A highly porous flexible Metal–Organic Framework with corundum topology†‡
Ronny
Grünker
,
Irena
Senkovska
,
Ralf
Biedermann
,
Nicole
Klein
,
Martin R.
Lohe
,
Philipp
Müller
and
Stefan
Kaskel
*
Dresden University of Technology, Department of Inorganic Chemistry, Bergstrasse 66, 01069 Dresden, Germany. E-mail: stefan.kaskel@chemie.tu-dresden.de; Fax: +49 351 463 37287; Tel: +49 351 463 34885
First published on 18th October 2010
Abstract
A flexible Metal–Organic Framework Zn4O(BenzTB)3/2 (DUT-13) was obtained by combination of a tetratopic linker and Zn4O6+ as connector. The material has a corundum topology and shows the highest pore volume among flexible MOFs.
In the last two decades a new class of materials, namely Metal–Organic Frameworks (MOFs), has set records concerning high specific surface area and pore volume.1–4 Compared to other porous materials like zeolites, mesoporous oxides or activated carbons, MOFs of the so called 3rd generation have flexible structures.5 The structural changes are often induced by guest molecules acting as external stimuli. Therefore those compounds are promising candidates for applications in gas separation,6,7 as drug delivery agents8 or sensors.9 A number of representatives for this generation have been reported. Regarding their topology they can be grouped into the following categories: interdigitated 2D-layer structures like Cu(dhbc)2(bpy),10Cd(bpndc)(bpy)11 and Zn2(ip)2(bpy)2,12 pillared-layer compounds like M2(L)2(dabco) (M = Zn, Ni, Cu; L = 1,4-bdc, 2,6-ndc)13 or Cu2(pzdc)2(dpyg),14 coordination polymers with channel systems like Ag3(atz)215 and the MIL-53 series,16 or interpenetrated compounds with moving networks caused by π,π-stackings of the linker molecules.7,17§ The compounds show distinguished flexibility, the so called “gate-pressure” effect. That means that the pore system shrinks or expands at a certain loading of guest molecules at given conditions.
Recently we reported a series of compounds based on the tetratopic N,N,N′,N′-benzidinetetrabenzoate (BenzTB4−) linker and paddle-wheel units (DUT-10/11/12, DUT = Dresden University of Technology), which show interesting fluorescence and selective gas adsorption properties. The compounds show an irreversible transformation during the desolvation procedure by shrinkage of the pore system. In this case the pore narrowing causes the selective gas adsorption properties.18 Furthermore, a doubly-interpenetrated magnesium contained framework (SNU-25) with selective gas adsorption properties was synthesized by Suh et al. using BenzTB4− as ligand.19
Herein, we report the synthesis and characterization of a new microporous Metal–Organic Framework, Zn4O(BenzTB)3/2 (DUT-13), based on the same linker but with exceptional high pore volume and a “reverse type” of flexibility: framework opening effect caused by adsorption of N2 (at −196 °C), CO2 (at −78 °C) and n-C4H10 (at 20 °C).
The N,N,N′,N′-benzidinetetrabenzoic acid (H4BenzTB) was synthesized as described earlier (see ESI‡).18 Single crystals of Zn4O(BenzTB)3/2 suitable for X-ray diffraction analysis were obtained by solvothermal reaction of H4BenzTB and zinc nitrate in N,N-diethylformamide (DEF) and glacial acetic acid as additive.‡ The compound crystallizes in the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c and shows a corundum topology (cor). The structure is built up by Zn4O(O2C)6 as Secondary Building Unit (SBU) and presents two different types of pores. The main larger pore centered at Wyckoff positions 6b (0,0,0) with C3i symmetry is constructed by eight Zn4O6+ clusters that are arranged in hexagonal bipyramidal geometry and connected by six BenzTB4− ligands. The dimension of this pore is 14.7 × 9.5 Å in diameter (van der Waals radii of corresponding atoms have been considered). The solvent-accessible volume calculated with PLATON20 is 53 995 Å3 corresponding to 82.4% of the unit cell volume. The pores form a rhombohedrally distorted cubic close packing (ccp) (Fig. S2, ESI‡).
c and shows a corundum topology (cor). The structure is built up by Zn4O(O2C)6 as Secondary Building Unit (SBU) and presents two different types of pores. The main larger pore centered at Wyckoff positions 6b (0,0,0) with C3i symmetry is constructed by eight Zn4O6+ clusters that are arranged in hexagonal bipyramidal geometry and connected by six BenzTB4− ligands. The dimension of this pore is 14.7 × 9.5 Å in diameter (van der Waals radii of corresponding atoms have been considered). The solvent-accessible volume calculated with PLATON20 is 53 995 Å3 corresponding to 82.4% of the unit cell volume. The pores form a rhombohedrally distorted cubic close packing (ccp) (Fig. S2, ESI‡).
