Homoleptic imidazolate frameworks 3∞[Sr1−xEux(Im)2]—hybrid materials with efficient and tuneable luminescence†‡
Alexander
Zurawski
a,
Marit
Mai
b,
Dominik
Baumann
a,
Claus
Feldmann
b and
Klaus
Müller-Buschbaum
*ac
aDepartment Chemie und Biochemie, Ludwig-Maximilians-Universität München, Butenandtstr. 5-13(D), 81377 München, Germany
bInstitut für Anorganische Chemie, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, 76131 Karlsruhe, Germany
cInstitut für Anorganische Chemie, Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: k.mueller-buschbaum@uni-wuerzburg.de; Fax: +49 931 31 84605; Tel: +49 931 31 88724
First published on 22nd October 2010
Abstract
Homoleptic frameworks of the formula 3∞[Sr1−xEux(Im)2] (1) (x = 0.01–1.0; Im− = imidazolate anion, C3H3N2−) are hybrid materials that exhibit an intensive green luminescence. Tuning of both emission wavelength and quantum yield is achieved by europium/strontium substitution so that a QE of 80% is reached at a Eu content of 5%. Even 100% pure europium imidazolate still shows 60% absolute quantum efficiency. Substitution of Sr/Eu shows that doping with metal cations can also be utilized for coordination compounds to optimize materials properties. The emission is finely tuneable in the region 495–508 nm via variation of the europium content. The series of frameworks 3∞[Sr1−xEux(Im)2] presents dense MOFs with the highest quantum yields reported for MOFs so far.
Framework and MOF chemistry1 have attracted attention, as interesting properties were reported like conductivity,2catalytic effects,3 luminescence4 and porosity.5 They are mainly known for oxygen coordinating ligands, mostly metal carboxylates6 which include the alkaline earth and 4f elements.7 Because of the oxophilicity of lanthanides oxygen-free multi-dimensional coordination networks are rarely found except for a few rare earth imidazolates and triazolates.8 Among transition metals the imidazole ring system is of exceptional interest together with several 3d metals as they adopt zeolite structures (ZIFs)9 that can be used for sorption and gas separation. Different from many solid state phosphors, coordination compounds can exhibit luminescence by metal ions although they contain 100% luminescence centres.10 An expected quenching by concentration is suppressed by ligand shielding. They are furthermore interesting luminescent hybrid materials, as emission can be achieved either via a fluorescence of the ligand system11 or the metal centres, mainly by the use of lanthanides.4 The excitation can benefit from antenna effects, viz. the ligand system is excited primarily followed by a transfer of the energy to the luminescence centres.12 However there are only little coordination compounds for which effective emission characterized by high quantum efficiencies has been reported.4,11,13 Mostly, no quantum yields were determined, although luminescence becomes important for MOFs concerning sensoring and lighting from UV to near IR.14,15
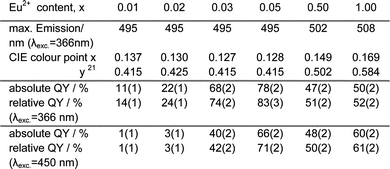
We now report a series of homoleptic imidazolate frameworks containing divalent strontium and europium that shows an exceptional combination of properties: an effective luminescence with the highest quantum yield reported for coordination polymers today, together with multiple excitation options including excitation maxima at the applicationally important wavelengths 370 and 460 nm (for Hg and blue LED excitation). The emission can be finely tuned in the region 495–508 nm (blue-green to green) via variation of the Eu content (Fig. 1). Furthermore a low quenching by concentration is observed, combined to a high thermal stability of the frameworks up to 530 °C.
