A ratiometric luminescent oxygen sensor based on a chemically functionalized quantum dot†‡
Matteo
Amelia
a,
Aurélie
Lavie-Cambot
b,
Nathan D.
McClenaghan
*b and
Alberto
Credi
*a
aDipartimento di Chimica “G.Ciamician”, Università di Bologna, Via Selmi 2, 40126 Bologna, Italy. E-mail: alberto.credi@unibo.it; Fax: +39 051 2099456
bInstitut des Sciences Moléculaires, University of Bordeaux/CNRS, UMR 5255, 33405 Talence cedex, France. E-mail: n.mc-clenaghan@ism.u-bordeaux1.fr; Tel: +33 540 00 33 21; Fax: +33 540 00 61 58
First published on 23rd August 2010
Abstract
Nanoconjugates composed of CdSe–ZnS core–shell nanocrystals and pyrenyl ligands are shown to exhibit a double photoluminescence. Owing to the different response of the two emission signals towards oxygen, the nanocrystals function as high dynamic range ratiometric luminescent O2 nanosensors.
The determination of oxygen concentration in solution is essential for a variety of applications ranging from life sciences to environmental sciences.1 However, as most methods require sophisticated equipment and/or careful calibration procedures, the development of oxygen sensing devices relying on simple and cheap instrumentation without the need of complex data collection and processing is an important problem in analytical sciences.1 In this context, ratiometric luminescent sensors2 (either homogeneous or heterogeneous) represent a convenient answer.
Quantum dots (QDs) are luminescent semiconductor nanocrystals that exhibit several advantages compared to molecular dyes, such as a size-tunable, intense and narrow emission in the UV-visible-NIR range, a broad and intense absorption spectrum, and a high photostability.3 QDs are usually prepared by solvothermal methods which involve the passivation of their surface by a monolayer of organic ligands to control their growth and prevent aggregation. The modification of this layer with functional molecular units, either by ligand exchange or by self-assembly, is a versatile strategy to implement functions such as sensing and switching.4,5 This approach, however, has yet to be fully developed and its application is limited to systems based either on Förster resonance energy transfer (FRET) from the QD to an attached fluorophore or, less frequently, on photoinduced electron transfer involving the QD and an attached molecular quencher.4,5
Very recently, Bawendi and Nocera reported the only available example of a QD-dye nanoconjugate that behaves as a luminescent oxygen sensor.6–8 This system also exhibits a ratiometric response,9 which is characterized by FRET from the QD to the attached dye (an osmium complex), and can be operated under two-photon excitation. While this work demonstrates the validity of the approach, more studies need to be undertaken to identify guidelines for adjusting properties and performances, and most importantly, to simplify the design and synthesis.
Here we describe the preparation and operation of a luminescent sensor for oxygen based on CdSe–ZnS core–shell QDs with pyrenyl units strongly chemisorbed to the nanocrystal surface. The inorganic and organic components are easy to make and assemble. The ratiometric response of the nanocrystals stems from the fact that the surface-bound fluorophore is strongly quenched by O210 while the QD emission is not,11 as for previously reported examples.6,8 However, in the current system, the emission signals originating from the QD and pyrenyl moieties are fully independent from one another (vide infra). This chromophore orthogonality offers a different functioning from related QD-chromophore nanoconjugates, and removes one of the energetic conditions associated with FRET systems.
