Recycling and reusing patterned self-assembled monolayers for cell culture†‡
Dawn M.
Johnson
and
Joshua A.
Maurer
*
Department of Chemistry and Center for Materials Innovation, Washington University in St. Louis, Campus Box 1134, One Brookings Drive, St. Louis, MO 63130, USA. E-mail: maurer@wustl.edu; Fax: +1 314-935-4481; Tel: +1 314-935-4695
First published on 4th November 2010
Abstract
Patterned self-assembled monolayers (SAMs) have been widely utilized for the study of cellular growth and behavior. While microcontact printing is a straightforward method of producing patterned substrates, the process is time consuming and requires the use of many techniques and specialized equipment. Here we present a method by which patterned substrates can be reused up to 15 times, saving both time and valuable resources.
The ability to produce patterned substrates suitable for cell culture has become highly desirable. Control of protein adsorption and cell growth allows for the study of cellular behavior and processes. Patterned substrates have been used to examine cellular confinement, cell migration, cell differentiation, and other important biological phenomena.1–8 As a result, these surfaces have the potential to significantly impact many fields of biology.
Reusable biosensors, DNA microarrays, and microfluidic devices have been developed to provide consistent results over the course of many cycles. Recently, major advances have occurred in these fields with the utilization of self-assembled monolayer chemistry for small molecule and protein detection. A reusable immunosensor for label-free detection of insulin has been produced with monolayers formed from polyethylene glycol (PEG) terminated alkanethiols on gold.9 This sensor can be reused more than 25 times without loss of function. Biosensors composed of short chain carboxylic acid terminated SAMs have been shown to be reusable up to 50 times when the thiol monomers forming SAMs are cycled on and off the substrate.10DNA microarrays have been developed through the use of SAM chemistry as well; allylmercaptan forms the surface to which thiol-terminated short DNA strands are covalently bound and can be released by pulsed plasma and re-formed.11 Each of these devices save time and resources because they are recyclable; however, many of them require release and reformation of the monolayer for reuse.
Here we have established a method by which patterned self-assembled monolayer substrates can be recycled up to fifteen times over the course of many days. We have developed two distinct methods for substrate recycling and reuse, which have the potential to be applied to a broad array of systems. Moreover, we have shown that it is possible to vary the cell lines utilized in cell confinement studies.
The production of patterned SAMs for cell culture is a multi-step process involving the use of a wide range of techniques. Photolithography is first used to create a patterned master from which a polydimethylsiloxane (PDMS) stamp can be cast. This stamp is then used to microcontact print hexadecanethiol onto an electron beam evaporated gold substrate and bare regions of the gold substrate are backfilled with an amide-linked glycol terminated thiol capable of resisting protein and cellular adhesion.12–14 Labeled protein can then be added to the surface to produce a well-defined, stable patterned substrate capable of supporting cell adhesion and growth. The production of a patterned substrate from the start of photolithography, through the process of microcontact printing, and finally to the addition of labeled protein can take anywhere from two days to two weeks or more. We have developed time- and cost-effective methods to create substrates that can be recycled and reused several times without disruption of the integrity of the patterned monolayer. Our system will allow any research laboratory to be able to take advantage of patterned substrates in their studies.
All patterned substrates were formed on glass coverslips coated with 50 Å titanium and 150 Å gold deposited by electron beam deposition. Microcontact printing of hexadecanethiol was followed by backfilling with the amide-linked glycol thiol; previously shown to resist protein adsorption and cell growth for up to 33 days.14 The patterned substrates were incubated with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of AlexaFluor 647 labeled and unlabeled human plasma fibronectin and CHO-K1 cells were plated onto the substrates.
1 mixture of AlexaFluor 647 labeled and unlabeled human plasma fibronectin and CHO-K1 cells were plated onto the substrates.
Recycling and reuse of the patterned substrates was completed in a simple two step cycle (Fig. 1). First, cells previously plated on the substrates were removed by TrypLE Express (a stable trypsin analogue) mediated release or by washing with 1% Triton X-100. Following release, new cells, either CHO-K1 or NIH/3T3, were plated onto the substrates. For each method, cycles of cell removal and cell plating were repeated until the patterned substrate no longer yielded consistent confinement.