![Selected and normalized excitation and emission spectra of 3∞[Sr1−xEux(Im)2] (1) (x = 0.05–1.0) in comparison to BaMgAl10O17 ∶ Eu, Mn as reference phosphor.](/image/article/2011/CC/c0cc02093a/c0cc02093a-f1.gif) | ||
Fig. 1 Selected and normalized excitation and emission spectra of 3∞[Sr1−xEux(Im)2] (1) (x = 0.05–1.0) in comparison to BaMgAl10O17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu, Mn as reference phosphor. Eu, Mn as reference phosphor. | ||
3∞[Sr1−xEux(Im)2] (x = 0.01–1.0; Im− = imidazolate anion, C3H3N2−) (1) are obtained by reactions of the metals europium and strontium together with a melt of the ligand 1H-imidazole in excellent yields up to 90%.§Solvent free reaction conditions avoid co-coordination of solvent molecules and drive the system towards homoleptic products.16Strontium and europium form isotypic compounds that allow complete mixing throughout all molar ratios as the two ions Sr2+ and Eu2+ have almost identical ionic radii.17 Also the monometallic frameworks 3∞[Sr(Im)2] (2) and 3∞[Eu(Im)2] (1) were prepared, corroborated by single crystal X-ray and powder diffraction. Although an oxidation to Eu3+ could be expected, the reaction finishes at a metal to ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Even upon excess of imidazole no reaction to Eu3+ is observed until decomposition.¶ For combination of Eu and Sr the ratio of the two metals can be setup and controlled by the use of liquid ammonia. Both metals dissolve under formation of ammonia complexes and electride solutions, so that perfect mixing on the atomic level is achieved.18||3∞[Sr1−xEux(Im)2] form at 160 °C. Excess imidazole can be evaporated with the MOFs being stable up to 530 °C.¶
2. Even upon excess of imidazole no reaction to Eu3+ is observed until decomposition.¶ For combination of Eu and Sr the ratio of the two metals can be setup and controlled by the use of liquid ammonia. Both metals dissolve under formation of ammonia complexes and electride solutions, so that perfect mixing on the atomic level is achieved.18||3∞[Sr1−xEux(Im)2] form at 160 °C. Excess imidazole can be evaporated with the MOFs being stable up to 530 °C.¶
In addition to 1 and 2 further Eu/Sr imidazolates were obtained. Imidazole containing networks of the formula 2∞[Sr1−xEux(Im)2(ImH)2] can be obtained at lower temperatures. Evidence is given again by X-ray single crystal diffraction of 2∞[Eu(Im)2(ImH)2] (3) and powder diffraction for Sr/Eu combinations. Alike HO– groups, HN– groups are known to function as quenchers. Accordingly, 3 is not a luminescent material and no emission is observed.
Luminescence of the series 3∞[Sr1−xEux(Im)2] (1) is based on a broad band excitation that ranges from λ = 250 nm to 460 nm. Independent from the europium content excitation via the imidazolate ligands is possible with excitation maxima at 270 and 366 nm. With an increasing europium content additional excitation maxima emerge at 345 and 450 nm. Thus both Eu and the ligand as an antenna effect can be used for excitation. For ligand excitation the energy is then transferred to the Eu ions. Emission is observed from europium centres only. Both 3∞[Sr(Im)2] (2) and 1H-imidazole were also investigated and neither displays a referring emission.** The broad emission band in 1 is typical for divalent europium as observed for SrSi2O2N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu2+ and Sr2Si5N8
Eu2+ and Sr2Si5N8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu2+.19 Emission derives from transitions between the 5d energy levels and the 4f8S7/2 levels. Different from 4f–4f transitions of Eu3+ these transitions are parity allowed, therefore strong in intensity, and influenced by the chemical surrounding by inclusion of the Eu 5d levels into the process.20 For imidazolate this results in an emission of 1 in the green region. Participation of Eu3+ in the emission can be excluded as the typical line emission 5D4 to the 7FJ states is not observed.20
Eu2+.19 Emission derives from transitions between the 5d energy levels and the 4f8S7/2 levels. Different from 4f–4f transitions of Eu3+ these transitions are parity allowed, therefore strong in intensity, and influenced by the chemical surrounding by inclusion of the Eu 5d levels into the process.20 For imidazolate this results in an emission of 1 in the green region. Participation of Eu3+ in the emission can be excluded as the typical line emission 5D4 to the 7FJ states is not observed.20
