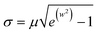Synthesis of gold nanoparticles by laser ablation of an Au foil inside and outside ionic liquids
Heberton
Wender
a,
Marcos L.
Andreazza
b,
Ricardo R. B.
Correia
*a,
Sérgio R.
Teixeira
*a and
Jairton
Dupont
*c
aInstituto de Física, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil. E-mail: durao@if.ufrgs.br; rego@if.ufrgs.br; Fax: +55 51 3308-6498; Tel: +55 51 3308-6498
bCCET, Universidade de Caxias do Sul (UCS), Caxias do Sul, RS, Brazil
cInstituto de Química, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil. E-mail: jairton.dupont@ufrgs.br; Fax: +55 51 3308-6521; Tel: +55 51 3308-6521
First published on 26th January 2011
Abstract
Stable gold nanoparticles (AuNPs) were prepared by simple laser ablation of an Au foil placed inside or outside four ionic liquids (ILs), without the addition of any external chemical reagent. Irregular spherical AuNPs with a diameter range of 5 to 20 nm were produced after laser ablation of an Au foil located inside or outside the ILs 1-n-butyl-3-methylimidazolium tetrafluoroborate (BMI·BF4), 1-n-butyl-3-methylimidazolium hexafluorophosphate (BMI·PF6) and 1-(3-cyanopropyl)-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ((BCN)MI·NTf2). Additionally, whereas laser ablation inside the IL 1-n-butyl-3-methylimidazolium dicyanamide BMI·N(CN)2 produced flower-like shaped nanoparticles of about 50 nm in size, ablation outside this IL presented similar results to the others ILs studied, as determined by TEM and UV-Vis. The size and shape of the prepared NPs were related to where NP nucleation and growth occurred, i.e., at the IL surface or within the IL. Indeed, the chemical composition of the IL/air interface and surface ion orientation played important roles in the stabilization of the AuNPs formed by laser ablation outside the ILs.
1. Introduction
Metal nanoparticles (NPs) play important roles in many different areas such as optics, electronics, catalysis, medical research, magnetism and information storage. The intrinsic properties of a metal nanoparticle are mainly determined by its size, shape, composition, crystalline structure, etc. The control of nanoparticle size and a better understanding of their chemical behavior have attracted considerable interest because of their size- and shape-dependent physicochemical properties.1–4Room temperature ionic liquids, especially imidazolium-based, have proven to be suitable media for the generation and stabilization of a plethora of soluble metal NPs.5–8 Indeed, transition-metal NPs with small sizes, narrow size distributions and different shapes have been prepared in ionic liquids (ILs) by the reduction of organometallic compounds with molecular hydrogen5,9 or hydrides,10,11decomposition of transition-metal complexes in the zero-valent state,12–16 metal bombardment17–19 or simple transfer of previously prepared water- or classical organic solvent-soluble colloids onto the ILs.20,21 Recently, it has been shown that stable metal NPs can be produced directly from physical vapor deposition (PVD) techniques onto ILs with the great advantages of eliminating chemical precursors, stabilizing agents and necessary further purification procedures.18,22–24Sputtering, thermal evaporation and laser ablation are, in this sense, cornerstone physical methods, which may produce an unrestricted sort of engineered NPs in IL matrices. Additionally, laser ablation inside liquid matrices allows for further interactions with laser pulses, which can reshape, fragmentize or combine NPs.17,25
The size and shape of metal NPs chemically prepared in a fluid are apparently related to the bulk structural organization of the IL, whereas those physically prepared on the IL are related to the IL surface organization. Therefore, the mechanism of NPs formation is intimately bound to the phase in which nucleation and growth occur, which in turn is dependent on the method used for their preparation. Usually, in chemical methods, both processes occur in the IL phase, whereas in the metal sputtering approach, nucleation and NPs growth tend to occur at the IL–vacuum interface,22,26 depending on the energy of the ejected atoms/clusters.22
Understanding the mechanisms of NPs growth by PVD onto ILs and, consequently, the factors that control NPs size, size distribution and shape are still a challenge to the design of new and promising nanomaterials. Although the laser ablation method can be performed with the precursor either inside or outside the IL (Fig. 1), this process has mainly been investigated with substrates embedded in the IL.17,24
 | ||
| Fig. 1 Focused laser incident on an Au foil surface located inside (a) or outside (b) an ionic liquid. | ||
In order to investigate the mechanism of NPs formation, the synthesis of AuNPs by laser ablation using four different ILs (Fig. 2) was performed with an Au foil placed either inside or outside of the IL (Fig. 1). Moreover, the formation of AuNPs was monitored in situ by digital imaging of the Au target and the IL.
