Isothermal detection of RNA with restriction endonucleases†‡
Lei
Yan
,
Shizuka
Nakayama
,
Saron
Yitbarek
,
Isabel
Greenfield
and
Herman O.
Sintim
*
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742, USA. E-mail: hsintim@umd.edu
First published on 1st October 2010
Abstract
Herein, we demonstrate how to detect nucleic acids that do not contain restriction endonuclease recognition sites with restriction endonucleases. We show that the topology of DNA probes used in this detection strategy remarkably affects the efficiency of RNA/DNA detection.
Restriction endonucleases (REases) have been the workhorses of several biotechnological applications for decades. In the last ten years, there has been an increasing interest in the use of REases for applications other than the traditional cloning experiments. For example, several researchers have described the detection of DNA and RNA1 as well as autonomous replication of DNA nanostructures1e utilizing various endonucleases. The drive towards the use of endonucleases for a non-PCR-based nucleic acid detection is fuelled by the need to develop strategies that can detect disease-related RNA/DNA at isothermal conditions without the need for expensive sample preparation or thermal cycling procedure, as is with the use of polymerases which can be inhibited by other biomolecules.2
Recently, we became interested in detecting RNA. The detection of RNA3 is not as straightforward as that of DNA.4 In typical RNA detection schemes, the RNA is first copied into DNA before PCR is used to amplify the cDNA. Also the facile degradation of RNA samples by adventitious RNAses into smaller fragments during sample preparation makes the use of PCR-based methods to detect RNA not straightforward as PCR is better suited for longer transcripts. We aimed to detect RNA using restriction endonucleases, although restriction endonucleases do not generally recognize or modify RNA molecules. With the exception of few,1f most technologies that utilize endonucleases to detect nucleic acids require the presence of specific sequences in the target analytes; therefore they cannot be used to detect most genes. In a recent communication, we described a junction probe (JP) technology which utilized the restriction endonuclease, BfuCI, to detect DNA templates which did not contain the cognate recognition sequence of BfuCI at isothermal conditions.5 Adaptation of the first generation JP technology to detect RNA was not as successful as the detection of DNA. This was partly because the first generation JP platform required very long assay times (several hours) in order to detect low concentrations of analytes and for biomolecules such as RNA which are not hydrolytically stable, such long assay times were not desirable. We therefore initiated a program to unravel salient design principles that would enable the development of a more efficient JP system.
The JP technology utilizes a strategy called TeHyP (an acronym for Template enhanced Hybridization Processes). For the TeHyP strategy, two probes which have limited regions of complementarity do not form a duplex at ambient temperature but can be made to hybridize to each other in the presence of an analyte to form tripartite 1 (see Scheme 1). If the region of complementarity between the two probes A and B contains a restriction endonuclease sequence, then the formation of tripartite structure 1 will lead to the generation of a cognate site that is cleaved by a REase to form tripartite 2 which is less stable than 1. Dissociation of 2 followed by a subsequent re-formation of 1 lead to amplified sensing of the template.
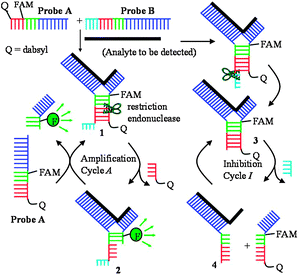 | ||
| Scheme 1 Amplification and inhibition cycles during JP amplified sensing. | ||
For the successful cleavage of the cognate restriction endonuclease site, 5′-GATC-3′ in JP structures by BfuCI, it is imperative that there exists an oligonucleotide overhang after the cognate REase site (see Scheme 1 and ESI‡, Fig. S1). We therefore rationalized that if the oligonucleotide overhang in JP tripartite is cleaved by the enzyme during catalysis, then blunt JP structures 3, which have been previously shown by us not to be a good substrate for BfuCI,5 will accumulate and inhibit the catalytic cycle (see Scheme 1). Incubation of JP probes A and B that had their respective 5′-ends labeled with fluorescein, matching DNA template and the restriction endonuclease enzyme BfuCI revealed that the enzyme could also cleave the single strand overhang of probe B to form tripartite 3 (refer to Scheme 1 and ESI‡, Fig. S2). Thus it was thought that by modifying the cleavage site of probe B with nuclease resistant moieties (see Fig. 1), the accumulation of blunt-end tripartites such as 3 would be reduced, invariably leading to an increase in JP amplification rate. Pleasingly, the modification of site B of JP with phosphoromonothioate (JP2) or phosphorodithioate (JP3) led to enhanced cleavage rate over the original JP structure (JP1) in which site B contained the native phosphate linkage (Fig. 1). The replacement of the exocyclic oxygen in the phosphate linkage at site B with either an acetate (JP4) or a methoxy group (JP5) completely prevented site B cleavage by BfuCI but the rates of JP4/5 cleavage by BfuCI were slightly less than that of the native phosphate moiety (JP1) (see Fig. 1). We hypothesize that non-steric mimics of the exocyclic oxygen of the phosphate moiety inhibit either endonuclease/DNA complex formation or the DNA cleavage step. In line with this hypothesis, phosphoromonothioate (JP2), which is a better isostere of phosphate than phosphorodithioate (JP3), was cleaved by BfuCI at a faster rate than JP3, see Fig. 1.