The emission maximum can be finely tuned by the content of europium over 14 nm and range from 495 nm for 1% Eu to 508 nm for 100% Eu and results in a pronounced shift of the colour points and thereby of the emission colour from blue green to bright green according to CIE (Commission Internationale de l'Eclairage).21 Most efficient emission is observed for Sr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 95
Eu = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 with a quantum yield of about 80% (λexc = 366 nm, see Table 1).** This is in the region of commercial phosphors like BaMgAl10O17
5 with a quantum yield of about 80% (λexc = 366 nm, see Table 1).** This is in the region of commercial phosphors like BaMgAl10O17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu: QE = 80–85%, Zn2SiO4
Eu: QE = 80–85%, Zn2SiO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn: 75–80%, YVO4
Mn: 75–80%, YVO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu: 65–70%, λexc = 254 nm,19 higher than the peak of the known Eu coordination compounds [Eu(nta)3(dmso)2]22 and twice as high as GWMOF-6,15,22 the MOF with the so far highest quantum yield (QE = 39%). Even for 100% Eu and no Sr content the quantum yield still is 60% for an excitation at 450 nm (blue LED). It is also remarkable that these quantum yields were determined for room temperature and not for low temperatures. Altogether 1 could be interesting even for solid lighting as a green phosphor component for a pcLED.
Eu: 65–70%, λexc = 254 nm,19 higher than the peak of the known Eu coordination compounds [Eu(nta)3(dmso)2]22 and twice as high as GWMOF-6,15,22 the MOF with the so far highest quantum yield (QE = 39%). Even for 100% Eu and no Sr content the quantum yield still is 60% for an excitation at 450 nm (blue LED). It is also remarkable that these quantum yields were determined for room temperature and not for low temperatures. Altogether 1 could be interesting even for solid lighting as a green phosphor component for a pcLED.
|
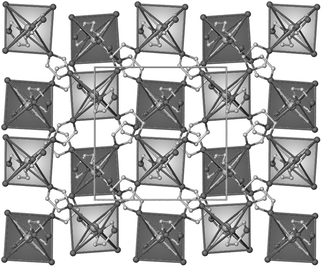
The complete series 3∞[Sr1−xEux(Im)2] (1) and 3∞[Sr(Im)2] (2) crystallize isotypic in the monoclinic space groupC2/c.†† This is responsible for an isopointal exchange of Euvs.Sr and the excellent doping possibilities. The metal ions are coordinated by six nitrogen atoms of six imidazolate anions. The imidazolate anions are committed in a μ3-η1:η1:η1 coordination mode and coordinated by three metal ions. Each Sr/EuN6 polyhedron is edge connected to two other polyhedra. This results in zig-zag chains of polyhedra that are interlinked vianitrogen atoms of the imidazolate ligands in the (a, b) plane to give 3D-framework structures (see Fig. 2). The overall structure is a dense framework.
![The crystal structure of the dense 3D-MOF 1 along [001] with coordination polyhedra. H atoms omitted for clarity.](/image/article/2011/CC/c0cc02093a/c0cc02093a-f2.gif) | ||
| Fig. 2 The crystal structure of the dense 3D-MOF 1 along [001] with coordination polyhedra. H atoms omitted for clarity. | ||
Distances M(Sr,Eu)–N of 1 range from 259.3(4) to 268.8(5) pm and are in the expected region for EuII.17 Additionally, an offset π-stacking is observed for each imidazolate ligand to a neighbouring aromatic ring. The distance between the centroids of neighbouring rings is only 277.4 pm, shorter than in molecular coordination compounds with N-heterocycles.23 According to Hunter and Sanders and the shift between both rings it can be classified as an electrostatic interaction,24 which is in good accordance to the Lewis acidic ionic character of Eu/Sr. The short ring distance is a result of a decrease in the electron density of the π-systems and of the electrostatic ring distraction. We believe π-stacking also influences the luminescence, but this effect and the low quenching by concentration cannot be fully interpreted by the literature.25
Prior to formation of 1 and 2, another Eu containing imidazolate was isolated at lower temperatures.§ Different from 1 and 2 this compound exhibits a 2D network structure of the formula 2∞[M(Im)2(ImH)2], M = Sr/Eu (3) and contains imidazole ligands as interplanar end-on ligands. The Eu/Sr atoms are octahedrally coordinated by N atoms, with the square plane of the octahedron containing four μ2-η1:η1-bridging imidazolate anions and the caps being N atoms of end-on imidazole molecules to form one layer of the structure. The next layer shows a parallel offset so that the SnF4 structure type is adopted (see Fig. 3). The Eu–N distances range from 258(2) to 265(2) pm being in the expected region for EuII.