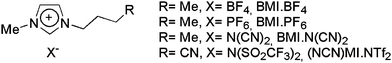 | ||
| Fig. 2 Structures of the ILs used in this study. | ||
2. Experimental
2.1 General considerations
All ILs used in this work were synthesized according to procedures in the literature.27–29 The morphology and size distribution of AuNPs were examined using Transmission Electron Microscopy (TEM) with a JEOL JEM1200 EXII operating at an acceleration voltage of 80 kV. Optical absorption spectra were measured by a Varian Cary 5000 UV-visible spectrophotometer using 1 mm optical path quartz cuvettes.2.2 Preparation of AuNPs
Gold colloids were prepared by laser ablation in different imidazolium-based ionic liquids with two distinct configurations in order to investigate the IL/air interface and IL bulk liquid phase on the nucleation and growth of AuNPs. To this end, a bulk gold foil was held against the back wall of an ordinary UV-Vis spectroscopy glass cell (10 mm), partially immersed within the IL. The oblique irradiation with a 1064 nm Nd:YAG laser with a fluence of 20 mJ cm−2 per pulse allowed ablation inside the IL and above the IL/air interface simply by sliding the cell parallel to its longer edge, as indicated by the arrow in Fig. 1. The Nd:YAG laser used in this work was a Spectra-Physics Lasers Quantum-Ray GCR-170, with 8.5 ns pulse duration (full width at half maximum) and pulse energy from about 18 mJ up to 540 mJ determined using an energy meter (FieldMax™II-P, Coherent Inc.). A spherical lens, with 10 cm focal length, was used to focus the laser beam. The target was placed in a displaced position in relation to the focus.The spot area on the surface of the target had an elliptical shape, with approximately, 0.73 mm at semi-major axis and 0.43 mm at semi-minor axis. The Au plate was irradiated by 6000 laser pulses at 5 Hz repetition rate to produce the gold colloids. When Au foil was inside the IL (Fig. 1(a)), the direct metal laser ablation process was similar to those described in the literature. However, with the Au foil outside the IL, the laser pulses illuminated a metal area above the IL surface and vaporized a small portion of the target. This sputtered material, which was ejected in a plasma plume extended above the surface, accumulated on the liquid interface (Fig. 1(b)), building up the metallic nanostructures.