Smaller pores (not detected by PLATON) with 6.8 × 5.0 Å in diameter are placed in the pseudo-octahedral holes of ccp (Fig. 1). They are formed by two Zn4O6+ clusters and three linker molecules. To the best of our knowledge DUT-13 is the first MOF with a complex pore system typical for rigid MOFs and a flexible framework (“gate-pressure” properties).
![Crystal structure of DUT-13 (a) the large hexagonal bipyramidal (red) and the small (yellow) pore; (b) arrangement of the large pores in a plane vertically along [0 0 1] in a ccp motif (red and yellow spheres indicate pore volume).](/image/article/2011/CC/c0cc02273j/c0cc02273j-f1.gif) | ||
| Fig. 1 Crystal structure of DUT-13 (a) the large hexagonal bipyramidal (red) and the small (yellow) pore; (b) arrangement of the large pores in a plane vertically along [0 0 1] in a ccp motif (red and yellow spheres indicate pore volume). | ||
To study the porosity of DUT-13, the nitrogen physisorption measurement was performed at −196 °C. For this purpose the synthesized material was washed with DEF, followed by an exchange of the solvent with absolute ethanol, and finally dried using a supercritical carbon dioxide technique.21 The nitrogen physisorption measurement reveals two steps in the adsorption branch of the isotherm (Fig. 2 and Fig. S3, ESI‡). Up to a relative pressure of 0.15 the observed isotherm corresponds to a classical type I isotherm reaching the plateau at about 600 cm3 g−1. At a pressure of 0.2 p/p0 (so called gate-opening pressure), a sudden rise in the isotherm occurs. The isotherm reaches saturation at 1279 cm3 g−1. The total pore volume estimated at p/p0 = 0.99 is 1.98 cm3 g−1 and therefore the highest value for flexible MOFs observed so far and among the highest values reported for MOFs (MOF-177: N2 saturation uptake (SU): 1350 cm3 g−1, pore volume (VP): 1.59 cm3 g−1;1 UMCM-2: SU: 1500 cm3 g−1, VP: 2.02 cm3 g−1;4 DUT-6: SU: 1380 cm3 g−1, VP: 2.02 cm3 g−1;3PCN-68: VP: 2.13 cm3 g−1).22 The desorption branch does not trace the adsorption branch forming a hysteresis loop which closes at relative pressure of 0.02 (gate-closing pressure). At lower pressures (p/p0 ≤ 0.01) a second smaller hysteresis is obtained. Several reruns of the N2 adsorption experiment using the same sample show a smaller hysteresis loop ending up in a type I like isotherm (see Fig. S4, ESI‡) with a maximum amount adsorbed of 524 cm3 g−1. Powder X-ray diffraction (PXRD) experiments after N2 adsorption indicate changes of the structure of DUT-13 (Fig. S10, ESI‡). The comparison of PXRD patterns after the first and third adsorption cycle reveals the partial conversion of the framework (or maybe conversion of the part of crystallites) into another phase during the first adsorption cycle and further transformation in the following cycles.