CdSe–ZnS QDs (core diameter, 5.8 nm; total diameter, 7.9 nm) capped with trioctylphosphineoxide (TOPO) (Fig. 1a) were prepared according to published procedures12,13 and characterized by electron microscopy, and absorption and photoluminescence (PL) spectroscopy (Fig. 2a and Table 1). Ligand PYI (Fig. 1b) consists of a 1-pyrenyl moiety attached to an imidazole surface docking unit via a four-CH2 bridge and was synthesized by reacting 1-(4-iodobutyl)pyrene with imidazole.14 As shown in Fig. 2b, PYI conserves the photophysical properties typical of the pyrene chromophore.10
 | ||
| Fig. 1 Schematic representation of (a) QD-TOPO and (b) QD-PYI. The various components are not on the same scale. | ||
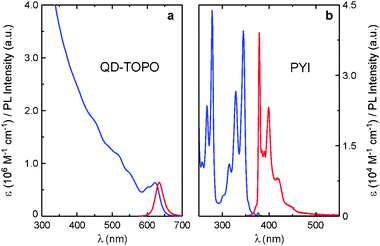 | ||
| Fig. 2 Absorption (blue) and photoluminescence (red) spectra of QD-TOPO (a) and PYI (b) in air-equilibrated CHCl3 at room temperature. Excitation was performed at 480 nm for QD-TOPO and at 335 nm for PYI. | ||
| Compound | λ max/nm | Φ PL c | τ 1/ns | τ 2/ns | τ av/nse |
|---|---|---|---|---|---|
| (b1/%)d | (b2/%)d | ||||
| a λ exc = 480 nm. b λ exc = 275 nm. c Photoluminescence quantum yield. d Relative contribution of the corresponding lifetime to the emission decay. e Average lifetime, calculated as τav = (τ1b1 + τ2b2)/100. | |||||
| QD-TOPO | 636a | 0.080 | 18.6 (40) | 3.8 (60) | 12.7 |
| PYI | 375b | 0.076 | 18.0 | — | — |
| QD-PYI | 632b | 0.020 | 23.5 (40) | 1.3 (60) | 14.6 |
| 378b | 0.018 | 18.6 (94) | 6.2 (6) | 17.8 |
Upon refluxing (at 65 °C) QD-TOPO with PYI in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of MeCN/CHCl3 under stirring and in an inert atmosphere (Ar), partial exchange of the TOPO ligands for PYI occurred.§ Successive purification steps12 afforded QD-PYI (Fig. 1b). From the absorption data we estimated that the average number of PYI ligands per QD is 55. The luminescence data of the QD-PYI nanohybrids and of their organic and inorganic components are summarized in Table 1.
1 mixture of MeCN/CHCl3 under stirring and in an inert atmosphere (Ar), partial exchange of the TOPO ligands for PYI occurred.§ Successive purification steps12 afforded QD-PYI (Fig. 1b). From the absorption data we estimated that the average number of PYI ligands per QD is 55. The luminescence data of the QD-PYI nanohybrids and of their organic and inorganic components are summarized in Table 1.
The absorption spectrum of QD-PYI, which is practically a linear combination of the absorption features of its components, shows the exciton peak of the CdSe core and the structured bands of the S2 ← S0 transition of the pyrene chromophore.14 The PL spectrum of QD-PYI obtained upon excitation at 275 nm, where both the nanocrystal and the pyrenyl moieties absorb, shows the pyrene-type fluorescence and the CdSe exciton luminescence (Fig. 3 and Table 1). These emissions exhibit very similar band shape and λmax compared to those observed for the separated QD-TOPO and PYI components, but have a somewhat lower quantum yield.
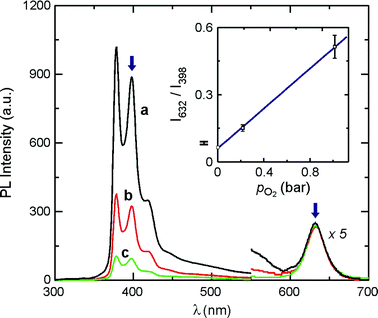 | ||
| Fig. 3 Emission spectra (λexc = 275 nm) of QD-PYI in CH3Cl in the presence of: 0 (a), 0.213 (b) and 1.013 bar O2. The inset shows the linear correlation between the ratiometric PL response (calculated from the intensity ratio at the wavelength values indicated by the blue arrows) and the O2 partial pressure. | ||
At first sight the pyrene-centered emission quenching may be ascribed to FRET to the lower lying CdSe exciton level; however, the expected corresponding shortening of the fluorescence lifetime is not observed. Moreover, the comparison between the absorption and excitation spectra of QD-PYI shows that the FRET process, if occurring at all, is very inefficient. In fact the quantum yield of the pyrene-type fluorescence in QD-PYI is very similar to that of PYI in hexane (0.022), in agreement with the fact that the pyrenyl units in the nanohybrid are surrounded by a hydrocarbon-like environment provided by the octyl chains of the TOPO ligands.