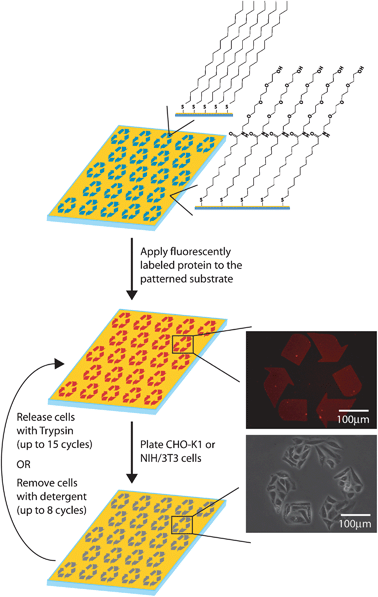 | ||
| Fig. 1 Schematic of the production of recyclable patterned substrates. | ||
In several trials, patterned substrates could be reused up to 15 times over the course of eleven days when cells were removed by TrypLE Express (Fig. 2). Both CHO-K1 and NIH/3T3 cells were cycled alternately. The cells were confined to 340 μm recycle symbols for 4–12 hours between release and reuse of the substrate. Typically, CHO-K1 cells were grown for 4 hours between cycles, while NIH/3T3 cells were grown for 12 hours due to variations in spreading and proliferation rates. No significant defects were found on the substrate until day 11 (cycle 15). A significant defect is observed upon cell spreading and proliferation, rather than attachment of cells possessing a round morphology. Cells exhibiting a round morphology were typically dead and easily rinsed off the substrate with Dulbecco's Phosphate Buffered Saline (DPBS). In previous studies using the amide-linked glycol, CHO-K1 cells cultured on these substrates and allowed to proliferate without disruption were confined to a pattern for up to 33 days.14 The discrepancy in surface stability is most likely due to the introduction of new cells. In this system, new, healthy cells are repeatedly introduced to the patterned substrate at high concentrations, providing a greater opportunity for cells to detect and attach at defect sites. The cells can then excrete extracellular proteins onto any defect site creating a larger and more accessible site for subsequent cell attachment and outgrowth. When substrates are used in a single long-term cell culture experiment, greater stability is observed as cells that detach are primarily unhealthy or dead.
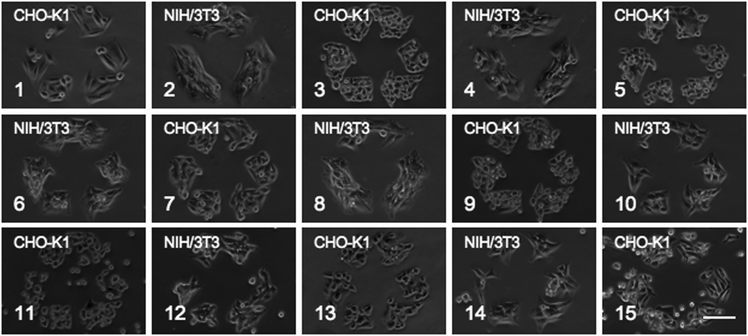 | ||
| Fig. 2 Fifteen TrypLE Express washing cycles of alternating patterned CHO-K1 and NIH/3T3 cells. Scale bar is 100 μm. | ||
When cells were removed with detergent (Triton X-100), the patterned substrates were stable for up to eight cycles (Fig. 3). As the number of cycles increased, degradation of the protein pattern was observed. The protein pattern was initially very sharp and became increasingly round and ragged along the edges affecting the area of cell growth (Fig. S1, ESI‡). After approximately six cycles, some areas of the substrate became confluent while other areas continued to confine cells to the recycle pattern. This is most likely due to disruption of the monolayer by the detergent. Triton X-100 could associate its hydrophilic head group into the amide-linked glycol thiol SAM, exposing its hydrophobic tail, thus forming defect sites throughout the background. This would allow cells to attach and proliferate outside the pattern.
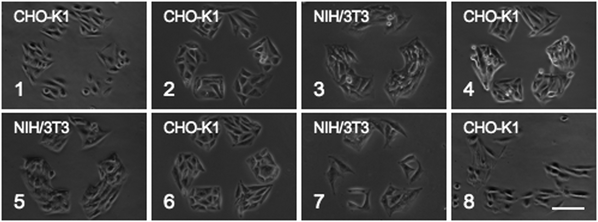 | ||
| Fig. 3 Eight detergent washing cycles of CHO-K1 and NIH/3T3 patterned cell growth. Scale bar is 100 μm. | ||
The TrypLE Express and Triton X-100 recycling methods were tested for SAMs comprised of (1-mercaptoundec-11-yl)tetra(ethyleneglycol), a commercially available ether-linked glycol thiol monomer commonly used in SAM formation. For both methods, only four cycles (in three days) could be completed before the integrity of the patterned monolayer was lost and cell growth was observed outside the pattern (Fig. S2, ESI‡). This suggests that the amide-linked glycol thiol is crucial to the creation of recyclable patterned monolayers.