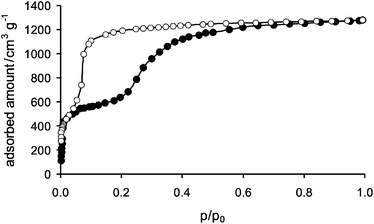 | ||
| Fig. 2 N2adsorption/desorption (●/○) isotherms of DUT-13 (−196 °C). | ||
The physisorption isotherm of n-butane measured at 20 °C under atmospheric pressure and dynamic conditions (n-butane diluted with nitrogen) is shown in Fig. S6, ESI.‡ At 15% n-butane by volume a first plateau in the isotherm with a storage capacity of 0.36 g g−1 is reached. Higher n-butane concentrations (40 to 80% n-butane by volume) lead to a further increase of the adsorption capacity to 0.76 g g−1. As shown for the nitrogen adsorption at −196 °C the desorption branch reveals a different inflection in comparison to the adsorption branch. A hysteresis loop is observed which closes at 8% n-butane by volume whereas the first plateau of the adsorption was almost reached. Even for lower concentrations (8 to 0% n-butane by volume) the branches are shifted. Similar to the nitrogen adsorption, the second and third run of the physisorption performed on the same sample does not match the first measurement with a decrease of the total adsorption capacity to 0.38 g g−1 at the third run (Fig. S7, ESI‡).
Interestingly, the adsorption of supercritical gases like hydrogen and methane at high pressures does not induce the gate opening (Fig. 3, Fig. S8, ESI‡) and has no influence on the crystal structure of DUT-13 as confirmed by PXRD analysis (Fig. S10, ESI‡).¶ The volumetric hydrogen adsorption measurement for DUT-13 reveals a maximum excess hydrogen storage capacity of 613 cm3 g−1 at 56 bar and −196 °C equal to 55 mg g−1 (5.23 wt%). With these values DUT-13 is ranking among the best MOFs for hydrogen storage (MOF-177: 75 mg g−1 at 60 bar,23PCN-68: 73 mg g−1 at 50 bar,22DUT-6: 60 mg g−1 at 50 bar and −196 °C).3
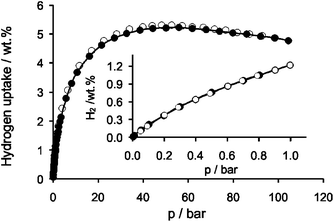 | ||
| Fig. 3 High pressure excess hydrogen (−196 °C) adsorption (●) and desorption (○) of DUT-13; inset: low pressure region up to 1 bar (−196 °C). | ||
For applications such as natural gas storage, the methane physisorption isotherm was measured by use of a magnetic suspension balance at 25 °C up to 150 bar (Fig. S8, ESI‡). At 35 bar the excess uptake of DUT-13 is 131 mg g−1 (71 cm3 cm−3 based on the crystallographic density). The maximum gravimetric amount of methane adsorbed is 198 mg g−1 at 90 bar equal to 107 cm3 cm−3. With these values DUT-13 is comparable to some of the best methane storage materials such as MIL-101 (239 mg g−1 at 125 bar),24DUT-6 (230 mg g−1 at 100 bar)3 and COF-102 (243 mg g−1 at 85 bar)25 in terms of gravimetric values.
Because of its property as a greenhouse gas, the removal of CO2 from exhaust gas is of increasing interest for industrial applications. At −78 °C DUT-13 stores up to 379 cm3 g−1 up to 1 bar (Fig. S9, ESI‡). Just as for nitrogen and n-butane a hysteresis in the desorption branch was observed. PXRD measurements show a structural change similar to that during N2 physisorption (Fig. S10, ESI‡). N2 physisorption after CO2 adsorption indicates that this material still has a high residual porosity up to 2532 m2 g−1 (BET 0.007 ≤ p/p0 ≤ 0.1) with a saturation N2 uptake of 769 cm3 g−1 and a typical type I isotherm (Fig. S5, ESI‡).
In conclusion, utilizing the tetracarboxylic acid H4BenzTB as linker we synthesized and characterized a new highly porous Metal–Organic Framework Zn4O(BenzTB)3/2 (DUT-13), showing flexibility as response to adsorption of certain gases. The MOF has a rare corundum topology not typical for flexible MOFs known so far. Physisorption measurements revealed high storage capabilities of DUT-13 for nitrogen (1279 cm3 g−1), hydrogen (613 cm3 g−1, 5.23 wt% at 56 bar) and n-butane (0.76 g g−1).
This work was financially supported by the German Research Foundation (SPP 1362). A special thank goes to the Helmholtz Zentrum Berlin (HZB) for the beam time at BESSY BL MX 14.2, Dr. Igor A. Baburin (Dresden University of Technology, Department of Physical Chemistry and Electrochemistry) for topological analysis and Dr. Gudrun Auffermann (Max Planck Institute for Chemical Physics of Solids) for the performance of the elemental analysis.