2.3 TEM sample preparation
The TEM samples were prepared by dissolving the Au/IL colloids in acetone at room temperature and then depositing them on a 400 mesh carbon-coated Cu grid. The histograms of the nanoparticle size distribution, assuming a spherical shape, were obtained from measurements of more than 200 particles and were reproduced in arbitrarily chosen regions of the grid.2.4 UV-Vis spectroscopy
The UV-visible absorption spectra of the described liquid samples were measured in a 1 mm thick quartz cell on a Cary 5000 spectrophotometer in a range between 300 and 800 nm. The spectra of the immersed nanoparticles in the IL were measured relative to the four respective liquid blank samples.3. Results and discussion
The formation of AuNPs was monitored by UV-Vis absorption spectroscopy. The spectra in Fig. 3 show the extinction after Au deposition either inside or outside BMI·BF4, BMI·PF6, BMI·N(CN)2 and (BCN)MI·NTf2 ILs, respectively, in the configurations shown in Fig. 1(a) and (b). All gold colloids displayed a single and broad absorption band centered near 550 nm due to the surface plasmon resonance (SPR) of AuNPs,30,31 except for the case inside BMI·PF6. The absorption band was very broad for laser ablation outside the ILs, corresponding to a larger NPs distribution and/or agglomeration processes. This band was broader than the one produced inside the IL (only (BCN)MI·NTf2 could not be compared due to the low absorption in the latter case).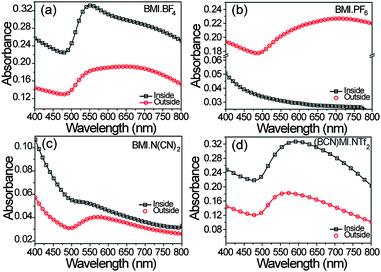 | ||
| Fig. 3 Absorption spectra of the AuNPs colloids after laser ablation for 10 minutes either inside or outside the ionic liquids BMI·BF4, BMI·PF6, BMI·N(CN)2 and (BCN)MI·NTf2. | ||
Both indicated that NPs growth probably occurs at the IL/air interface instead inside the IL bulk liquid phase, similar to what has been observed for AuNPs produced by sputtering deposition onto castor oil.23
In the case of the larger AuNPs produced by laser ablation inside (BCN)MI·NTf2, a fragmentation process may have occurred, changing the AuNPs size and size distribution, consequently increasing the density of NPs.17
In order to investigate the form factor, size and size distribution of AuNPs, TEM analyses were conducted in all colloids produced. Fig. 4 shows representative TEM images of the three BMI cation-based ILs after laser ablation inside or outside the IL. It can be seen that after laser ablation inside and outside the ILs BMI·BF4 and BMI·PF6, spherical AuNPs were produced. However, in the IL BMI·N(CN)2, a contrasting result was observed. Laser ablation inside BMI·N(CN)2 could produce flower-like AuNPs of approximately 50 nm in diameter instead of only spherical NPs as obtained outside the same IL that can account for the very weak absorption band observed in Fig. 3(c) “inside”. All the histograms clearly show a lognormal distribution (eqn (1)) for the population of NPs. The parameters of NPs population fit are presented in Table 1.
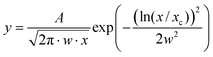 | (1) |
 | ||
| Fig. 4 TEM images of AuNPs obtained by laser ablation under the same experimental conditions inside (a, c and e) or outside (b, d and f) the ILs BMI·BF4, BMI·PF6 and BMI·N(CN)2, respectively. The insets on the charts are the respective histograms, except in (e) where is presented a magnified image. | ||
| IL | Au foil position | Mean (nm)
|
Standard deviation
|
|---|---|---|---|
| BMI·BF4 | Inside | 22.0 | 17.5 |
| Outside | 12.6 | 8.3 | |
| BMI·PF6 | Inside | 8.1 | 4.3 |
| Outside | 9.0 | 3.8 | |
| BMI·N(CN)2 | Inside | Flower-like | Flower-like |
| Outside | 6.4 | 2.7 | |
| (BCN)MI·NTf2 | Inside | 5.2 and 20.5 | 1.1 and 2.1 |
| Outside | 9.1 | 3.8 |
Additionally, we were motivated to study the chemical functionalization of an IL in the synthesis and stabilization of AuNPs produced by laser ablation. Fig. 5 shows representative TEM images after laser ablation inside and outside the functionalized ionic liquid (BCN)MI·NTf2. The AuNPs were spherical with a bimodal distribution with mean diameters of 5.2 nm (σ = 1.1 nm) and 20.5 nm (σ = 2.1 nm) after Au laser ablation inside the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N functionalized IL, and 9.1 nm (σ = 3.8 nm) outside. As observed in the histograms, laser ablation inside this IL apparently fragmented the AuNPs of mean diameters around 30–50 nm, thus increasing the NPs population around 5.2 nm.