Each method for cell removal and recycling of the patterned substrate is applicable to different types of studies. The trypsin recycling method is ideal for studies where the main objective is to retain the integrity of the SAM. Since trypsinization does not kill cells, this method would be preferred for research studies that utilize the same cell line for multiple trials. In contrast, the Triton X-100 recycling method is best for experiments involving multiple cell lines. As 1% Triton X-100 rapidly kills cells, the original cell line is no longer viable and can be completely replaced with a subsequent cell line. This protocol can be used in studies where the initial cell line would interfere with the growth of the subsequent cell line.
This system of recycling and reusing patterned SAMs provides the ability to rapidly explore cell culture on patterned substrates. Our recycling strategies are compatible with a variety of cell types, as demonstrated through the use of CHO-K1 and NIH/3T3 cell lines. These methods reuse the same patterned substrate providing greater consistency of the pattern and allowing for reproducibility between experiments. In addition, our methods drastically reduce the time between trials. A typical patterning experiment takes at least 12–14 hours for substrate preparation. Recycling patterned substrates reduces this time to just five or ten minutes. Here we have shown that recycling patterned substrates will save both time and resources while maintaining well-confined cell studies. Advances in research technology and methodology like those shown here allow the scientific community to perform more research studies using fewer disposable resources.
This work was supported by the National Institute of Mental Health (1R01MH085495). We thank Matthew K. Strulson for synthesizing the amide-linked glycol thiol and ether-linked glycol thiol used during these studies and Natalie A. LaFranzo for providing insightful discussion.
Notes and references
- A. Brock, E. Chang, C. Ho, P. LeDuc, X. Jiang, G. Whitesides and D. Ingber, Langmuir, 2003, 19, 1611–1617 CrossRef CAS.
- N. Faucheux, R. Schweiss, K. Lutzow, C. Werner and T. Groth, Biomaterials, 2004, 25, 2721–2730 CrossRef CAS.
- Y. Luk, M. Kato and M. Mrksich, Langmuir, 2000, 16, 9604–9608 CrossRef.
- M. Mrksich, C. Chen, Y. Xia, L. Dike, D. Ingber and G. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 10775–10778 CrossRef CAS.
- M. Mrksich and G. Whitesides, Annual Review of Biophysics and Biomolecular Structure, 1996, 25, 55–78 CrossRef CAS.
- K. Prime and G. Whitesides, Science, 1991, 252, 1164–1167 CrossRef CAS.
- K. Simon, E. Burton, Y. Han, J. Li, A. Huang and Y. Luk, J. Am. Chem. Soc., 2007, 129, 4892–4893 CrossRef CAS.
- C. Chen, M. Mrksich, S. Huang, G. Whitesides and D. Ingber, Science, 1997, 276, 1425–1428 CrossRef CAS.
- K. Gobi, H. Iwasaka and N. Miura, Biosens. Bioelectron., 2007, 22, 1382–1389 CrossRef CAS.
- S. Choi and J. Chae, Biosens. Bioelectron., 2009, 25, 527–531 CrossRef CAS.
- W. Schofield, J. McGettrick, T. Bradley, J. Badyal and S. Przyborski, J. Am. Chem. Soc., 2006, 128, 2280–2285 CrossRef CAS.
- M. Mrksich, L. Dike, J. Tien, D. Ingber and G. Whitesides, Exp. Cell Res., 1997, 235, 305–313 CrossRef CAS.
- K. Prime and G. Whitesides, J. Am. Chem. Soc., 1993, 115, 10714–10721 CrossRef CAS.
- M. K. Strulson, D. M. Johnson and J. A. Maurer, 2010, submitted.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm |
| ‡ Electronic supplementary information (ESI) available: Detailed experimental procedures and supplemental data. See DOI: 10.1039/c0cc02262d |
| This journal is © The Royal Society of Chemistry 2011 |