Notes and references
- H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. B. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523 CrossRef CAS.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040 CrossRef CAS; K. Koh, A. G. Wong-Foy and A. J. Matzger, Angew. Chem., Int. Ed., 2008, 47, 677 CrossRef CAS.
- N. Klein, I. Senkovska, K. Gedrich, U. Stoeck, A. Henschel, U. Mueller and S. Kaskel, Angew. Chem., Int. Ed., 2009, 48, 9954 CrossRef CAS.
- K. Koh, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2009, 131, 4184 CrossRef CAS.
- S. Bureekaew, S. Shimomura and S. Kitagawa, Sci. Technol. Adv. Mater., 2008, 9 Search PubMed , no pp given; S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 Search PubMed.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, Angew. Chem., Int. Ed., 2006, 45, 1390 CrossRef CAS.
- P. Küsgens, M. Rose, I. Senkovska, H. Fröde, A. Henschel, S. Siegle and S. Kaskel, Microporous Mesoporous Mater., 2009, 120, 325 CrossRef; P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regi, M. Sebban, F. Taulelle and G. Férey, J. Am. Chem. Soc., 2008, 130, 6774 CrossRef CAS; P. Horcajada, C. Serre, M. Vallet-Regi, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974 CrossRef CAS; R. C. Huxford, J. D. Rocca and W.-B. Lin, Curr. Opin. Chem. Biol., 2010, 14, 262 CrossRef CAS.
- M. D. Allendorf, R. J. T. Houk, L. Andruszkiewicz, A. A. Talin, J. Pikarsky, A. Choudhury, K. A. Gall and P. J. Hesketh, J. Am. Chem. Soc., 2008, 130, 14404 CrossRef CAS; A. Lan, K. Li, H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. Hong and J. Li, Angew. Chem., Int. Ed., 2009, 48, 2334 CAS.
- R. Kitaura, K. Seki, G. Akiyama and S. Kitagawa, Angew. Chem., Int. Ed., 2003, 42, 428 CrossRef CAS.
- D. Tanaka, K. Nakagawa, M. Higuchi, S. Horike, Y. Kubota, T. C. Kobayashi, M. Takata and S. Kitagawa, Angew. Chem., Int. Ed., 2008, 47, 3914 CrossRef CAS.
- S. Horike, D. Tanaka, K. Nakagawa and S. Kitagawa, Chem. Commun., 2007, 3395 RSC.
- H. Chun, D. N. Dybtsev, H. Kim and K. Kim, Chem.–Eur. J., 2005, 11, 3521 CrossRef CAS; D. N. Dybtsev, H. Chun and K. Kim, Angew. Chem., Int. Ed., 2004, 43, 5033 CrossRef CAS; N. Klein, C. Herzog, M. Sabo, I. Senkovska, J. Getzschmann, S. Paasch, M. R. Lohe, E. Brunner and S. Kaskel, Phys. Chem. Chem. Phys., 2010, 12, 11778 RSC; K. Seki and W. Mori, J. Phys. Chem. B, 2002, 106, 1380 CrossRef CAS; T. Takei, T. Ii, J. Kawashima, T. Ohmura, M. Ichikawa, M. Hosoe, Y. Shinya, I. Kanoya and W. Mori, Chem. Lett., 2007, 1136 CrossRef CAS; K. Uemura, Y. Yamasaki, Y. Komagawa, K. Tanaka and H. Kita, Angew. Chem., Int. Ed., 2007, 46, 6662 CrossRef CAS; H. Wang, J. Getzschmann, I. Senkovska and S. Kaskel, Microporous Mesoporous Mater., 2008, 116, 653 CrossRef CAS.
- R. Kitaura, K. Fujimoto, S.-i. Noro, M. Kondo and S. Kitagawa, Angew. Chem., Int. Ed., 2002, 41, 133 CrossRef CAS.
- J.-P. Zhang, Y.-Y. Lin, W.-X. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2005, 127, 14162 CrossRef CAS.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Férey, Chem.–Eur. J., 2004, 10, 1373 CrossRef CAS; C. Serre, F. Millange, C. Thouvenot, M. Nogues, G. Marsolier, D. Loueer and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519 CrossRef CAS.
