLO materials based on 1. However, studies are currently in progress in order to grow ordered films based on suitable functionalization derivatives of these very promising chromophores. In fact, we already verified in the past that it is possible to grow NLO active thin films based on strongly dipolar chromophores according to both solution17 and vacuum18 strategies.
Notes and references
- S. R. Marder, J. Mater. Chem., 2009, 19, 7392 RSC.
- J. Luo, X.-H. Zhou and A. K.-Y. Jen, J. Mater. Chem., 2009, 19, 7410 RSC.
- H. Kang, P. Zu, Y. Yang, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2004, 126, 15974 CrossRef CAS and references therein.
- J. A. Davies, A. Elangovan, P. A. Sullivan, B. C. Olbricht, D. H. Bale, T. R. Ewy, C. M. Isborn, B. E. Eichinger, B. H. Robinson, P. J. Reid, X. Li and L. R. Dalton, J. Am. Chem. Soc., 2008, 130, 10565 CrossRef CAS.
- S. R. Marder, B. Kippelen, A. K.-Y. Jen and N. Peyghambarian, Nature, 1997, 388, 845 CrossRef CAS.
- S. J. Benight, D. H. Bale, B. C. Olbricht and L. R. Dalton, J. Mater. Chem., 2009, 19, 7466 RSC.
- H. Kang, A. Facchetti, H. Jiang, E. Cariati, S. Righetto, R. Ugo, C. Zuccaccia, A. Macchioni, C. L. Stern, Z. Liu, S.-T. Ho, E. C. Brown, M. A. Ratner and T. J. Marks, J. Am. Chem. Soc., 2007, 129, 3267 CrossRef CAS.
- C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH, 2003 Search PubMed.
- W. Holzer and C. Jäger, Monatsh. Chem., 1992, 123, 1027 CrossRef CAS.
- L. Oudar and D. S. Chemla, J. Chem. Phys., 1977, 66, 2664 CrossRef CAS.
- A. Abbotto, L. Beverina, S. Bradamante, A. Facchetti, C. Klein, G. A. Pagani, M. Redi-Abshiro and R. Wortmann, Chem.–Eur. J., 2003, 9, 1991 CrossRef CAS.
- L. R. Dalton, A. W. Harper, R. Ghosn, W. H. Steier, M. Ziari, H. Fetterman, Y. Shi, R. V. Mustacich, A. K.-Y. Jen and K. J. Shea, Chem. Mater., 1995, 7, 1060 CrossRef CAS.
- Database of average bond lengths in organic compounds: F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1 Search PubMed.
- Selected bond distances: C1–N2 1.3312(14); C1–C2 1.455(2); C2–C3 1.3887(17); C3–C4 1.367(2); C3–H3 0.978(15); C4–N1 1.3390(17); C4–H4 0.997(17); C5–N1 1.477(3); C5–H5A 1.05(4); C5–H5B 1.04(4); C5–H5C 0.98(4); N2–N3 1.3406(17). Selected bond angles: N2–C1–N2 111.99(16); N2–C1–C2 124.01(8); C3–C2–C3 117.39(18); C3–C2–C1 121.30(9); C4–C3–C2 120.31(14); C4–C3–H3 118.5(8); C2–C3–H3 121.2(8); N1–C4–C3 120.73(14); N1–C4–H4 115.8(9); C3–C4–H4 123.5(9); N1–C5–H5A 107.4(19); N1–C5–H5B 106.0(18); H5A–C5–H5B 109(2); N1–C5–H5C 106(2); H5A–C5–H5C 113(2); H5B–C5–H5C 115(2); C4–N1–C4 120.54(17); C4–N1–C5 119.73(9); C1–N2–N3 104.55(12); N3–N3–N2 109.46(7).
- T. M. Klapötke, M. Stein and J. Stierstorfer, Z. Anorg. Allg. Chem., 2008, 634, 1711 CrossRef.
- S. Fukuzumi, O. Inada, N. Satoh, T. Suenobu and H. Imahori, J. Am. Chem. Soc., 2002, 124, 9181 CrossRef CAS.
- A. Facchetti, M. E. Van Der Boom, A. Abbotto, L. Beverina, T. J. Marks and G. A. Pagani, Langmuir, 2001, 17, 5939–5942 CrossRef CAS.
- A. Facchetti, E. Annoni, L. Beverina, M. Morone, P. Zhu, T. J. Marks and G. A. Pagani, Nat. Mater., 2004, 3, 910–917 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm |
| ‡ Electronic supplementary information (ESI) available: Synthetic and characterization details for the preparation of derivatives 1 and 3. DSC-TGA traces. Details of the nonlinear optical characterization. CCDC 779200. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc01652g |
| This journal is © The Royal Society of Chemistry 2011 |