![The cleavage rates of junction probes with different modifications at site B are significantly different. [Probes A] = [probe B] = 1 μM, [DNA template] = 0.2 μM, [BfuCI] = 0.033 U μL−1.](/image/article/2011/CC/c0cc02208j/c0cc02208j-f1.gif) | ||
| Fig. 1 The cleavage rates of junction probes with different modifications at site B are significantly different. [Probes A] = [probe B] = 1 μM, [DNA template] = 0.2 μM, [BfuCI] = 0.033 U μL−1. | ||
Most type II REases require Mg2+ as a cofactor, although other divalent cations such as Zn2+, Mn2+, Co2+, and Cd2+ can replace Mg2+. The replacement of Mg2+ cofactor with the aforementioned divalent cations did not improve the REase cleavage rate. In fact, the rate of JP cleavage by BfuCI was significantly reduced (by a factor close to five, data not shown) when Mg2+ was replaced with Zn2+, Mn2+or Co2+.
Analyses of the X-ray crystal structures of several REases whose structures are deposited in the protein data bank (PDB) revealed that the majority of these REases contact bases or phosphate moieties that lie a few nucleotides away from their cognate recognition sequences. For example, EcoRI makes contact with phosphate linkages that lie two nucleotides away from the cognate recognition sequence.6 Therefore, it was expected that the sequence content of the single strand overhang of JP tripartite would influence the rate of REase cleavage of JP structures. In line with this expectation, JP tripartite with four adenine overhang at the 5′-end of probe B (JP2) was cleaved three times faster than those containing four guanines (JPS1), cytosines (JPS2) or thymines (JPS3) (see ESI‡, Fig. S3). As the sequences that lie adjacent to the cleavage site in JP tripartites significantly influence the cleavage rate of JP structures we proceeded to investigate how other architectures, such as duplex overhangs, would influence the REase cleavage rate of JP tripartites (see Fig. 2). REases can form multimers in solution and can also bind a single recognition site or two DNA sites simultaneously.7 It has been demonstrated that a growing number of type II restriction endonucleases require a second cognate recognition site for allosteric activation.8 The crystal structure of the REase, BfuCI, has not been solved and its full biochemical characterization has not been undertaken, to the best of our knowledge. We therefore designed junction probe structures JP6–11 to contain an additional recognition site in the duplex overhang at the 5′-end of probe B to investigate if the presence of an additional cognate binding site would allosterically activate the cleavage of JP structures by BfuCI. As probe B is not cleaved during JP catalysis, it would be advantageous if probe B remains affixed to the target analyte during the catalytic process. Therefore, it is desirable that the second REase cognate recognition site that is placed on the 5′-duplex overhang of probe B contains nuclease resistance sites so that the duplex moiety remains intact during catalysis. Consequently, in JP6–11, the second recognition site on the duplex overhang was modified with phosphoromonothioate at the two cleavage sites. JP7 was cleaved twice as fast as JP6. Although a factor of two is a modest enhancement, it indicates that BfuCI might belong to the growing class of type II REases that are allosterically activated by a second recognition site. The first generation junction probe platform required several hours for cleavage to be completed whereas the second generation design only required minutes for complete cleavage at the same probe/template concentrations.