The four-fold quenching of the CdSe exciton emission in QD-PYI compared to QD-TOPO can be ascribed to the replacement of TOPO with the PYI ligands, which afford a less effective surface passivation. Such an explanation is consistent with the slight increase of the emission lifetime.15 In principle, the exciton level could also be quenched by energy transfer to the lower lying triplet excited state of the pyrenyl moiety.10 In this case, however, a shortening of the CdSe emission lifetime would be expected; indeed, more detailed experiments would be required to investigate the possibility of this non-dominant pathway.
As shown in Fig. 3, the luminescence spectrum of QD-PYI is profoundly influenced by the O2 concentration, thereby suggesting the use of QD-PYI as luminescent oxygen nanosensors. The oxygen quenching of the pyrene-type fluorescence can be interpreted in terms of the Stern–Volmer relation and is therefore of dynamic nature, as confirmed by emission lifetime measurements.‡ The quenching rate constant resulted to be kq = 1.1 × 1010 M−1 s−1.9 Conversely, the CdSe emission is practically unaffected by the presence of oxygen (Fig. 3). Therefore, the CdSe emission can be used as an internal reference for the normalization of the pyrene-type fluorescent signal. The optical output of the nanosensor is calculated as the ratio between the CdSe emission at 632 nm and the pyrene-type fluorescence at 398 nm. The ratiometric response as a function of the O2 pressure is shown in the inset of Fig. 3. The linear fit of the data points yields the following equation,
| I632/I398 = (0.061 ± 0.004) + (0.445 ± 0.007) × pO2 | (1) |
In summary, we have prepared and investigated CdSe–ZnS QDs derivatized with pyrenyl moieties. In CHCl3 solution these nanohybrids show two emission bands that exhibit a differential response to O2 concentration, thus providing a basis for ratiometric sensing. Owing to the strong quenching effect of oxygen on pyrene fluorescence, our system possesses a substantially higher dynamic range in its optical output compared to a previously reported similarly constructed device.6 The present QDs are strongly hydrophobic because their surface is mostly covered by TOPO molecules; however, several strategies exist3 to impart water solubility by introducing hydrophilic ligands. The strategy reported herein, which equally shows an example of surface-bound fluorophore-QD orthogonality, is both versatile and simple, and can be further developed to construct luminescent QD-molecule nanohybrids with tailored functionalities for sensing and switching purposes in different environments.
Financial support from the University of Bologna, Fondazione Carisbo, MIUR (PRIN 2006), MAE (DGPCC), Université Franco-Italienne (Galilée Programme), European Research Council under the EC FP7 programme (ERC starting grant no. 208702 to N.D.McC.), Région Aquitaine, Ministère de la Recherche et de l'Enseignement Supérieur (to A.L.-C.) is gratefully acknowledged.
Notes and references
- (a) Y. Amao, Microchim. Acta, 2003, 143, 1 CrossRef CAS; (b) A. Mills, Chem. Soc. Rev., 2005, 34, 1003 RSC; (c) J. R. Stetter and J. Li, Chem. Rev., 2008, 108, 352 CrossRef CAS.
