Structural and theoretical insights into the perturbation of uranium–rhenium bonds by dative Lewis base ancillary ligands†‡
Dipti
Patel
,
David M.
King
,
Benedict M.
Gardner
,
Jonathan
McMaster
,
William
Lewis
,
Alexander J.
Blake
and
Stephen T.
Liddle
*
School of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. E-mail: stephen.liddle@nottingham.ac.uk; Fax: +44 (0)115-951-3563; Tel: +44 (0)115-846-7167
First published on 5th August 2010
Abstract
Amine-elimination gave the two uranium–rhenium complexes [(TsXy)(THF)nURe(η5-C5H5)2] [TsXy = HC(SiMe2N-3,5-Me2C6H3)3; n = 0 or 1]; structural and theoretical analyses, and comparison to [(TrenTMS)URe(η5-C5H5)2] [TrenTMS = N(CH2CH2NSiMe3)3], reveal an increasing σ-component to the U–Re bond upon removal of dative ancillary ligands from uranium with the π-component remaining essentially invariant.
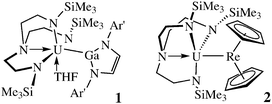
Understanding the nature and reactivity of metal–metal bonds is fundamentally important.1 Metal–metal bonds involving d- and p-block elements are widespread and well understood, and even examples from the s-block are known.2 However, despite the tendency of metal–metal complexes to display novel bonding and reactivity, complexes containing uranium–metal bonds are scarce.3–5 Structurally characterised uranium–metal bonds were previously limited to the p-block derivatives [{(Ar)(But)N}3USi(SiMe3)3] (Ar = 3,5-Me2C6H3),6 [(η5-C5H5)3USnPh3],7 and [(η5-C5H4SiMe3)3UAl(η5-C5Me5)].8
We have been investigating the chemistry of tripodal triamido uranium complexes,9 and have employed Tren-based ligands to isolate the U–Ga complex [(TrenTMS)(THF)UGa(NAr′CH)2] [1, Ar′ = 2,6-Pri2C6H3; TrenTMS = N(CH2CH2NSiMe3)3];10 and the U–Re complex [(TrenTMS)URe(η5-C5H5)2] (2).11 Both of these complexes are notable because they exhibit σ- and π-components within the U–M bonds. Complex 2 was the first, and remains the only, structurally characterised uranium–transition metal (U–TM) bond.4 Little is known of the strength and nature of such bonds, but this information is vital to exploit any intrinsic reactivity patterns associated with uranium–metal bonds.
Ligand design is of paramount importance to designing well defined uranium complexes. We have therefore sought to employ a range of tripodal ligands to support U–M bonds. We recently reported tris(N-arylamidodimethylsilyl)methane uranium amide and halide complexes as precursors to U–M bonds.12 Here, we extend this chemistry with two new U–Re bonds, and comparison with 2 presents the first opportunity to systematically examine the impact on the U–Re bond that results from modification of the type and number of dative ancillary ligands at uranium.
We prepared 2 using salt elimination;11 however, some TM-anions react sluggishly using this route so we explored amine-elimination13 as a general entry to U–TM bonds, Scheme 1. Treatment of [(TsXy)(THF)U(NCy2)] (3)12 [TsXy = HC(SiMe2NAr)3; Ar = 3,5-Me2C6H3; Cy = cyclohexyl] with one equivalent of [(η5-C5H5)2ReH]14 in THF gave, after work-up and recrystallisation from hexane, the red complex [(TsXy)(THF)URe(η5-C5H5)2] (4) in 68% crystalline yield.‡ The characterisation data support this formulation; the 1H NMR spectrum of 4 spans the range +34 to −71 ppm and the room temperature solution magnetic moment of 3.02 μB is characteristic of a U(IV) complex.15
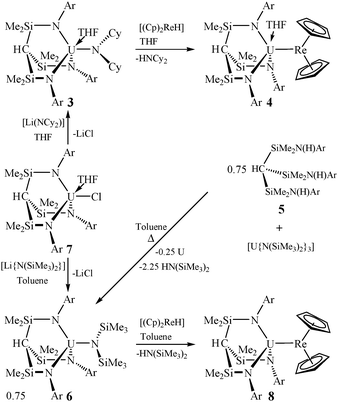 | ||
| Scheme 1 Synthesis of 4, 6, and 8. | ||
The molecular structure of 4 is illustrated in Fig. 1a with pertinent metrical data.§ The salient feature of 4 is the unsupported U–Re bond and retention of a THF molecule coordinated to uranium, which adopts a distorted trigonal bipyramidal geometry. This contrasts to 2 which is THF-free but contains a coordinated trialkyl amine.11 Two molecules of 4 crystallise in the asymmetric unit, but bond lengths and angles are similar and both U–Re bonds are longer than in 8. In order to prepare a solvent-free U–Re complex we designed and prepared a new uranium amide precursor.
