A facile synthesis of mesoporous crystalline tin oxide films involving a base-triggered formation of sol–gel building blocks†
Dorothee Irmgard
Fried‡
a,
Alesja
Ivanova
a,
Vesna
Müller
a,
Jiri
Rathousky
b,
Bernd M.
Smarsly
c and
Dina
Fattakhova-Rohlfing
*a
aDepartment of Chemistry and Center for Nanoscience (CeNS), University of Munich (LMU), Butenandtstrasse 5-11 (E), 81377, Munich, Germany. E-mail: Dina.Fattakhova@cup.uni-muenchen.de; Fax: +49 89 218077622; Tel: +49 89 2180 77805
bJ. Heyrovský Institute of Physical Chemistry, v.v.i., Academy of Sciences of the Czech Republic, Dolejskova 3, 18223, Prague 8, Czech Republic. E-mail: jiri.rathousky@jh-inst.cas.cz; Fax: +420 286582307; Tel: +420 266053945
cInstitute of Physical Chemistry, Justus Liebig University, Heinrich-Buff-Ring-58, 35390, Giessen, Germany. E-mail: Bernd.Smarsly@phys.chemie.uni-giessen.de; Fax: +49 0641 9934509; Tel: +49 0641 9934590
First published on 24th January 2011
Abstract
We have developed a new facile procedure for manufacturing crystalline thin films of SnO2 with a uniform mesoporous architecture and full crystallinity of the walls. The procedure is based on the evaporation-induced self-assembly (EISA) of prehydrolyzed tin oxide precursor directed by a commercially available Pluronic polymer. The formation of the tin oxide precursor, which can be self-assembled into a mesoporous structure, is achieved by an addition of ammonium hydroxide to a tin tetrachloride solution. The relative concentration of ammonium hydroxide as well as the duration and temperature of the hydrolysis reaction influence significantly the properties of hydrolyzed tin oxide species and the mesostructure assembled from them. The films coated from these precursor solutions and calcined at 300 °C to 400 °C exhibit a well-developed worm-like porosity with a wall to wall distance of ca. 18 nm, a surface area of up to 50 cm2 cm−2 (corresponding to 55 ± 5 m2 g−1), and high crystallinity.
Introduction
Evaporation-induced self-assembly (EISA) is one of the most versatile routes to the preparation of functional metal oxide films with a large interface area and a well-defined nanoporous architecture.1–5 Spontaneous self-organization of inorganic building blocks (metal oxide precursors) and micelles of amphiphilic molecules (structure-directing agents) enables the formation of highly ordered periodic nanostructures in a very simple way, avoiding the expensive nanolithography or vacuum technologies. The self-assembling ability of the components strongly depends on their surface properties and their mutual interactions. The control and understanding of these parameters is a key issue in the formation of desirable nanostructures.Inorganic building blocks are conventionally prepared by sol–gel reactions of suitable metal compounds.1,2,6–8 The formed hydrolyzed species can easily assemble into periodic structures and afterwards condense to a continuous inorganic framework. However, the control over the nature of the formed products is generally low. Consequently, the recent research efforts have been aimed at the preparation of more defined building blocks in order to gain more control over the self-assembly and the mesostructure of the formed films.9–15
One of the processes which requires a precise control over the self-assembly is the formation of tin oxide films with a well-developed porosity and high crystallinity. Tin oxide is one of the technologically important semiconducting materials, which is widely used for sensors and catalysts,16–18 due to the marked change in its electrical conductivity upon the interaction with reducing or oxidizing species. However, the formation of such films using the self-assembly approach has been found far from straightforward.19–27 The mesostructure ordering of sol–gel derived tin oxide precursors requires the use of surfactants with a very strong hydrophilic-hydrophobic contrast such as small neutral and ionic surfactants,21,22,28–32 which, however, provide mesostructures with either a relatively small pore size, or with only a partial crystallinity of the walls and a low thermal stability. Highly crystalline films with a periodically ordered large pores23 can be obtained with a special KLE type amphiphilic polymer,4,33 which is however not yet commercially available. The preparation of crystalline mesoporous tin oxide films with a larger pore size using commercially available Pluronic polymers has provided either only partially crystalline films,3,26,34 or has required a rather elaborate and time-consuming post-synthesis treatment.35–37 Moreover, the choice of available tin oxide precursors is rather restricted, mostly limited to tin tetrachloride due to the high cost of tin alkoxides.