- Recent examples: (a) Z. Xu, K.-H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601–610 CrossRef CAS; (b) F. Westerlund, C. B. Hildebrandt, T. J. Sorensen and B. W. Laursen, Chem.–Eur. J., 2010, 16, 2992 CrossRef CAS; (c) Z. Xu, J. Yoon and D. R. Spring, Chem. Commun., 2010, 46, 2563 RSC; (d) R. Guliyev, A. Coskun and E. U. Akkaya, J. Am. Chem. Soc., 2009, 131, 9007 CrossRef CAS; (e) C. S. Bonnet and T. Gunnlaugsson, New J. Chem., 2009, 33, 1025 RSC; (f) J. Han, A. Loudet, R. Barhoumi, R. C. Burghardt and K. Burgess, J. Am. Chem. Soc., 2009, 131, 1642 CrossRef CAS; (g) G. L. Law, R. Pal, L. O. Palsson, D. Parker and K. L. Wong, Chem. Commun., 2009, 7321 RSC; (h) X. Zhang, Y. Xiao and X. Qian, Angew. Chem., Int. Ed., 2008, 47, 8025 CrossRef CAS; (i) S. Sreejith, K. P. Divya and A. Ajayaghosh, Angew. Chem., Int. Ed., 2008, 47, 7883 CrossRef CAS.
- (a) A. L. Efros and M. Rosen, Annu. Rev. Mater. Sci., 2000, 30, 475 CrossRef CAS; (b) X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS.
- (a) A. R. Clapp, I. L. Medintz and H. Mattoussi, ChemPhysChem, 2006, 7, 47 CrossRef CAS; (b) R. C. Somers, M. G. Bawendi and D. G. Nocera, Chem. Soc. Rev., 2007, 36, 579 RSC; (c) J. F. Callan, A. P. de Silva, R. C. Mulrooney and B. Mc Caughan, J. Inclusion Phenom. Macrocyclic Chem., 2007, 58, 257 CrossRef CAS; (d) I. Yildiz, M. Tomasulo and F. M. Raymo, J. Mater. Chem., 2008, 18, 5577 RSC; (e) R. Gill, M. Zayats and I. Willner, Angew. Chem., Int. Ed., 2008, 47, 7602 CrossRef CAS; (f) I. L. Medintz and H. Mattoussi, Phys. Chem. Chem. Phys., 2009, 11, 17 RSC; (g) I. Yildiz, E. Deniz and F. M. Raymo, Chem. Soc. Rev., 2009, 38, 1859 RSC.
- (a) Z. Erno, I. Yildiz, B. Gorodetsky, F. M. Raymo and N. R. Branda, Photochem. Photobiol. Sci., 2010, 9, 249 RSC; (b) D. Geissler, L. J. Charbonnière, R. F. Ziessel, N. G. Butlin, H.-G. Löhmannsröben and N. Hildebrandt, Angew. Chem., Int. Ed., 2010, 49, 1396 CAS; (c) J. E. Halpert, J. R. Tischler, G. Nair, B. J. Walker, W. Liu, V. Bulovic and M. G. Bawendi, J. Phys. Chem. C, 2009, 113, 9986 CrossRef CAS; (d) R. Freeman, T. Finder and I. Willner, Angew. Chem., Int. Ed., 2009, 48, 7818 CrossRef CAS; (e) I. L. Medintz, T. Pons, K. Susumu, K. Boeneman, A. M. Dennis, D. Farrell, J. R. Deschamps, J. S. Melinger, G. Bao and H. Mattoussi, J. Phys. Chem. C, 2009, 113, 18552 CrossRef CAS; (f) R. Freeman, T. Finder, L. Bahshi and I. Willner, Nano Lett., 2009, 9, 2073 CrossRef CAS; (g) B. Gadenne, I. Yildiz, M. Amelia, F. Ciesa, A. Secchi, A. Arduini, A. Credi and F. M. Raymo, J. Mater. Chem., 2008, 18, 2022 RSC; (h) I. Yildiz, M. Tomasulo and F. M. Raymo, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11457 CrossRef CAS; (i) R. C. Mulrooney, N. Singh, N. Kaur and J. F. Callan, Chem. Commun., 2009, 686 RSC.
- E. J. McLaurin, A. B. Greytak, M. G. Bawendi and D. G. Nocera, J. Am. Chem. Soc., 2009, 131, 12994 CrossRef CAS.