![Molecular structures of 4 (a: values for second molecule in asymmetric unit in []), 6 (b), and 8 (c) with displacement ellipsoids at 40% and selective atom labelling. U⋯C contacts and hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (°) for 4: U(1)–Re(1) 3.0021(8) [2.9541(8)], U(1)–N(1) 2.261(12) [2.279(11)], U(1)–N(2) 2.256(12) [2.297(12)], U(1)–N(3) 2.288(13) [2.308(11)], U(1)–O(1) 2.531(9) [2.573(9)], and Ct–Re(1)–Ct 166.3(4) [167.1(15)], for 6: U(1)–N(1) 2.253(2), U(1)–N(2) 2.212(2), U(1)–N(3) 2.242(2), and U(1)–N(4) 2.257(2), for 8: U(1)–Re(1) 2.9307(8), U(1)–N(1) 2.239(12), U(1)–N(2) 2.207(12), U(1)–N(3) 2.232(12), and Ct–Re(1)–Ct 166.9(6).](/image/article/2011/CC/c0cc01387k/c0cc01387k-f1.gif) | ||
| Fig. 1 Molecular structures§ of 4 (a: values for second molecule in asymmetric unit in []), 6 (b), and 8 (c) with displacement ellipsoids at 40% and selective atom labelling. U⋯C contacts and hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (°) for 4: U(1)–Re(1) 3.0021(8) [2.9541(8)], U(1)–N(1) 2.261(12) [2.279(11)], U(1)–N(2) 2.256(12) [2.297(12)], U(1)–N(3) 2.288(13) [2.308(11)], U(1)–O(1) 2.531(9) [2.573(9)], and Ct–Re(1)–Ct 166.3(4) [167.1(15)], for 6: U(1)–N(1) 2.253(2), U(1)–N(2) 2.212(2), U(1)–N(3) 2.242(2), and U(1)–N(4) 2.257(2), for 8: U(1)–Re(1) 2.9307(8), U(1)–N(1) 2.239(12), U(1)–N(2) 2.207(12), U(1)–N(3) 2.232(12), and Ct–Re(1)–Ct 166.9(6). | ||
Reaction of pro-ligand 5 with trivalent [U{N(SiMe3)2}3]16 in toluene afforded red-brown, tetravalent [(TsXy)UN(SiMe3)2] (6), with concomitant formation of finely divided uranium from disproportionation, in 42% crystalline yield after work-up. Alternatively, reaction of [(TsXy)(THF)UCl] (7)12 with one equivalent of [LiN(SiMe3)2] in toluene afforded 6 in 40% crystalline yield following work-up.‡ The characterisation data for 6 support the formulation; the room temperature solution magnetic moment of 3.09 μB for 6 is similar to that for 3, and the 1H NMR spectrum occupies the range of +4 to −30 ppm.
Fig. 1b shows the molecular structure§ of 6 with significant metrical parameters. In contrast to 3, 6 is THF-free and the pseudo-tetrahedral uranium centre is supplemented by a contact to a xylylipso-carbon [U(1)⋯C(4) = 3.001(3) Å] to ameliorate its electron deficiency. Since 6 was found to be free of ancillary Lewis base ligands it was judged to be an excellent precursor to solvent-free uranium–metal bonds.
Accordingly, treatment of 6 with one equivalent of [(η5-C5H5)2ReH]14 in toluene afforded [(TsXy)URe(η5-C5H5)2] (8) as red blocks in 35% crystalline yield following work-up and recrystallisation from hexane.‡ The room temperature solution magnetic moment of 3.06 μB for 8 is intermediate to those of 4 and 6. The 1H NMR spectrum is similar to that observed for 4, spanning +15 to −68 ppm, except THF resonances are absent.