![The presence of REase recognition site in the duplex overhang at the 5′-end of probe B results in enhanced cleavage of junction probes by BfuCI. The second recognition site in the duplex overhang is made nuclease-resistant viaphosphoromonothioate modification. [Probes A] = [probe B] = 1 μM, [DNA template] = 0.5 μM, [BfuCI] = 0.033 U μL−1.](/image/article/2011/CC/c0cc02208j/c0cc02208j-f2.gif) | ||
| Fig. 2 The presence of REase recognition site in the duplex overhang at the 5′-end of probe B results in enhanced cleavage of junction probes by BfuCI. The second recognition site in the duplex overhang is made nuclease-resistant viaphosphoromonothioate modification. [Probes A] = [probe B] = 1 μM, [DNA template] = 0.5 μM, [BfuCI] = 0.033 U μL−1. | ||
The addition of a third recognition site in the duplex overhang region of probe B (see JP8) did not lead to any further enhancement of cleavage rate. The architecture of the 5′-overhang is also important for JP cleavage. Overhangs that contain additional recognition sites but that differ in structure from the canonical duplex overhang in JP7 led to a dramatic decrease in JP cleavage rate; indicating that steric encumbrance is not tolerated by the enzyme (see Fig. 2, JP9–11). For the proposed catalytic cycle of the junction probe technology, the two fragments of probe A that result from REase cleavage fall off tripartite structure 2 and is replaced by a new uncleaved probe A (see Scheme 1). It therefore follows that factors that influence either the on-rate of probe A or the off-rate of the cleavage product of probe A will affect the rate of JP amplification. We therefore investigated the effect of probe A–template base-pairing number (i.e. the number of base pairs between probe A and the template; designated as x in Fig. S4, ESI‡) on JP cleavage rate. Our expectation was that the JP cleavage rate would increase as x increases (due to increase in on-rate) up to a particular x value and begin to decrease as the value of x increases further (because the off-rate for the cleaved tripartite 2, see Scheme 1, would decrease as x increases). In line with this expectation, the rate of JP cleavage by BfuCI is as follows: x = 7 < 8 < 9 < 10 > 11 > 12 (see ESI‡, Fig. S4).
The motivation of our program is to develop a non-PCR-based and an efficient platform that can detect RNA at isothermal conditions. Along this line, we aimed to detect RNA without extensive sample preparation due to aforementioned problems associated with extensive handling of samples that contain RNA. Secondly, we also aimed to provide a simple detection platform for the detection of microRNA, which is traditionally more difficult to detect because of its comparatively short length and requires specially designed primers for effective RT-PCR detection assays of microRNA.9 Pleasingly, the new junction probe technology that incorporates a second “protected” cleavage site can detect microRNAs such as miR-16 (see Fig. 3a). Importantly, the fluorescence signal generated in the absence of miR-16 was insignificant. This reiterates that although the two JP detection probes have regions of complementarity, they do not associate to an appreciable degree to cause significant background noise. The new JP technology can also detect ribosomal RNA in total E. coliRNA. In this case, probes that were designed to detect E. coliribosomal RNA detected E. coli total RNA better than that of control RNA from V. harveyi (see Fig. 3b). Importantly, REase JP can detect total RNA in a bacterial cell lysate (see ESI‡, Fig. S5) without the need for sophisticated sample clean-up. The second generation JP reported herein can also detect single nucleotide polymorphism in DNA (SNP; see ESI‡, Fig. S6).
![(a) Detection of miRNA. NT = no template was added; (b) E. coli-specific probes detect E. coliribosomal RNA better than that of control RNA from V. harveyi. (a): [Probe A] = [probe B] = 1 μM, [template] = 10 nM, [BfuCI] = 0.1 U μL−1. (b): [Probes A] = [probe B] = 200 nM, [BfuCI] = 0.1 U μL−1.](/image/article/2011/CC/c0cc02208j/c0cc02208j-f3.gif) | ||
| Fig. 3 (a) Detection of miRNA. NT = no template was added; (b) E. coli-specific probes detect E. coliribosomal RNA better than that of control RNA from V. harveyi. (a): [Probe A] = [probe B] = 1 μM, [template] = 10 nM, [BfuCI] = 0.1 U μL−1. (b): [Probes A] = [probe B] = 200 nM, [BfuCI] = 0.1 U μL−1. | ||
In conclusion, REases are generally robust enzymes and have the potential to be used on crude samples without any extensive and costly sample preparation methods. Although the use of nucleases to detect nucleic acid has some precedent in the literature, detailed studies that have looked into how specific topologies affect the efficiency of nuclease-based detection methods are lacking. In this report, we demonstrate that the efficiency of REase-based bio-analyte detection systems can be remarkably improved via the incorporation of specific probe architectures near the enzyme's cognate recognition site. Future work will investigate if the design principles uncovered herein are applicable to other commercially available REases. To date thousands of REases have been reported (over 3500 have been discovered so far); making REase-based detection platforms more amenable to optimization (due to the embarrassment of riches) than other endonuclease-based assays which use specific endonucleases which might be limited in number and diversity.1f The development of isothermal amplification systems for the detection of bioanalytes will continue to attract interest.10 PCR has excellent sensitivity which is almost impossible to rival so these technologies are not meant to replace PCR-based methods but to complement PCR-strategies and to be used in scenarios where PCR is not appropriate due to either technical or financial reasons.
