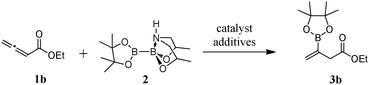
Regio- and stereoselective copper-catalyzed β-borylation of allenoates by a preactivated diboron†‡
Steven B.
Thorpe
,
Xi
Guo
and
Webster L.
Santos
*
Department of Chemistry, Virginia Tech, Blacksburg, Virginia 24061, USA. E-mail: santosw@vt.edu; Fax: +1 540 231 3255; Tel: +1 540 231 5041
First published on 20th September 2010
Abstract
A mild and efficient copper-catalyzed borylation of electron deficient allenoates using an sp2–sp3 mixed hybridized diboron regioselectively installs a boron moiety on the β-position with exclusive (Z)-double bond geometry.
In recent years, allenes have emerged as an important structural unit in the construction of complex molecules.1 For example, cycloaddition, radical, nucleophilic addition and metalation reactions demonstrate the versatility and unique reactivity of allenes.2 Because of their potential in organic synthesis and other applications, their preparation is subject to intense investigation. In particular, the reactivity of electron-deficient allenes can be tuned to produce either β,γ-unsaturated carbonyl compounds or γ-addition products depending on the reaction conditions. For example, Shibasaki and co-workers elegantly developed the construction of highly functionalized δ-lactones with tetrasubstituted chiral centers using a three component assembly of dialkylzincs, allenic esters and ketones.3 Jorgensen et al. demonstrated the organocatalytic asymmetric conjugate addition to electron-deficient allenes to form tertiary and quaternary stereogenic centers.4 These reactions produce β,γ-unsaturated carbonyl compounds, and examples in the literature are limited. However, the regioselectivity of the reaction can be reversed. Indeed, Lewis bases such as phosphines catalyze γ-additions of carbon, nitrogen, oxygen and sulfur nucleophiles to allenoates.5
Transition metal-catalyzed reactions of electrophilic allenoates generate molecular scaffolds with functional and structural diversity.1a,6 For example, 2,3-allenyl carboxylic acids cycloisomerize in the presence of Cu(I) source7 or undergo halolactonization with CuX2 (X = Br, Cl).8 These intermediates are useful building blocks in the synthesis of more elaborate chemical structures, especially with cross-coupling reactions. As complementary reacting partners, organoboron compounds increasingly provide access to the formation of difficult carbon–carbon bonds. Indeed, the Suzuki–Miyaura coupling reaction, which employs a boronic acid and organohalide, is a powerful method for the construction of complex molecules partly because of its functional group tolerance.9 Thus far, silaboration,10 cyanoboration,11 and diboration12 have been achieved with allenes using transition metals. To date, however, direct borylation of electron-deficient allenes has not been reported. We recently disclosed the synthesis of an internally activated, sp2–sp3 hybridized diboron compound, PDIPA diboron (pinacolato diisopropanolaminato diboron, 2), and demonstrated its utility in the copper-catalyzed, β-borylation of α,β-unsaturated conjugated compounds.13 In pursuit of expanding the scope of this reaction to more intricate substrates, we investigated the utility of 2 to the borylation of electrophilic allenoates. These substrates are attractive because they give rise to β,γ-unsaturated carbonyl compounds, as opposed to acetylenic esters which produce α,β-unsaturated carbonyl compounds,14 allowing for further functionalization of the resulting nonconjugated olefin. In addition, we were excited by the possibility that a racemic mixture of the starting allenoate forms a product containing a defined double bond geometry and a vinyl boronic ester, which is potentially a Suzuki–Miyaura cross-coupling partner.9