To confirm the structure of 8 we carried out an X-ray diffraction study and the molecular structure is depicted in Fig. 1c with selected metrical data.§ Complex 8 is solvent-free, and the uranium centre adopts a distorted tetrahedral geometry. Abatement of the electron deficient nature of uranium in 8 is suggested by agostic contacts to the closest two cyclopentadienyl C–H bonds [U(1)⋯C(32) = 2.894(12); U(1)⋯C(37) = 2.952(12) Å].
In order to gain further insight into the nature of the U–Re bonds in 4 and 8, and to make comparisons with 2, we carried out DFT calculations on 4 and 8. Calculated data are listed in Table 1 and compare well to the experimentally determined structures; we thus conclude that the DFT calculations provide a qualitative description of the electronic structures. With combined experimental and theoretical analyses in hand, several trends can be identified even though the nature of the tripodal ligand changes from 2 to 4 and 8.
| Complex (ref. 11 for complex 2) | Calculated U–Re and U–Namide (av.) bond lengths (Å) | Nalewajski–Mrozek bond indices | Atomic charges (averaged for N) | Atomic spin densities (averaged for N) | Orbital contributions to σ (HOMO −4) and π (HOMO −3) components of the uranium–rhenium bond (U/Re% contributions) | U–Re bond interaction energy (kJ mol−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U–Re | U–Namide | U–Re | U–N | U | Re | N | U | Re | N | σ | Total | π | Total | ||
| 2 | 3.0514 | 2.3032 | 1.15 | 1.34 | +1.77 | +0.36 | −1.40 | +2.28 | −0.09 | −0.05 | 11.3/54.4 | 65.7 | 10.4/58.8 | 69.2 | −561.31 |
| 4 | 3.0258 | 2.2965 | 1.18 | 1.34 | +2.15 | +0.35 | −1.38 | +2.31 | −0.09 | −0.06 | 15.0/56.7 | 71.7 | 11.8/57.4 | 69.2 | −599.53 |
| 8 | 3.0028 | 2.2632 | 1.30 | 1.37 | +2.10 | +0.30 | −1.40 | +2.37 | −0.13 | −0.07 | 17.5/57.3 | 74.8 | 13.1/56.6 | 69.7 | −657.51 |
The U–Re bond length contracts from 3.0475(4) Å in 2 to 3.0021(8) [2.9541(8)] Å in 4 following exchange of a trialkylamine for THF, which corresponds to a reduction of ∼0.04 [∼0.09] Å. Following the loss of THF, the U–Re bond contracts by 0.07 [0.02] Å to 2.9307(8) Å in 8.17 Overall, this corresponds to a U–Re bond distance decrease of 0.11 Å from 2 to 8. This trend is qualitatively matched by the DFT calculations, Table 1. The contracted U–Re bond distances in the calculated structures are matched by increased Nalewajski–Mrozek bond indices,18 which were computed to be 1.15 (2), 1.18 (4), and 1.30 (8), respectively. The average value of the U–Namide bond indices (∼1.35) suggests some π-bonding to uranium and they vary only a modest amount in 2, 4, and 8. The suggested agostic U⋯CHCp contacts from the experimentally determined structure of 7 are supported by computed bond indices of 0.24 and 0.23 from U(1) to C(32) and C(37), respectively. A significant change is observed in the Mulliken charge at uranium following removal of the trialkylamine donor from 2 to 4, which is followed by a more modest change following removal of the THF to give 8; a modest decrease is noted for rhenium and almost no change is observed for the amide nitrogen atoms. The Mulliken population analyses show that the spin densities at uranium are consistently greater than would be expected for a 3H4 f2uranium(IV) centre and were computed to be +2.28 (2), +2.31 (4), and +2.37 (8). These values show a net transfer of electron density to uranium, and that this increases from 2 to 4 to 8. In line with this observation, the rhenium and nitrogen centres exhibit small decreases in their computed spin densities.