 | ||
| Fig. 3 The crystal structure of 3. Coordination polyhedra of two different layers are marked in light grey and dark grey. | ||
3 is a low temperature phase in the system Sr/Eu/imidazole. Upon thermal treatment it is converted into the homoleptic dense MOF structure of 1. Conversion is combined to a stepwise release of imidazole and can be observed with thermal analysis and powder diffraction.¶ With the NH groups of the imidazole molecules being known quenchers, 3 does not show any luminescence. Accordingly a suitable reaction temperature is vital to obtain the highly luminescent hybrid materials 3∞[Sr1−xEux(Im)2] (1).
Notes and references
- G. Ferey, Dalton Trans., 2009, 4400 RSC; U Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastre, J. Mater. Chem., 2006, 16, 626 RSC; M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keefe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef; J.-P. Zhang and S. Kitagawa, J. Am. Chem. Soc., 2008, 130, 907 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS.
- R. Eberhardt, M. Allmendiger, M. Zintl, C. Troll, G. A. Luinstra and B. Rieger, Macromol. Chem. Phys., 2004, 205, 42 CrossRef CAS; J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982 CrossRef CAS.
- B. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui and G. Qian, Angew. Chem., Int. Ed., 2009, 48, 500 CrossRef CAS; F. Gándara, A. de Andrés, B. Gómez-Lor, E. Gutiérrez-Puebla, M. Iglesias, M. A. Monge, D. M. Proserpio and N. Snejko, Cryst. Growth Des., 2008, 8, 378 CrossRef CAS; C. Serre, F. Millange, C. Thouvenot, N. Gardant, F. Pelle and G. Ferey, J. Mater. Chem., 2004, 14, 1540 RSC; C. R. De Silva, J. Li, Z. Zheng and L. R. Corrales, J. Phys. Chem. A, 2008, 112, 4527 CrossRef CAS; C. J. Höller, M. Mai, C. Feldmann and K. Müller-Buschbaum, Dalton Trans., 2010, 39, 461 RSC.
- M. O'Keefe and O. M. Yaghi, Nature, 1999, 402, 276 CrossRef CAS; G. Ferey, Chem. Mater., 2001, 13, 3084 CrossRef CAS; K. Biradha and M. Fujita, Angew. Chem., Int. Ed., 2002, 41, 3392 CrossRef CAS; H. Althues and S. Kaskel, Langmuir, 2002, 18, 7428 CrossRef CAS.
- O. M. Yaghi, H. Li, C. Davis, T. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS; A. J. Blake, N. R. Champness, P. Hubberstey, W. S. Li, M. A. Withersby and M. Schröder, Coord. Chem. Rev., 1999, 183, 117 CrossRef CAS; M. Eddaoudi, J. Kim, J. B. Wachter, H. K. Chae, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2001, 123, 4368 CrossRef CAS.
- D.-L. Long, A. J. Blake, N. R. Champness, C. Wilson and M. Schröder, Angew. Chem., Int. Ed., 2001, 40, 2444 CAS; L. Pan, N. Zheng, Y. Wu, S. Han, R. Yang, X. Huang and J. Li, Inorg. Chem., 2001, 40, 828 CrossRef CAS; Y. Kim and D.-Y. Jung, Chem. Commun., 2002, 908 RSC; J. Liu, E. A. Meyer, J. A. Cowan and S. G. Shore, Chem. Commun., 1998, 2043 RSC.