To explore the feasibility of the borylation reaction, we investigated reaction conditions to generate β-borylated β,γ-unsaturated ethyl ester 3b using ethyl 2,3-butadienoate (1b) as the substrate. As shown in Table 1, agents with strong sigma bond donor capacity to activate bis(pinacolato)diboron such as N-heterocyclic carbenes (NHC),15(IMe)CO216 and (ICy)BF4,17 promoted the borylation reaction with good yields (entries 1 and 2). However, the use of a strong base to generate the carbene can be a disadvantage for sensitive substrates. In addition, NHC ligands are expensive and difficult to handle. With the effort to use milder reaction conditions, we investigated whether a preactivated diboron13 also effects the desired transformation. To our delight, treating allenoate 1b with a catalytic amount of CuCl, trifluoroethanol (TFE) and 2 in dichloromethane produced 3b in excellent yield (entry 3).18 Running the reaction in the absence of TFE successfully provided the product (entries 4 and 12). Other additives such as base and phosphine ligand were effective although a copper stabilizing DPEphos dramatically decreased the product yield (entries 5–7). As expected, when the reaction was attempted without a copper catalyst, the reaction was sluggish (entry 8). Evaluation of the effect of solvent revealed that the reaction was tolerant of aprotic solvents used, although the reaction performed in THF was most effective (entries 4, 9 and 10). We also screened the effect of temperature on the reaction and discovered that the borylation reaction is efficient at room temperature in the presence of TFE (compare entries 1–10 vs. 11). CuCl as the copper source appears to be important since CuBr or other Cu(II) sources afforded the product in moderate yields (entries 12–14). Finally, an examination of other transition metals such as Rh, Pt, Ni and Ag resulted in minor or undetectable product formation (entries 15–18). Thus, subsequent investigations used 10 mol% CuCl, 4 equiv. TFE, allenoate substrate and 2 in THF at room temperature.
| Entry | Catalyst | Solvent | Additives (equiv.) | Conv%b |
|---|---|---|---|---|
| a PDIPA diboron (2, 1.2 equiv.), catalyst (0.1 equiv.), and additives were suspended in solvent and stirred for 5 minutes. Ethyl 2,3-butadienoate (1.1 equiv.) was then added and the reaction was stirred for 2 hours at the indicated temperature. b Conversion was determined by GC analysis of the crude material. c The reaction was performed at rt and bis(pinacolato)diboron was used instead of 2. d The reaction was heated to 40 °C. e The reaction was heated to 70 °C. f The reaction was performed at rt. Abbreviations: DPEphos = bis(2-diphenylphosphinophenyl)ether; (IMe)CO2 = 1,3-dimethylimidazolium carboxylate; (ICy)BF4 = 1,3-dicyclohexylimidazolium tetrafluoroborate; TFE = 2,2,2-trifluoroethanol; ND = none detected. | ||||
| 1 | CuCl c | ACN | (IMe)CO2 (0.1) | 81.3 |
| 2 | CuCl c | THF | (ICy)BF4 (.05) | |
| NaOtBu (0.1) | 95.1 | |||
| 3 | CuCl d | CH2Cl2 | TFE (4) | 96.9 |
| 4 | CuCl d | CH2Cl2 | — | 96.4 |
| 5 | CuCl d | CH2Cl2 | NaOtBu (0.1) | 98.3 |
| 6 | CuCl d | CH2Cl2 | DPEphos (0.1) | 45.7 |
| 7 | CuCl d | CH2Cl2 | NaOtBu (0.1) | |
| TFE (4) | 97.5 | |||
| 8 | —d | CH2Cl2 | — | 4.9 |
| 9 | CuCl d | DMF | — | 78.0 |
| 10 | CuCl e | THF | — | 98.3 |
| 11 | CuCl f | THF | TFE (4) | 96.7 |
| 12 | CuBr e | THF | — | 87.8 |
| 13 | CuBr2e | THF | — | 76.6 |
| 14 | CuCl2e | THF | — | 86.7 |
| 15 | [Rh(cod)Cl]2e | THF | — | ND |
| 16 | Pt(cod)Cl2e | THF | — | ND |
| 17 | Ni(cod) e | THF | — | ND |
| 18 | Ag(NO3) e | THF | — | 1.5 |
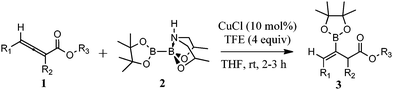
With the optimized conditions in hand, we next investigated the scope of the reaction using various allenoates. The results of our study are summarized in Table 2. Increasing the size of the ester moiety from methyl to ethyl on unsubstituted allenoates provided the β-borylated β,γ-unsaturated ethyl esters 3a and b in good yields (entries 1 and 2). A change to the bulkier 3-phenylpropyl ester 1c resulted in slight decrease in yield with some unreacted starting material isolated (entry 3). Gratifyingly, the presence of a bulky phenyl substituent on the γ-position allowed the borylation to proceed in moderate yields (entries 4–6). When the phenyl substituent was replaced with a less sterically demanding methyl group, the product was isolated in good yield (58–78%, entries 7–10). The presence of a bulky o-nitrobenzyl moiety resulted in a much lower yield in part because this group is sensitive to light (entry 11). Finally, α,γ-disubstituted ethyl allenoate 1l was converted to the product in moderate yield presumably because of the bulkiness of the substrate (entry 12).