Inspection of the Kohn–Sham orbitals of 4 and 8‡ shows that, like 2, similar σ- and π-components are present in the U–Re bonds and they are localised to HOMOs −4 (principally ∼54% Re 5dz2 and ∼15% U 6dz2and 5fz3) and −3 (mainly ∼56% Re 5dyz and ∼12% U 5dyz and 5fz2y) in each complex, respectively. Analysis of the frontier orbitals involved in the σ-components (Table 1) shows that as Lewis base ancillaries are removed the contributions to HOMO −4 from uranium- and rhenium-based orbitals increase so the total contribution to these molecular orbitals grows. In contrast, the total contribution from uranium- and rhenium-based orbitals to the HOMO −3 π-molecular orbital stays almost constant for 2, 4, and 8 (Table 1). Thus, as uranium becomes more electron deficient on moving from 2 to 4 to 8, the principal mechanism by which this is ameliorated appears to be through the U–Re σ-bond. We examined the U–Re bonds in 2, 4, and 8 using energy decomposition analyses and values are given in Table 1. This gave calculated U–Re interaction energies of −561.31, −599.53, and −657.51 kJ mol−1, respectively, for 2, 4, and 8, which follows the anticipated trend as revealed by the U–Re bond distances.
To summarise, we have prepared two new U–Re complexes by amine-elimination with systematic variation of the nature and number of dative Lewis base ancillary ligands at uranium. Combined experimental and theoretical analyses have given insights into the perturbation of U–Re bonds by dative Lewis base ancillary ligands. The data suggest that increased electron deficiency at uranium is ameliorated principally through the σ-component of the U–Re bond, whilst the π-component remains essentially invariant.
We thank the Royal Society, the Engineering and Physical Sciences Research Council, the European Research Council, the University of Nottingham, and the UK National Nuclear Laboratory for generously supporting this work.
Notes and references
- F. A. Cotton and R. A. Walton, Multiple Bonds between Metal Atoms, Oxford University Press, New York, 2nd edn, 1993 Search PubMed.
- S. Green, C. Jones and A. Stasch, Science, 2007, 318, 1754 CrossRef CAS.
- (a) S. T. Liddle and D. P. Mills, Dalton Trans., 2009, 5592 RSC; (b) S. T. Liddle, Philos. Trans. R. Soc., A, 2009, 465, 1673 CAS.
- Spectroscopically characterised examples: (a) M. Santos, J. Marçalo, A. Pires de Matos, J. K. Gibson and R. G. Haire, Eur. J. Inorg. Chem., 2006, 3346 CrossRef CAS; (b) S. P. Nolan, M. Porchia and T. J. Marks, Organometallics, 1991, 10, 1450 CrossRef CAS; (c) M. Porchia, N. Brianese, U. Casellato, F. Ossola, G. Rossetto, P. Zanella and R. Graziani, J. Chem. Soc., Dalton Trans., 1989, 677 RSC; (d) R. S. Sternal and T. J. Marks, Organometallics, 1987, 6, 2621 CrossRef CAS; (e) M. Porchia, F. Ossola, G. Rossetto, P. Zanella and N. Brianese, J. Chem. Soc., Chem. Commun., 1987, 550 RSC; (f) R. L. Bennett, M. I. Bruce and F. G. A. Stone, J. Organomet. Chem., 1971, 26, 355 CrossRef CAS.
- For Th–TM bonds see: (a) R. S. Sternal, C. P. Brock and T. J. Marks, J. Am. Chem. Soc., 1985, 107, 8270 CrossRef CAS; (b) P. J. Hay, R. R. Ryan, K. V. Salazar, D. A. Wrobleski and A. P. Sattelberger, J. Am. Chem. Soc., 1986, 108, 313 CrossRef CAS; (c) J. M. Ritchey, A. J. Zozulin, D. A. Wrobleski, R. R. Ryan, H. J. Wasserman, D. C. Moody and R. T. Paine, J. Am. Chem. Soc., 1985, 107, 501 CrossRef.
- P. L. Diaconescu, A. L. Odom, T. Agapie and C. C. Cummins, Organometallics, 2001, 20, 4993 CrossRef CAS.
- M. Porchia, U. Casellato, F. Ossola, G. Rossetto, P. Zanella and R. Graziani, J. Chem. Soc., Chem. Commun., 1986, 1034 RSC.
- (a) S. G. Minasian, J. L. Krinsky, J. D. Rinehart, R. Copping, T. Tyliszczak, M. Janousch, D. K. Shuh and J. Arnold, J. Am. Chem. Soc., 2009, 131, 13767 CrossRef CAS; (b) S. G. Minasian, J. L. Krinsky, V. A. Williams and J. Arnold, J. Am. Chem. Soc., 2008, 130, 10086 CrossRef CAS.