We thank the University of Maryland and NSF (CHE0746446) for Percy Julian fellowship to SY and IG and Azco Biotech for generous supply of materials.
Notes and references
- (a) J. H. Kim, R. A. Estabrook, G. Braun, B. R. Lee and N. O. Reich, Chem. Commun., 2007, 4342–4344 RSC; (b) K. Jang, H. Lee, H. Jin, Y. Park and J. Nam, Small, 2009, 5, 2665–2668 CrossRef CAS; (c) C. J. Fuery, H. L. Impey, N. J. Roberts, T. L. Applegate, R. L. Ward, N. J. Hawkins, C. A. Sheehan, R. O'Grady and A. V. Todd, Clin. Chem. (Washington, DC, U. S.), 2000, 46, 620–624 CAS; (d) A. G. Kanaras, Z. Wang, A. D. Bates, R. Cosstick and M. Brust, Angew. Chem., Int. Ed., 2003, 42, 191–194 CrossRef CAS; (e) Y. Weizmann, Z. Cheglakov, V. Pavlov and I. Willner, Angew. Chem., Int. Ed., 2006, 45, 2238–2242 CrossRef CAS; (f) V. Lyamichev, A. L. Mast, J. G. Hall, J. R. Prudent, M. W. Kaiser, T. Takova, R. W. Kwiatkowski, T. J. Sander, M. de Arruda, D. A. Arco, B. P. Neri and M. A. D. Brow, Nat. Biotechnol., 1999, 17, 292–296 CrossRef CAS.
- W. A. Al-Soud and P. Radstrom, J. Clin. Microbiol., 2001, 39, 485–493 CrossRef.
- S. M. Spencer, L. Lin, C. Chiang, Z. Peng, P. Hesketh, J. Salon and Z. Huang, ChemBioChem, 2010, 11, 1378–1382 CrossRef CAS.
- (a) S. Cai, C. Lau and J. Lu, Anal. Chem., 2010, 82, 7178–7184 CrossRef CAS; (b) Y. Hara, T. Fujii, H. Kashida, K. Sekiguchi, X. Liang, K. Niwa, T. Takase, Y. Yoshida and H. Asanuma, Angew. Chem., Int. Ed., 2010, 49, 5502–5506 CrossRef CAS.
- S. Nakayama, L. Yan and H. O. Sintim, J. Am. Chem. Soc., 2008, 130, 12560–12561 CrossRef CAS.
- M. Koziolkiewicz and W. J. Stec, Biochemistry, 1992, 31, 9460–9466 CrossRef CAS.
- D. H. Kruger, G. J. Barcak, M. Reuter and H. O. Smith, Nucleic Acids Res., 1988, 16, 3997–4008 CrossRef CAS.
- (a) M. Deibert, S. Grazulis, G. Sasnauskas, V. Siksnys and R. Huber, Nat. Struct. Biol., 2000, 7, 792–799 CrossRef CAS; (b) D. T. Bilcock, L. E. Daniels, A. J. Bath and S. E. Halford, J. Biol. Chem., 1999, 274, 36379–36386 CrossRef CAS.
- C. Chen, D. A. Ridzon, A. J. Broomer, Z. Zhou, D. H. Lee, J. T. Nguyen, M. Barbisin, N. L. Xu, V. R. Mahuvakar, M. R. Andersen, K. Q. Lao, K. J. Livak and K. J. Guegler, Nucleic Acids Res., 2005, 33, e179 CrossRef.
- (a) S. Nakayama and H. O. Sintim, J. Am. Chem. Soc., 2009, 131, 10320–10333 CrossRef CAS; (b) S. Nakayama and H. O. Sintim, Mol. BioSyst., 2010, 6, 89–91 RSC; (c) R. M. Franzini and E. T. Kool, J. Am. Chem. Soc., 2009, 131, 16021–16023 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: DNA sequences, experimental details. See DOI: 10.1039/c0cc02208j |
| This journal is © The Royal Society of Chemistry 2011 |