In the present work, we have proposed that the weak mesostructuring ability of Pluronic polymers is due to an unfavorable interaction of its micelles with the precursor tin oxide species. Therefore, we have aimed at the optimization of the interaction strength and the affinity to self-assemble by the variation of the size, charge and hydrophilicity of the tin oxide precursor. We have found that the key issue in the whole process is the sol–gel formation of suitable colloidal particles of tin oxide precursor, which act as the building units of the assembled mesostructures. Their formation was triggered by the addition of ammonium hydroxide and controlled by its amount and the reaction time.
Results and discussion
The formation of the condensed tin oxide framework via hydrolysis of tin tetrachloride is described by the general Scheme 1. | ||
| Scheme 1 | ||
This seemingly simple reaction equation, however, summarizes a very complex process, which involves a sequence of coupled hydrolysis and branched condensation reactions, as well as the gelation and agglomeration of the formed tin oxide species.7,38–42 Each elementary reaction step has a different reaction rate, and any single equilibrium step can be shifted by a change in the reaction conditions. The mechanism of the sol–gel reactions was intensively studied for a large number of metal ions including silicon, titanium, aluminium, tungsten etc,7 but the reports on the initial steps of the sol–gel process of tin tetrachloride are rather scarce, the reaction mechanism being poorly understood.40,43–45 In our work, we have concentrated on the determination of the effect of various processing parameters on the formation of the precursors in order to obtain some basic empirical knowledge, how to control this process and to obtain suitable building blocks for the formation of tin oxide mesostructures. The precursor solution included tin tetrachloride as a tin source, water as a hydrolysis reactant, ammonium hydroxide as a hydrolysis catalyst, Pluronic F127 as a structure-directing agent and either ethanol, n-butanol or their mixture as solvents. The varied process parameters included the ammonium hydroxide and Pluronic F127 to tin chloride molar ratios, the aging time and temperature of the precursor solution, as well as the solvents used.
Sols of the tin oxide precursor were coated on several substrates. The best mesostructure periodicity according to the small angle XRD measurements was obtained when the Pluronic to tin oxide molar ratio was 0.8. As a solvent for the preparation of colloidal sols, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v mixture of ethanol and n-butanol was found the most suitable. When either pure n-butanol or ethanol was used as a solvent, a phase separation or precipitation occurred after the addition of ammonium hydroxide, which we attribute to the formation of polycondensed oxo-hydroxide products.
1 v/v mixture of ethanol and n-butanol was found the most suitable. When either pure n-butanol or ethanol was used as a solvent, a phase separation or precipitation occurred after the addition of ammonium hydroxide, which we attribute to the formation of polycondensed oxo-hydroxide products.
The hydroxide to tin ratio, the aging time and temperature were found to be the most important parameters. The formation of periodic mesostructure occurred only when ammonium hydroxide was added to the solution, the OH/Sn molar ratio of 2.6 ± 0.1 being found the best (Fig. 1a–c). A further increase in the OH/Sn ratio led to irreversible precipitation of the hydrolyzed tin compound, and the films prepared from such solutions do not show any mesostructure ordering (Fig. 1c).
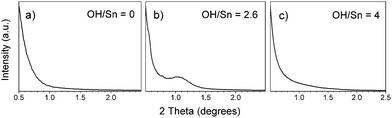 | ||
| Fig. 1 Small angle diffractograms of tin oxide films calcined at 400 °C prepared from NH4OH-hydrolyzed sols using different NH4OH/Sn ratios (indicated in labels) and aged at room temperature for one day. | ||
The coating solution has to be aged for a certain time in order to form hydrolyzed tin oligomers suitable as building blocks in the EISA process. At room temperature, the minimum aging time is about 20 h. The solution pH, which increases initially after the addition of ammonium hydroxide, decreases continuously during the aging process, which can be attributed to the hydrolysis reaction releasing HCl according to Scheme 1 (Fig. S1 in the Supporting information). The aging process can be accelerated by an increase in the aging temperature. At 40 °C, 3 h are already sufficient to obtain an ordered mesostructure (Fig. 2b). At 60 °C, however, the hydrolysis and condensation processes became too rapid and difficult to control, which prevented the formation of ordered mesoporous films (Fig. 2c). Generally, the acceleration of the aging process in this system by increasing the temperature leads to a deterioration of the mesostructure ordering of the films, as follows from the broadening of the diffraction peaks at small angles (Fig. 2a–c). The deterioration is apparently due to the less controllable course of the hydrolysis and condensation processes, leading to a broader distribution of the shape and size of the formed oligomers.