N functionalized IL, and 9.1 nm (σ = 3.8 nm) outside. As observed in the histograms, laser ablation inside this IL apparently fragmented the AuNPs of mean diameters around 30–50 nm, thus increasing the NPs population around 5.2 nm.
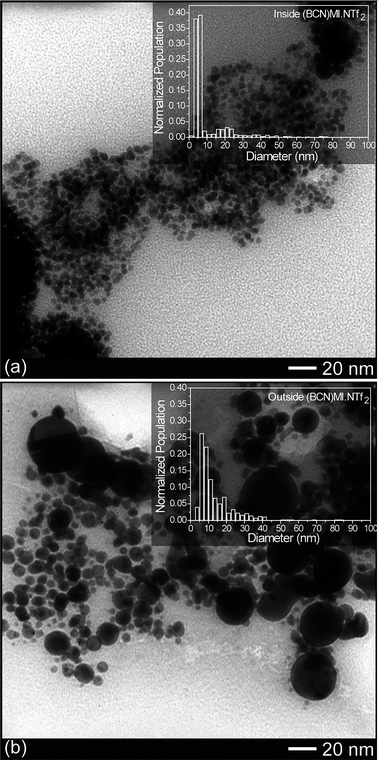 | ||
| Fig. 5 TEM images of AuNPs obtained by laser ablation for 10 minutes inside (a) and outside (b) the IL (BCN)MI·NTf2. The insets of each figure are the respective histograms. | ||
Therefore, laser ablation inside the ionic liquids showed similar results reported for other fluids such as water.25 The large size distribution can be easily diminished since the fragmentation process resulting in smaller AuNPs can be achieved by further laser ablation. As cited above, a previous study has described Au laser ablation inside the ILs EMI·BF4, BMI·BF4 and OMI·BF4 with laser pulses of 1024 and 532 nm.24 They found that among the almost spherical NPs with a diameter of 3.6 nm (σ = 0.9 nm), rod-like NPs were produced in BMI·BF4 after 532 nm laser ablation. Additionally, a reduction of the particle size by further laser irradiation increased the stability of the AuNPs. In our case, 1264 nm ablation into BMI·BF4 resulted in irregular spherical AuNPs with a diameter of 22.0 nm (σ = 17.5 nm). As shown in Table 1, AuNPs of 8.1 nm (σ = 4.3 nm) were produced by laser ablation inside the IL BMI·PF6. Comparing the anion effects on the AuNPs after laser ablation inside these two ILs, BMI·PF6 generated smaller NPs than BMI·BF4. This is in contrast with what has been observed in the synthesis of NPs in ILs by the hydrogen reduction of inorganic salt precursors, where a linear increase in the NPs size with the IL anion molecular volume was achieved.32 Therefore, these data indicate and confirm that the mechanism of NPs formation and stabilization through laser ablation is quite different from that of chemical reduction.
Laser ablation inside the IL BMI·N(CN)2 was the unique case where the formation of flower-like33 AuNPs was revealed. Since “pure” imidazolium-based ILs should be considered as three dimensional networks of cations and anions linked by weak interactions, such as hydrogen bonds and van der Waals and Coulomb forces, the AuNPs formed in BMI·N(CN)2 probably experienced weak particle–particle interactions that were correlated with the weak anion coordination ability. This is currently being investigated further in terms of the observed anisotropic shape growth. Nevertheless, to the best of our knowledge, this is the first time that flower-like AuNPs synthesis in an imidazolium-based ionic liquid has been reported. These flower-shaped NPs are of special interest because they have showed improved electrocatalytic activity.34 In the case of the nitrile cation-functionalized IL ((BCN)MI·NTf2), it was clear that further fragmentation processes occurred after ablation, since a second small average size distribution (∼5 nm) emerged. This effect corresponded to breaking the larger AuNPs with diameter in the range of 20–40 nm, which were also the size of the large particles observed after laser ablation outside this IL. It is to note that the dynamics of the fragmentation process arises mainly from electron ejections what lead to a charging of the surface of the bigger NPs that then disintegrate to form smaller-size NPs.35,36 (BCN)MI·NTf2 specially favored the fragmentation since laser ablation was conducted on the same conditions for all ILs. Apparently, no effects of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N functional group in NPs coordination were observed. Therefore, we have shown that laser ablation inside ILs with different anions can produce NPs of different shapes, sizes and size distributions, and that anion coordination ability plays an important role in NPs nucleation and growth in the IL bulk liquid phase.