| Entry | R1 | R2 | R3 | Product | Z/Eb | %c Yield (%)d |
|---|---|---|---|---|---|---|
| a For detailed synthetic procedure, see ESI.2 b The ratio of E/Z isomers were determined by GC and the geometry of the product was assigned based on NOESY experiments. c Isolated yield. d Corrected yield with recovered starting material. e Reaction was stirred 24 hours before workup. Product contaminated with 22% α,β-unsaturated isomer. | ||||||
| 1 | H | H | Me | 3a | — | 59 |
| 2 | H | H | Et | 3b | — | 60 |
| 3 | H | H | (CH2)3Ph | 3c | — | 41 (71) |
| 4 | Ph | H | Me | 3d | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
41 |
| 5 | Ph | H | Et | 3e | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
46 |
| 6 | Ph | H | (CH2)3Ph | 3f | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
14 (45) |
| 7 | Me | H | Me | 3g | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
72 |
| 8 | Me | H | Et | 3h | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
58 |
| 9 | Me | H | i Pr | 3i | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
78 |
| 10 | Me | H | t Bu | 3j | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
71 |
| 11 | Me | H | o-Nitrobenzyl | 3k | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
33 (75) |
| 12 | Me | Me | Et | 3l | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
38e (81) |
It is important to highlight that the copper catalyzed β-borylation reaction proceeded not only regioselectively but also diastereoselectively. In contrast with phosphine-catalyzed reactions to electrophilic allenoates that afford γ-substituted products,5 the copper-catalyzed reaction regioselectively installed the boryl moiety on the β-position. In addition, under the reaction conditions of our investigation, we did not observe any cycloisomerization products seen in other copper-catalyzed reactions with allenoates (vide infra).7 Interestingly, the geometry of the double bond on the resulting product was determined to be Z based on NOESY experiments, and 1H NMR and GC-MS analysis of the crude reaction mixture showed exclusive formation of this product.19 The stereoselectivity of the reaction can be rationalized by analysis of the approach of the boryl–copper intermediate (Fig. 1). Since the two double bonds are orthogonal to each other, boryl addition to the β-carbon is expected to occur on the opposite side of the γ-substituent of the allenoate because of a strong steric interaction. Furthermore, 1,3-allylic strain in the (E)-isomer should favor the formation of the more stable (Z)-isomer. Indeed, only the (Z)-product was observed. Finally, we were pleased to find that although the allenoate substrates were used as racemic mixtures, the reaction provides a single product.