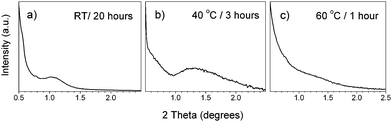 | ||
| Fig. 2 Small angle diffractograms of tin oxide films calcined at 400 °C prepared from NH4OH-hydrolyzed sols using a fixed OH/Sn ratio of 2.6 and aged at different temperatures and times indicated in labels. | ||
By the variation of the processing parameters the best mesostructure ordering was obtained when the coating solution contained tin tetrachloride and ammonium hydroxide at a OH/Sn molar ratio of 2.6 ± 0.1 in a 1: 1 v/v mixture of ethanol and n-butanol, and was aged for one day at room temperature. The films coated from such a solution were calcined in air at 300 to 400 °C in order to condense and crystallize the inorganic framework and to remove the template (see TGA analysis of the coating solution showing that the template is combusted at the temperatures below 300 °C, Figure S3). The detection of a diffraction peak at small angles in the XRD pattern suggests that the films obtained at such conditions are ordered, but the presence of just one broad peak indicates a low degree of periodicity. The formation of a mesoporous structure of the films calcined at 300 °C is apparent from the SEM and TEM images. The SEM images show the uniform crack-free surface of the films featuring channel-like pores with a low degree of pore organization. The FFT of the images reveals a periodic repeating unit of 18 ± 0.7 nm (Fig. 3a, b). The TEM proves a uniform pore structure (Fig. 4), the FFT exhibiting a ring corresponding to a periodicity of 17 ± 0.5 nm (Fig. 4a, inset). The HR-TEM images demonstrate that the walls are completely crystalline, being composed of nanocrystals 2.5–3 nm in size (Fig. 4c and Fig. S4).
 | ||
| Fig. 3 SEM images (top view) of the mesoporous SnO2 films prepared from OH-hydrolyzed solutions (OH/Sn molar ratio of 2.6, aging for one day) calcined at 300 °C (a, b) and 400 °C (c, d), demonstrating their mesoporous structure and crack-free character. Bright areas show the SnO2 network whereas the black ones the pores. The insets show the corresponding FFT images. | ||
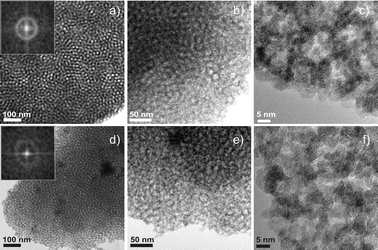 | ||
| Fig. 4 TEM images of the mesoporous SnO2 films prepared from OH-hydrolyzed solutions (OH/Sn molar ratio of 2.6, aged for one day at room temperature) after calcination at 300 °C (a–c) and 400 °C (d–f). The dark areas correspond to the SnO2 network, while the white ones to the pores. The insets show the corresponding FFT images. | ||
The mesostructure is preserved after the calcination at 400 °C, as can be seen from the SEM, TEM, small angle XRD and Kr adsorption data. Surprisingly, the SEM images of the films (top-views) show that the mesostructure unit increased to 18.5 ± 0.5 nm (see Fourier transform in the Fig. 3c, inset) and the pores became more open. This can be attributed to the densification of the inorganic walls due to the further crystallization, leading to an asymmetric change in the pore shape. At the same time, the size of the crystals composing the walls increased to 3–3.5 nm as follows from the HR-TEM (Fig. 3f). Similar to the HR-TEM images, the XRD measurements of the films prove that they are crystalline if calcined above 300 °C – the XRD patterns contain broad diffraction lines typical of cassiterite (P42/mnm, JCPDS card no. 41-1445). A quantitative XRD analysis using a crystalline standard as a reference and comparing the (110) cassiterite peak integral (Fig. 5a) shows that the films' crystallinity increased two-times if the calcination temperature was increased from 300 to 400 °C. (For details see the Experimental part).