N functional group in NPs coordination were observed. Therefore, we have shown that laser ablation inside ILs with different anions can produce NPs of different shapes, sizes and size distributions, and that anion coordination ability plays an important role in NPs nucleation and growth in the IL bulk liquid phase.
One of the main objectives was to perform laser ablation outside the ILs to investigate the mechanism of NPs formation, i.e., to determine if the Au atoms/clusters nucleated on the IL surfaces or directly in the bulk liquid phase. Recently, in the process of sputtering deposition, it was proposed that the Au atoms pulled away from the sputter target and could penetrate directly into the bulk liquid phase or remain at the liquid surface, thus starting the mechanism of nucleation. It was found that this directly depended on the kinetic energy of the atoms when they arrived at the liquid surface.22 A correlation was observed between AuNPs size and IL surface chemical composition, rather than surface tension and viscosity, which were secondary parameters. Based on these results, we initially considered that the atoms and/or clusters evaporated from the Au foil by the laser pulses would remain on the IL surface, where they would start to nucleate and grow. To verify that behavior, the deposition processes on the IL surface were recorded by a strategically placed camera during the experiments. The results show that, in the first few minutes, the ILs surfaces displayed a reddish color and the bulk liquid phases remained transparent (data not shown). Only after approximately three minutes did the IL bulk liquid phase start to present a reddish color, indicating that AuNPs first form near the surface of the ILs then diffuse to the bulk liquid phases.
Therefore, the chemical composition and ion orientation at the IL surface play important roles in the stabilization of AuNPs formed by laser ablation outside ILs, as shown in the sputtering case.22 While sputtering deposition of Au onto BMI·BF4 and BMI·PF6 resulted in AuNPs of mean diameters 3.6 nm (σ = 0.4 nm) and 3.7 nm (σ = 0.4 nm), respectively, Au laser ablation resulted in AuNPs of mean diameters 12.6 nm (σ = 8.3 nm) and 9.0 nm (σ = 3.8 nm), respectively, as determined by TEM. Thus, the AuNPs produced by laser ablation outside ILs had larger sizes and size distributions compared to sputtering deposition. Nevertheless, these physical methods presented remarkable differences: (1) sputtered material resulting from the Ar+ collision are in the majority Au atoms with a defined kinetic energy distribution, while for laser ablation, few atoms, molecules and clusters are in this regime stripped off from the Au bulk surface with a large size distribution resulting from the thermal process; (2) while sputtering was performed at a low pressure glow discharge, laser ablation was performed directly into an air atmosphere and air/Au atom collisions need to be considered.
The ILs BMI·BF4 and BMI·PF6 have similar anion and cation populations at the IL/air interface, as observed in the literature,37–39 presenting similar results when sputtering or laser ablation was performed onto their surfaces. However, Au laser ablation outside BMI·N(CN)2 produced AuNPs with a mean diameter of 6.4 nm (σ = 2.7 nm), which were smaller than those observed for the other ILs studied. This reinforces the supposition that surface chemical composition can control NPs nucleation and growth, since N(CN)2 anion clearly propitiated a different surface composition in this IL. It is noteworthy that the surface tensions of BMI·BF4, BMI·PF6 and BMI·N(CN)2 are 0.0436, 0.0488 and 0.0486 N m−1, respectively, measured at 298 K. Apparently, no correlation was found between ILs surface tensions and NPs size, as in the sputtering protocol.22 Therefore, the anion coordination ability, surface composition and ion orientation effects on NPs formation by laser ablation need to be considered in future investigations.