- K. Müller-Buschbaum, Y. Mokaddem, F. M. Schappacher and R. Pöttgen, Angew. Chem., Int. Ed., 2007, 46, 4385 CrossRef; K. Müller-Buschbaum and Y. Mokaddem, Chem. Commun., 2006, 2060 RSC; K. Müller-Buschbaum, Z. Naturforsch., B, 2006, 61, 792.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. D. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keefe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186 CrossRef CAS.
- M.-S. Wang, S.-P. Guo, Y. Li, L.-Z. Cai, J.-P. Zou, G. Xu, W.-W. Zhou, F.-K. Zheng and G.-C. Guo, J. Am. Chem. Soc., 2009, 131, 13572 CrossRef CAS; A. Lan, K. Li, H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. Hong and J. Li, Angew. Chem., 2009, 121, 2370 CrossRef; Y.-Q. Huang, B. Ding, H.-B. Song, B. Zhao, P. Ren, P. Cheng and H.-G. Wang, Chem. Commun., 2006, 4906 RSC.
- C. Janiak, Dalton Trans., 2003, 2781 RSC; Z.-Q. Lia, L.-G. Qiu, W. Wanga, T. Xua, Y. Wua and X. Jiang, Inorg. Chem. Commun., 2008, 11, 1375 CrossRef CAS; K. C. Stylianou, R. Heck, S. Y. Chong, J. Bacsa, J. T. A. Jones, Y. Z. Khimyak, D. Bradshaw and M. J. Rosseinsky, J. Am. Chem. Soc., 2010, 132, 4119 CrossRef CAS.
- Y. Kuroda, K. Sugou and K. Sasaki, J. Am. Chem. Soc., 2000, 122, 7833 CrossRef CAS; R. J. Abergel, A. D'Alo, C. N. Pak Leung, D. K. Shuh and K. N. Raymond, Inorg. Chem., 2009, 48, 10868 CrossRef CAS.
- K. Binnemans, Chem. Rev., 2009, 109, 4283 CrossRef CAS; M. Giraud, E. S. Andreiadis, A. S. Fisyuk, R. Demadrille, J. Pecaut, D. Imbert and M. Mazzanti, Inorg. Chem., 2008, 47, 3952 CrossRef CAS.
- A. I. Voloshin, N. M. Shavaleev and V. P. Kazakov, J. Lumin., 2001, 93, 191 CrossRef CAS; Y. Zhang, H. Shi, Y. Ke and Y. Cao, J. Lumin., 2007, 124, 51 CrossRef CAS; C. A. Bauer, T. V. Timofeeva, T. B. Settersten, B. D. Patterson, V. H. Liu, B. A. Simmons and M. D. Allendorf, J. Am. Chem. Soc., 2007, 129, 7136 CrossRef CAS; L. Yang, Y. Zhao, Y. Su and J. Wu, Spectrochim. Acta, Part A, 2002, 58, 2803 CrossRef.
- M. D. Allendorf, C. A. Bauer, R. K. Bhaktaa and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC; K. Müller-Buschbaum, S. G. Torres, P. Larsen and C. Wickleder, Chem. Mater., 2007, 19, 655 CrossRef.
- K. Müller-Buschbaum, Z. Anorg. Allg. Chem., 2005, 631, 811 CrossRef; K. Müller-Buschbaum and C. C. Quitmann, Inorg. Chem., 2006, 45, 2678 CrossRef; G. B. Deacon, A. Gitlits, B. W. Skelton and A. H. White, Chem. Commun., 1999, 1213 RSC.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- F. C. Schmidt, F. J. Studer and J. Sottysiak, J. Am. Chem. Soc., 1938, 60, 2780 CrossRef CAS; R. Juza and C. Hadenfeldt, Naturwissenschaften, 1968, 55, 229 CrossRef CAS; C. C. Quitmann and K. Müller-Buschbaum, Angew. Chem., Int. Ed., 2004, 43, 5994 CrossRef CAS.