- B. M. Gardner, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, J. Am. Chem. Soc., 2009, 131, 10388 CrossRef CAS.
- S. T. Liddle, J. McMaster, D. P. Mills, A. J. Blake, C. Jones and W. D. Woodul, Angew. Chem., Int. Ed., 2009, 48, 1077 CrossRef CAS.
- B. M. Gardner, J. McMaster, W. Lewis and S. T. Liddle, Chem. Commun., 2009, 2851 RSC.
- D. Patel, W. Lewis, A. J. Blake and S. T. Liddle, Dalton Trans., 2010, 39, 6638 RSC.
- Amine-elimination afforded a U–Sn bond, see ref. 7. Alkane elimination has afforded yttrium– and ytterbium–rhenium bonds, see: M. V. Butovskii, O. L. Tok, F. R. Wagner and R. Kempe, Angew. Chem., Int. Ed., 2008, 47, 6469 Search PubMed.
- M. L. H. Green, L. Pratt and G. Wilkinson, J. Chem. Soc., 1958, 3916 RSC.
- I. Castro-Rodriguez, K. Olsen, P. Gantzel and K. Meyer, J. Am. Chem. Soc., 2003, 125, 4565 CrossRef CAS.
- R. A. Andersen, Inorg. Chem., 1979, 18, 1507 CrossRef CAS.
- The sum of uranium and rhenium covalent radii is calculated to be 3.01 Å, see: P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 186 Search PubMed.
- A. Michalak, R. L. DeKock and T. Ziegler, J. Phys. Chem. A, 2008, 112, 7256 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Full synthetic, spectroscopic, crystallographic, and computational details for 4, 6, and 8. CCDC 777012–777014. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc01387k |
§ Crystal data for 4: C45H64N3OReSi3U·0.5C6H14, M = 1214.58, triclinic, a = 17.0448(4), b = 18.6938(4), c = 19.3062(5) Å, α = 63.838(3)°, β = 89.402(2)°, γ = 63.080(2)°, U = 4783.5(2) Å3, T = 90 K, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, 67 , Z = 2, 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 044 reflections measured, 17 044 reflections measured, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172 unique reflections (Rint = 0.100), R (F2 > 2σ) = 0.0680, Rw (F2, all data) = 0.1680. CCDC 777012. For 6: C37H64N4Si5U, M = 943.40, monoclinic, a = 16.1847(4), b = 11.3466(2), c = 23.8954(5) Å, β = 94.371(2)°, U = 4375.42(16) Å3, T = 90 K, space groupP21/c, Z = 4, 44 172 unique reflections (Rint = 0.100), R (F2 > 2σ) = 0.0680, Rw (F2, all data) = 0.1680. CCDC 777012. For 6: C37H64N4Si5U, M = 943.40, monoclinic, a = 16.1847(4), b = 11.3466(2), c = 23.8954(5) Å, β = 94.371(2)°, U = 4375.42(16) Å3, T = 90 K, space groupP21/c, Z = 4, 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 442 reflections measured, 7716 unique reflections (Rint = 0.027), R (F2 > 2σ) = 0.0205, Rw (F2, all data) = 0.0532. CCDC 777013. For 8: C41H56N3ReSi3U, M = 1099.39, monoclinic, a = 15.9356(4), b = 15.1651(5), c = 33.4591(10) Å, β = 91.052(3)°, U = 8084.5(4) Å3, T = 90 K, space groupC2/c, Z = 8, 25 442 reflections measured, 7716 unique reflections (Rint = 0.027), R (F2 > 2σ) = 0.0205, Rw (F2, all data) = 0.0532. CCDC 777013. For 8: C41H56N3ReSi3U, M = 1099.39, monoclinic, a = 15.9356(4), b = 15.1651(5), c = 33.4591(10) Å, β = 91.052(3)°, U = 8084.5(4) Å3, T = 90 K, space groupC2/c, Z = 8, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 376 reflections measured, 7278 unique reflections (Rint = 0.085), R (F2 > 2σ) = 0.0764, Rw (F2, all data) = 0.1824. CCDC 777014. 376 reflections measured, 7278 unique reflections (Rint = 0.085), R (F2 > 2σ) = 0.0764, Rw (F2, all data) = 0.1824. CCDC 777014. |
| This journal is © The Royal Society of Chemistry 2011 |