4. Conclusions
In summary, the size and shape of the nanoparticles prepared by laser ablation of Au foils depend mainly on the nature of the IL counter-anion and where the ablation is performed, i.e., with the Au foil placed inside or outside the ionic liquid. Laser ablation with the Au foil inside the ILs produced irregular spherical AuNPs without the addition of any external chemical reagent. The IL BMI·N(CN)2 was the only case where AuNPs were generated with a different shape (flower-like) that probably is due to the lower coordination ability of the N(CN)2 anion. In the reactions performed with the Au foil placed outside the ILs, the formation of AuNPs occurred at the IL surface, and the sizes and shapes of the AuNPs were almost completely dependent on the surface compositions of the IL, as previously shown for sputtering. In these cases, irregular spherical-shaped AuNPs of mean diameters from 5.4 to 12.6 nm were obtained.Acknowledgements
Thanks are due to the following Brazilian agencies for their financial support: CNPq, FAPERGS and CAPES. The authors also thank the CME-UFRGS for use of the TEM and the Laboratório de Altas Pressões e Materiais Avançados for laser equipment.References
- G. Schmid, Endeavour, 1990, 14, 172–178 CAS.
- A. Roucoux, J. Schulz and H. Patin, Chem. Rev., 2002, 102, 3757–3778 CrossRef CAS.
- H. Bonnemann and R. M. Richards, Eur. J. Inorg. Chem., 2001, 2455 CrossRef CAS.
- O. A. Belyakova and Y. L. Slovokhotov, Russ. Chem. Bull., 2003, 52, 2299–2327 Search PubMed.
- J. Dupont, G. S. Fonseca, A. P. Umpierre, P. F. P. Fichtner and S. R. Teixeira, J. Am. Chem. Soc., 2002, 124, 4228–4229 CrossRef CAS.
- P. Migowski and J. Dupont, Chem.–Eur. J., 2007, 13, 32–39 CrossRef.
- J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780–1804 RSC.
- E. Redel, M. Walter, R. Thomann, C. Vollmer, L. Hussein, H. Scherer, M. Kruger and C. Janiak, Chem.–Eur. J., 2009, 15, 10047–10059 CrossRef CAS.
- G. S. Fonseca, G. Machado, S. R. Teixeira, G. H. Fecher, J. Morais, M. C. M. Alves and J. Dupont, J. Colloid Interface Sci., 2006, 301, 193–204 CrossRef CAS.
- Y. Wang and H. Yang, J. Am. Chem. Soc., 2005, 127, 5316–5317 CrossRef CAS.
- P. Dash and R. W. J. Scott, Chem. Commun., 2009, 812–814 RSC.
- E. Redel, M. Walter, R. Thomann, L. Hussein, M. Kruger and C. Janiak, Chem. Commun., 2010, 46, 1159–1161 RSC.
- C. W. Scheeren, G. Machado, J. Dupont, P. F. P. Fichtner and S. R. Texeira, Inorg. Chem., 2003, 42, 4738–4742 CrossRef CAS.
- J. Kramer, E. Redel, R. Thomann and C. Janiak, Organometallics, 2008, 27, 1976–1978 CrossRef.
- M. Scariot, D. O. Silva, J. D. Scholten, G. Machado, S. R. Teixeira, M. A. Novak, G. Ebeling and J. Dupont, Angew. Chem., Int. Ed., 2008, 47, 9075–9078 CrossRef CAS.
- P. Migowski, G. Machado, S. R. Texeira, M. C. M. Alves, J. Morais, A. Traverse and J. Dupont, Phys. Chem. Chem. Phys., 2007, 9, 4814–4821 RSC.