- For other nanoparticle-based oxygen sensors, see: (a) M. P. Coogan, J. B. Court, V. L. Gray, A. J. Hayes, S. H. Lloyd, C. O. Millet, S. J. A. Pope and D. Lloyd, Photochem. Photobiol. Sci., 2010, 9, 103 RSC; (b) S. Simoncelli, G. Kuzmanich, M. N. Gard and M. A. Garcia-Garibay, J. Phys. Org. Chem., 2010, 23, 376 CAS; (c) G. Zhang, G. M. Palmer, M. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747 CrossRef CAS; (d) C. Wu, B. Bull, K. Christensen and J. McNeill, Angew. Chem., Int. Ed., 2009, 48, 2741 CrossRef CAS; (e) Y. E. L. Koo, Y. F. Cao, R. Kopelman, S. M. Koo, M. Brasuel and M. A. Philbert, Anal. Chem., 2004, 76, 2498 CrossRef CAS.
- For an oxygen-sensitive composite material containing a film of QDs, see: X. Wang, X. Chen, Z. Xie and X. Wang, Angew. Chem., Int. Ed., 2008, 47, 7450 Search PubMed.
- Ratiometric pH sensors based on QDs: (a) T. Jin, A. Sasaki, M. Kinjo and J. Miyazaki, Chem. Commun., 2010, 46, 2408 RSC; (b) Y. Wu, S. Chakraborthy, R. A. Gropeanu, J. Wilhelmi, Y. Xu, K. S. Er, S. L. Kuan, K. Koynov, Y. Chan and T. Weil, J. Am. Chem. Soc., 2010, 132, 5012 CrossRef CAS; (c) P. T. Snee, R. C. Somers, G. Nair, J. P. Zimmer, M. G. Bawendi and D. G. Nocera, J. Am. Chem. Soc., 2006, 128, 13320 CrossRef CAS.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of photochemistry, CRC Press, Boca Raton, 3rd edn, 2006 Search PubMed.
- G. W. Walker, V. C. Sundar, C. M. Rudzinski, M. G. Bawendi and D. G. Nocera, Appl. Phys. Lett., 2003, 83, 3555 CrossRef CAS.
- (a) Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2002, 124, 3343 CrossRef CAS; (b) C. R. Bullen and P. Mulvaney, Nano Lett., 2004, 4, 2303 CrossRef CAS.
- J. J. Li, Y. A. Wang, W. Guo, J. C. Keay, T. D. Mishima, M. B. Johnson and X. Peng, J. Am. Chem. Soc., 2003, 125, 12567 CrossRef CAS.
- See the ESI† and J. Larsen, F. Puntoriero, T. Pascher, N. McClenaghan, S. Campagna, E. Åkesson and V. Sundström, ChemPhysChem, 2007, 8, 2643 Search PubMed.
- M. A. Hines and P. Guyot-Sionnest, J. Phys. Chem., 1996, 100, 468 CrossRef CAS.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater., 2003, 15, 2854 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Syntheses, absorption, TEM images, luminescence decays, Stern–Volmer plots, the evaluation of the number of pyrene per quantum dot. See DOI: 10.1039/c0cc02163f |
| § Synthesis of the CdSe–ZnS QDs: the preparation of the CdSe core nanocrystals was performed according to established procedures12 with slight modifications.14 The ZnS shell was deposited following the SILAR procedure.13 The diameter of the core was determined from the excitonic peak position,16 whereas the shell thickness was calculated assuming a ZnS layer thickness of 0.35 nm. General procedure for the preparation of QD-PYI: a CHCl3 solution of QD-TOPO was mixed with a solution of PYI in MeCN. This mixture was subjected to continuous stirring and argon purging at 65 °C for 24 hours, then stirred at room temperature for an additional two days. The final product was precipitated with methanol in order to remove the unreacted nanocrystals, and washed at least three times with MeCN to remove the unreacted PYI ligand. The efficacy of the purification procedure was checked by monitoring the washing solutions spectrophotometrically. |
| This journal is © The Royal Society of Chemistry 2011 |