 | ||
| Fig. 5 Crystallinity and porosity of SnO2 films coated from OH-hydrolyzed solutions (OH/Sn molar ratio of 2.6, aged for one day at room temperature) calcined at 300 °C (red) and 400 °C (blue): wide angle XRD patterns, normalized according to the LiF signal taken as an inner standard (a) and Kr adsorption isotherms (b). | ||
The texture properties and the accessibility of the internal surface of the tin oxide films were studied by krypton adsorption at 77 K. The Kr adsorption isotherms on the films calcined at 300 and 400 °C (Fig. 5b) show that both films exhibit a well-developed mesoporosity without any pore-blocking. As the slope of the hysteresis loop of the isotherms on both films is rather flat ranging from the relative pressure as low as 0.1 up to 1, these samples exhibit a broad pore size distribution, including also rather small pores. However, there are several characteristic differences in the film porosity due to different calcination temperatures. The increase in the calcination temperature leads to an increase in the specific surface area related to 1 cm2 of the support by ca. 22% (from 40 to 49 cm2 cm−2), as well as to an increase in the pore volume by ca. 29%. The character of the porosity also changes to a certain extent. The hysteresis loop for the film calcined at 400 °C is slightly shifted to higher relative pressures (a lower adsorption at relative pressure under 0.4), and that of the film calcined at 300 °C becomes narrower with increasing pressure and shows a greater tendency to form a plateau. Consequently it can be deduced that the film calcined at 300 °C contains a greater percentage of smaller pores and a smaller percentage of pores larger than ca. 10 nm (which are not filled with krypton at ca. 77 K) than that treated at 400 °C. Similar data were also obtained from the adsorption isotherms of toluene on the films deposited on the piezoelectric quartz crystals (Fig. S2 in the Supporting information). The toluene adsorption isotherms provide surface area of 45 ± 5 and 55 ± 5 m2 g−1 for the films calcined at 300 °C and 400 °C, respectively, which corresponds well to the 66 m2 g−1 found for pure bulk mesoporous SnO2 assembled from crystalline nanoparticles.9
The films obtained by the described procedure are not completely transparent. The optical quality of the films is influenced by the amount of ammonium hydroxide added and by the aging conditions. The films prepared from a solution, which does not contain any ammonium hydroxide, are completely transparent (Fig. 6), but do not have any mesoporosity. While the solutions aged with the added ammonium hydroxide are clear, the mesoporous films coated from them turn opaque after calcination. The optical quality of the films was found to deteriorate with the increasing amount of ammonium hydroxide added, with the increasing aging temperature and aging time, and also due to the presence of the Pluronic polymer in the reaction mixture during the aging process. The deteriorated optical quality can be attributed to the presence of larger aggregates which scatter the incident light. The acceleration of the hydrolysis/condensation processes makes the course of the reaction less controllable, leading to a formation of larger amounts of aggregated species and thus to decreased films' transparency.
 | ||
| Fig. 6 Films prepared from solutions with a different OH/Sn molar ratio (indicated in the labels) and aged for one day at room temperature. The films were calcined at 400 °C. | ||
In order to shed some light on the role of hydroxide ions in a formation of the SnO2 mesoporous structure, we performed dynamic light scattering (DLS) measurements of the reaction solutions used for the films coating. The DLS monitoring of the solution containing 2.6 mol mol−1 of OH/Sn detected the formation of species ca. 3 nm in size shortly after the addition of the ammonium hydroxide to the tin tetrachloride solution (Fig. 7). The size of the species continuously increased with the reaction time, reaching a maximum of 8 ± 3 nm after ca. 19 h, i.e. the time of the precursor solution aging required for the mesostructure to be formed. A further prolonging of the aging time did not lead to any substantial change in the particle size distribution. Similar analysis of the solutions, which do not contain any ammonium hydroxide, did not detect any formation of comparable species in the same time range. Therefore, we suggest that the ca. 8 nm oligomers are important mesostructure precursors suitable for the self-assembly around the Pluronic micelles. The formation of oligomers of such a size by the hydrolysis and oligomerization catalyzed by bases seems a general characteristic for various metal oxides and differs from more common acid-catalyzed processes.7 The size of the species observed in the DLS is bigger than the size of the crystallites composing the walls of the mesoporous SnO2 film. This seeming contradiction can be however understood taking into account the significant increase in the density upon the crystallization of the amorphous phase. Also, one has to consider that certain assumptions are to be made in the calculation of the particle size by DLS, aggravating the precise size determination of amorphous particles. Besides the mentioned 8 nm species, a formation of much larger species roughly 100 to 600 nm in size was also detected in a limited extent, which could correspond to the higher condensation products or agglomerates of smaller colloidal particles as described by Pulcinelli et al.39–41 The presence of these species can explain the gaze of the films obtained from these solutions.