- M. A. Gelesky, A. P. Umpierre, G. Machado, R. R. B. Correia, W. C. Magno, J. Morais, G. Ebeling and J. Dupont, J. Am. Chem. Soc., 2005, 127, 4588–4589 CrossRef CAS.
- T. Torimoto, K. Okazaki, T. Kiyama, K. Hirahara, N. Tanaka and S. Kuwabata, Appl. Phys. Lett., 2006, 89, 243117 CrossRef.
- S. Kuwabata, T. Tsuda and T. Torimoto, J. Phys. Chem. Lett., 2010, 1, 3177–3188 Search PubMed.
- G. T. Wei, Z. S. Yang, C. Y. Lee, H. Y. Yang and C. R. C. Wang, J. Am. Chem. Soc., 2004, 126, 5036–5037 CrossRef CAS.
- D. B. Zhao, Z. F. Fei, W. H. Ang and P. J. Dyson, Small, 2006, 2, 879–883 CrossRef CAS.
- H. Wender, L. F. de Oliveira, P. Migowski, A. F. Feil, E. Lissner, M. H. G. Prechtl, S. R. Teixeira and J. Dupont, J. Phys. Chem. C, 2010, 114, 11764–11768 CrossRef CAS.
- H. Wender, L. F. de Oliveira, A. F. Feil, E. Lissner, P. Migowski, M. R. Meneghetti, S. R. Teixeira and J. Dupont, Chem. Commun., 2010, 46, 7019–7021 RSC.
- Y. Kimura, H. Takata, M. Terazima, T. Ogawa and S. Isoda, Chem. Lett., 2007, 36, 1130–1131 CrossRef CAS.
- J.-P. Sylvestre, S. Poulin, A. V. Kabashin, E. Sacher, M. Meunier and J. H. T. Luong, J. Phys. Chem. B, 2004, 108, 16864–16869 CrossRef CAS.
- K. Richter, A. Birkner and A.-V. Mudring, Angew. Chem., Int. Ed., 2010, 49, 2431–2435 CAS.
- P. A. Z. Suarez, J. E. L. Dullius, S. Einloft, R. F. DeSouza and J. Dupont, Polyhedron, 1996, 15, 1217–1219 CrossRef CAS.
- C. C. Cassol, G. Ebeling, B. Ferrera and J. Dupont, Adv. Synth. Catal., 2006, 348, 243–248 CrossRef CAS.
- D. Zhao, Z. Fei, R. Scopelliti and P. J. Dyson, Inorg. Chem., 2004, 43, 2197–2205 CrossRef CAS.
- H. Itoh, K. Naka and Y. Chujo, J. Am. Chem. Soc., 2004, 126, 3026–3027 CrossRef CAS.
- P. Mulvaney, Langmuir, 1996, 12, 788–800 CrossRef CAS.
- E. Redel, R. Thomann and C. Janiak, Inorg. Chem., 2007, 47, 14–16.
- M. S. Bakshi, J. Phys. Chem. C, 2009, 113, 10921–10928 CrossRef CAS.
- A. K. Das and C. R. Raj, J. Electroanal. Chem., 2010, 638, 189–194 CrossRef CAS.
- P. V. Kamat, M. Flumiani and G. V. Hartland, J. Phys. Chem. B, 1998, 102, 3123–3128 CrossRef CAS.
- F. Giammanco, E. Giorgetti, P. Marsili and A. Giusti, J. Phys. Chem. C, 2010, 114, 3354–3363 CrossRef CAS.
- S. Rivera-Rubero and S. Baldelli, J. Phys. Chem. B, 2006, 110, 4756–4765 CrossRef CAS.
- C. Aliaga, C. S. Santos and S. Baldelli, Phys. Chem. Chem. Phys., 2007, 9, 3683–3700 RSC.
- C. S. Santos and S. Baldelli, Chem. Soc. Rev., 2010, 39, 2136–2145 RSC.
| This journal is © The Royal Society of Chemistry 2011 |