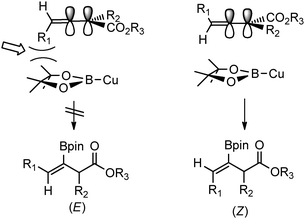 | ||
| Fig. 1 Steric interactions lead to exclusive formation of the (Z)-isomer. | ||
A possible catalytic cycle for the β-borylation reaction is shown in Fig. 2. Preactivated sp2–sp3 hybridized diboron 2 is sufficiently activated to transmetalate with CuCl to generate nucleophilic boryl species 4. DFT calculations on the copper-catalyzed borylation of related α,β-unsaturated carbonyl compounds20 suggest the formation of metalated intermediates 6/7 that can be protonated to yield the desired β-borylated β,γ-unsaturated ester 8 with the simultaneous regeneration of copper–alkoxide that continues the catalytic cycle. The essential role of TFE is to accelerate the reaction by protonation of 6/7 and formation of copper–alkoxide.18
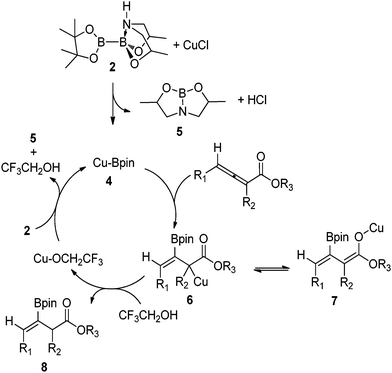 | ||
| Fig. 2 Proposed reaction mechanism. | ||
To demonstrate the utility of the regio- and stereoselective borylation reaction, Suzuki–Miyaura cross coupling reaction between 3g and iodobenzene was attempted. In this case, the coupled product 9 was isolated in quantitative yield (Fig. 3). In contrast, 9 was previously synthesized as a mixture of E/Z isomers at 52% yield from a cyclopropanone acetal derivative.21
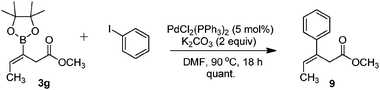 | ||
| Fig. 3 Coupling of β-boronic ester with iodobenzene. | ||
In conclusion, an efficient and catalytic copper-catalyzed regioselective borylation of allenoates was developed. To the best of our knowledge, this reaction is the first example of a boryl addition to electrophilic allenoates. The reaction provided β-borylated β,γ-unsaturated esters with exclusive (Z)-double bond geometry. These borylated products are useful intermediates for subsequent elaboration to more complex structures.
We gratefully acknowledge financial support from Virginia Tech Department of Chemistry and the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust.
Notes and references
- For reviews, see: (a) S. Ma, Acc. Chem. Res., 2003, 36, 701 CrossRef CAS; (b) R. Zimmer, C. U. Dinesh, E. Nandanan and F. A. Khan, Chem. Rev., 2000, 100, 3067 CrossRef; (c) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC.
- For a review, see: S. Ma, Chem. Rev., 2005, 105, 2829 Search PubMed.
- (a) K. Oisaki, D. Zhao, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2007, 129, 7439 CrossRef CAS; (b) D. Zhao, K. Oisaki, M. Kanai and M. Shibasaki, Tetrahedron Lett., 2006, 47, 1403 CrossRef CAS; (c) D. Zhao, K. Oisaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2006, 128, 14440 CrossRef CAS.
- P. Elsner, L. Bernardi, G. D. Salla, J. Overgaard and K. A. Jorgensen, J. Am. Chem. Soc., 2008, 130, 4897 CrossRef CAS.
- (a) B. M. Trost and C. J. Li, J. Am. Chem. Soc., 1994, 116, 3167 CrossRef CAS; (b) B. M. Trost and C. J. Li, J. Am. Chem. Soc., 1994, 116, 10819 CrossRef CAS; (c) C. Zhang and X. Lu, Synlett, 1995, 645 CrossRef CAS; (d) Z. Chen, G. Zhu, Q. Jiang, D. Xiao, P. Cao and X. Zhang, J. Org. Chem., 1998, 63, 5631 CrossRef CAS; (e) S. W. Smith and G. C. Fu, J. Am. Chem. Soc., 2009, 131, 14231 CrossRef CAS; (f) B. Liu, R. Davis, B. Joshi and D. W. Reynolds, J. Org. Chem., 2002, 67, 4595 CrossRef CAS; (g) C. Lu and X. Lu, Org. Lett., 2002, 4, 4677 CrossRef CAS; (h) Y. K. Chung and G. C. Fu, Angew. Chem., Int. Ed., 2009, 48, 2225 CrossRef CAS; (i) J. Sun and G. C. Fu, J. Am. Chem. Soc., 2010, 132, 4568 CrossRef CAS.