 | ||
| Fig. 7 DLS particle size distribution of a colloidal solution containing ammonium hydroxide (OH/Sn molar ratio of 2.6) and aged at room temperature for different times as indicated in the labels. | ||
The accelerated aging of tin tetrachloride based sols by adding a certain amount of ammonium hydroxide enables to obtain suitable building units and to assemble fully crystalline mechanically stable SnO2 films with a uniform accessible mesoporosity in a very simple procedure. Based on the obtained data, the role of ammonium hydroxide in this process is not completely clear. The hydroxide to tin molar ratio of 2.6 used in the optimized procedure is below the stoichiometric ratio of 1: 4 needed to replace all the chloride atoms in the tin tetrachloride molecule. As the solution pH after the addition of the mentioned sub-stoichiometric amount of ammonium hydroxide is still very low, ca. 0.8–1.6, the hydrolysis and condensation processes still proceed at very acidic conditions. The interpretation of the results is complicated by the fact that the sol–gel chemistry of tin tetrachloride is not well understood, and the conclusions about the hydrolysis mechanism are rather contradictory. It is well established that the hydrolysis of tin ion is much faster than that of, e.g., silicon ion. It seems, however, that the course of the hydrolysis and condensation of tin differs from the reaction scheme of other metal ions. Thus, the transition metal oxide precursors for the EISA process are usually prepared at strongly acidic conditions in order to suppress hydrolysis and to stabilize the condensation products in the form of small oligomers.6,46 Tin compounds, however, were found to be still present as monomers or dimers at these conditions.40,44 We suggest that the addition of hydroxide ions can lead to two possible different scenarios. In the first one, it acts as a reactant for the neutralization of HCl released in the reaction of SnCl4 with alcohols or water, thus shifting the total equilibrium to the right and triggering the hydrolysis. In the second one, hydroxide ions react directly with the tin cation leading to the formation of tin hydroxide, which can further condensate to higher molecular weight products. In both cases, the reaction conditions are still very acidic, which makes our process different from the basic hydrolysis of tin ion leading as a rule to the formation of compact tin oxide particles several hundreds of nanometres in size.
Besides the role of hydroxide ion in the formation mechanism of hydrolyzed tin compounds, the role of ammonium cation needs to be investigated. Our attempts to replace ammonium hydroxide by some other bases such as tetramethylammonium or sodium hydroxide of similar concentration led to a rapid formation of precipitate which did not yield any mesostructure after the addition of Pluronic polymers.
Conclusions
We have developed a new facile procedure for manufacturing mesoporous crystalline thin films of SnO2 by the EISA process using cheap and easily available chemicals, especially tin tetrachloride as a source of tin oxide and Pluronic F127 as a structure directing agent. The procedure developed differs from the common base-catalyzed hydrolysis and condensation of tin compounds, but rather resembles the techniques based on preformed nanocrystals. The crucial factor is the formation of a specific oligomeric precursor ca. 8 nm in size, whose preparation was triggered by the addition of ammonium hydroxide to an alcoholic solution of tin tetrachloride. Despite the addition of hydroxide, the pH of the alcoholic solution is still very low, 1.2–1.4. Such a precursor is suitable for the assembly directed by the Pluronic polymer and afterwards relatively easily convertible to a stable mesoporous framework.The periodicity of the mesostructure ordering and the transparency of the films obtained by the developed approach are inferior to those of films fabricated from the same precursors using delayed humidity (DHT) post-treatment.35–37 The developed protocol however offers a practical advantage of a facile and fast preparation. The obtained films could be of a significant interest for applications where the high crystallinity, small crystal size, large pore size, accessible porosity and high surface area are of primary importance, such as in sensors and catalysis. Further optimization of the films' ordering and their optical properties would require a much deeper insight into the formation mechanism of the precursor species, into their structure and their interaction with the template. Moreover, the developed approach involving formation of the pre-defined metal oxide building blocks can become an efficient way for nanostructuring metal oxides, whose sol–gel chemistry is difficult to control.