- B. Chen, S. Ma, E. J. Hurtado, E. B. Lobkovsky, C. Liang, H. Zhu and S. Dai, Inorg. Chem., 2007, 46, 8705 CrossRef CAS; B. Chen, S. Ma, F. Zapata, E. B. Lobkovsky and J. Yang, Inorg. Chem., 2006, 45, 5718 CrossRef CAS; M. R. Kishan, J. Tian, P. K. Thallapally, C. A. Fernandez, S. J. Dalgarno, J. E. Warren, B. P. McGrail and J. L. Atwood, Chem. Commun., 2010, 46, 538 RSC; T. M. Reineke, M. Eddaoudi, D. Moler, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2000, 122, 4843 CrossRef CAS; K. Seki, Phys. Chem. Chem. Phys., 2002, 4, 1968 RSC.
- R. Grünker, I. Senkovska, R. Biedermann, N. Klein, A. Klausch, I. A. Baburin, U. Mueller and S. Kaskel, Eur. J. Inorg. Chem., 2010, 3835 CrossRef.
- Y. E. Cheon, J. Park and M. P. Suh, Chem. Commun., 2009, 5436 RSC.
- P. Van der Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194 CrossRef.
- A. P. Nelson, O. K. Farha, K. L. Mulfort and J. T. Hupp, J. Am. Chem. Soc., 2009, 131, 458 CrossRef CAS.
- D. Yuan, D. Zhao, D. Sun and H.-C. Zhou, Angew. Chem., Int. Ed., 2010, 49, 5357 CrossRef CAS.
- H. Furukawa, M. A. Miller and O. M. Yaghi, J. Mater. Chem., 2007, 17, 3197 RSC; A. G. Wong-Foy, A. J. Matzger and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 3494 CrossRef CAS.
- P. L. Llewellyn, S. Bourrelly, C. Serre, A. Vimont, M. Daturi, L. Hamon, G. De Weireld, J.-S. Chang, D.-Y. Hong, Y. K. Hwang, S. H. Jhung and G. Férey, Langmuir, 2008, 24, 7245 CrossRef.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Synthetic procedures for H4BenzTB and DUT-13, further structural illustrations, PXRD patterns, TGA curves and additional adsorption isotherms. CCDC 784971. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc02273j |
| § Abbreviations: bpy = 4,4′-bipyridine, dhbc = 2,5-dihydroxybenzenedicarboxylate, bpndc = benzophenone-4,4′-dicarboxylate, ip = isophthalate, 1,4-bdc = 1,4-benzenedicarboxylate, 2,6-ndc = 2,6-naphthalenedicarboxylate, dabco = 1,4-diazabicyclo[2.2.2]octane, pzdc = pyrazine-2,3-dicarboxylate, dpyg = 1,2-di(4-pyridyl)-glycol, atz = 3-amino-1,2,4-triazolate, btc = 1,3,5-benzenetricarboxylate. |
¶ Crystal data for DUT-13: M = 1268.40 g mol−1, trigonal, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, a = 25.682(4), c = 114.90(2) Å, V = 65 c, a = 25.682(4), c = 114.90(2) Å, V = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630(18) Å3, Z = 12, T = 293(2) K, ρ = 0.385 g cm−3, μ = 0.450 cm−1 (solvent free), λ = 0.88561 Å, 1.22° < θ < 35.92°, 167 630(18) Å3, Z = 12, T = 293(2) K, ρ = 0.385 g cm−3, μ = 0.450 cm−1 (solvent free), λ = 0.88561 Å, 1.22° < θ < 35.92°, 167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 measured reflections, 16 128 measured reflections, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 481 independent, data were processed with PLATON19 Squeeze routine Rint = 0.048, R1 (observed reflections) = 0.0542, wR2 (all reflections) = 0.1644, max./min. residual electron density: 0.458/−0.522. 481 independent, data were processed with PLATON19 Squeeze routine Rint = 0.048, R1 (observed reflections) = 0.0542, wR2 (all reflections) = 0.1644, max./min. residual electron density: 0.458/−0.522. |
| This journal is © The Royal Society of Chemistry 2011 |