- S. Ma, Acc. Chem. Res., 2009, 42, 1679 CrossRef CAS.
- S. Ma, Z. Yu and S. Wu, Tetrahedron, 2001, 57, 1585 CrossRef CAS.
- S. Ma and S. Wu, J. Org. Chem., 1999, 64, 9314 CrossRef CAS.
- D. Hall, Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine, Wiley-VCH GmbH & Co., Weinheim, 2005 Search PubMed.
- (a) M. Suginome and Y. Ito, J. Organomet. Chem., 2003, 680, 43 CrossRef CAS; (b) S.-Y. Onozawa, Y. Hatanaka and M. Tanaka, Chem. Commun., 1999, 1863 RSC; (c) M. Suginome, Y. Ohmori and Y. Ito, Synlett, 1999, 1567; (d) K.-J. Chang, D. K. Rayabarapu, F.-Y. Yang and C.-H. Cheng, J. Am. Chem. Soc., 2004, 127, 126; (e) M. Suginome, T. Ohmura, Y. Miyake, S. I. Mitani, Y. Ito and M. Murakami, J. Am. Chem. Soc., 2003, 125, 11174 CrossRef CAS.
- A. Yamamoto, Y. Ikeda and M. Suginome, Tetrahedron Lett., 2009, 50, 3168 CrossRef CAS.
- (a) A. R. Woodward, H. E. Burks, L. M. Chan and J. P. Morken, Org. Lett., 2005, 7, 5505 CrossRef CAS; (b) F.-Y. Yang and C.-H. Cheng, J. Am. Chem. Soc., 2001, 123, 761 CrossRef CAS; (c) T. Ishiyama, T. Kitano and N. Miyaura, Tetrahedron Lett., 1998, 39, 2357 CrossRef CAS; (d) H. E. Burks, S. Liu and J. P. Morken, J. Am. Chem. Soc., 2007, 129, 8766 CrossRef CAS; (e) N. F. Pelz, A. R. Woodward, H. E. Burks, J. D. Sieber and J. P. Morken, J. Am. Chem. Soc., 2004, 126, 16328 CrossRef CAS.
- M. Gao, S. B. Thorpe and W. L. Santos, Org. Lett., 2009, 11, 3478 CrossRef CAS.
- J.-E. Lee, J. Kwon and J. Yun, Chem. Commun., 2008, 733 RSC.
- A. Bonet, H. Gulyas and E. Fernandez, Angew. Chem., Int. Ed., 2010, 49, 5130 CrossRef CAS.
- A. M. Voutchkova, M. Feliz, E. Clot, O. Eisenstein and R. H. Crabtree, J. Am. Chem. Soc., 2007, 129, 12834 CrossRef CAS.
- K. S. Lee, A. R. Zhugralin and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 7253 CrossRef CAS.
- Detailed kinetic studies of the borylation of α,β-unsaturated carbonyls with 2 show that TFE is the best protic additive. M. Gao, S. B. Thorpe, C. Kleeberg, T. B. Marder, W. L. Santos, submitted.
- See ESI‡ for details.
- L. Dang, H. T. Zhao, Z. Y. Lin and T. B. Marder, Organometallics, 2008, 27, 1178 CrossRef CAS.
- A. Oku, M. Abe and M. Iwamoto, J. Org. Chem., 1994, 59, 7445 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Detailed experimental procedures, synthesis and characterization of unknown allenoates, analytical data for β-borylated β,γ-unsaturated esters. See DOI: 10.1039/c0cc02270e |
| This journal is © The Royal Society of Chemistry 2011 |