- R. Mueller-Mach, G. Mueller, M. R. Krames, H. A. Höppe, F. Stadler, W. Schnick, T. Juestel and P. Schmidt, Phys. Status Solidi A, 2005, 202, 1727 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer, 1994 Search PubMed.
- S. Shionoya and W. M. Yen, Phosphor Handbook, CRC Press, 1999 Search PubMed.
- L. D. Carlos, C. de Mello Donega, R. Q. Albuquerque, S. Alves Jr., J. F. S. Menezes and O. L. Malta, Mol. Phys., 2003, 101, 1037 CrossRef CAS; D. T. de Lill, N. S. Gunning and C. L. Cahill, Inorg. Chem., 2005, 44, 258 CrossRef CAS.
- C. Janiak, J. Chem. Soc., Dalton Trans., 2000, 3885 RSC.
- C. A. Hunter and J. K. M. Sanders, J. Am. Chem. Soc., 1990, 112, 5525 CrossRef CAS.
- R. Büchner, C. T. Cunningham, J. S. Field, R. J. Haines, D. R. McMillin and G. C. Summerton, J. Chem. Soc., Dalton Trans., 1999, 711 RSC; N. W. Alcock, P. R. Barker, J. M. Haider, M. J. Hannon, C. L. Painting, Z. Pikramenou, E. A. Plummer, K. Rissanen and P. Saarenketo, J. Chem. Soc., Dalton Trans., 2000, 1447 RSC.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Crystal structure determinations and depictions, Rietveld refinements, luminescence and IR spectroscopy results, experimental details. CCDC 758602–758604. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc02093a |
| § Reactions of the 4f metal europium, and the alkaline earth metal strontium with amine melts are redox reactions that give hydrogen gas in addition to the amide products.16 Detailed description on the Experimental of 1–3 is found in the ESI‡ including the results of IR and CHN-analysis. |
| ¶ The trivalent oxidation state is stable for Eu (E0LnII/III = 0.35 V). But reactions with 1H-imidazole stop at Eu2+, as proven by the luminescence being characteristic for Eu2+. No further oxidation is observed until decomposition at 530 °C. 1–3 are stable vs. dry air and vs. normal air over six hours, after which hydrolysis is observed. Thermal properties were determined by simultaneous DTA/TG and powder diffraction. For detailed information please check the ESI.‡ |
|| Mixing of the metals Sr and Eu is essential for the luminescence properties of 1 but limited upon grinding. The problem is overcome by activation of the metals by electride formation in liquid NH3.16,18 The metals equally dissolve under formation of ammine complexes [M(NH3)x]2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 and solvated electrons identified by a dark blue solution. Upon removal of ammonia the metals are reformed as very small metal particles that fairly improve the mixing for the subsequent reaction with imidazole. 18 and solvated electrons identified by a dark blue solution. Upon removal of ammonia the metals are reformed as very small metal particles that fairly improve the mixing for the subsequent reaction with imidazole. |
| ** Excitation of Eu2+ leads to population of the 4f65d1-level followed by relaxation without emission to the t2g 4f65d1-level. Here emission occurs to the 8S7/2 4f7 ground state. Because of coordination of the imidazolate anions, lowering of the 5d levels in energy below the 6P 4f7-level occurs. Parity forbidden f–f transitions are therefore neglectable. In addition to the series of compounds 1, excitation and emission spectra were also recorded for 3∞[Sr(Im)2] (2) and the ligand 1H-imidazole. The characteristic emission of 1 can neither be detected for 2 nor for the free ligand. For further information, please see the ESI.‡ |
| †† Crystallographic data: for detailed crystallographic data on 1–3, please refer to the ESI‡ including interatomic distances and angles. |
| This journal is © The Royal Society of Chemistry 2011 |