Experimental
Materials
Ammonium hydroxide (2 M), n-butanol, ethanol, Pluronic F127 and tin(IV) chloride (99,9%) were purchased from Aldrich, and used without further purification.Preparation of mesoporous tin(IV) oxide thin films
0.4 g of SnCl4 (1.54 mmol) was dissolved in 2 mL of ethanol, afterwards 0.2 mL (11.1 mmol) of distilled water was added. After stirring for about 5 min, 0.8, 1.8, 2.0, 3.0 or 4.0 mmol of 2M ammonium hydroxide was added (the optimum amount leading to the formation of mesoporous structure was 2.0 ml, pH after the ammonia addition equalled 1.32). At first the solution was turbid, but became clear again after ca. 30 min. The mixture was stirred for up to 20 h at 20 °C to 60 °C, afterwards 0.18 g of Pluronic F127 (80%, calculated as a weight ratio of Pluronic to the amount of SnO2 formed) in 2 ml of butanol was added. The dissolution of Pluronic was accelerated by warming up the reaction vessel to 60 °C. The films were prepared by dip-coating glass or Si wafers with the prepared solution at 23 ± 2 °C and a relative humidity of 55 ± 5% at a withdrawal rate of 1.8 mm s−1. The deposited films were calcined at 300 to 400 °C for 30 min. The calcination program included the first ramp with the heating rate of 1 °C min−1 to 100 °C, dwell at 100 °C for 8h, and the second ramp to the final temperature with the heating rate of 3 °C min−1.Characterization methods
Small angle X-ray diffraction measurements of the thin films on substrates were performed on a SCINTAG XDS 2000 diffractometer with Ni-filtered Cu-Kα-radiation (λ = 1.5406 Å) with a θ/θ geometry and a scintillation detector operated at 40 kV and 30 mA. Wide angle X-ray diffraction was carried out in the reflection mode using a Bruker D8 Discover diffractometer with Ni-filtered Cu-Kα-radiation equipped with a Vantec-1 position sensitive detector. The quantitative crystallinity measurements were performed using the internal standard method with LiF as internal standard.47,48 For this purpose, the dip-coating solutions were cast into a wide calcination vessel, dried over night at 60 °C, and calcined at 300 °C or 400 °C (with a ramp of 0.6 °C min−1) for 30 min, respectively. The resulting film or powder was ground and thoroughly mixed with 40 wt% of LiF. X-ray diffraction patterns were recorded using a STOE STADI P COMBI diffractometer. The diffraction peaks of (110) cassiterite and (111) LiF were fitted with a Gaussian peak shape and integrated. The integral of (110) cassiterite was divided by that of (111) LiF.Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) were performed using a FEI Titan 80–300 electron microscope equipped with a field emission gun operated at 300 kV. The samples were prepared by scraping the thin films off the substrate onto a Plano holey carbon coated copper grid. Scanning electron microscopy (SEM) images of the films on a Si substrate were obtained with a JEOL JSM-6500F scanning electron microscope equipped with a field emission gun operated at 4 kV.
Film thickness was determined using a Veeco Dektak 150 profilometer equipped with a diamond stylus (12.5 μm radius) in the contact mode. The average thickness of the obtained films was ca. 200 nm. The porosity of the films was determined by the analysis of adsorption isotherms of Kr at the boiling point of liquid nitrogen (approx. 77 K) using an ASAP 2010 apparatus (Micromeritics). The BET surface area was calculated using the cross-sectional area of krypton of 0.210 nm2. The toluene adsorption was carried out using a self-built quartz crystal microbalance (QCM) system. For this purpose, the precursor solutions were spin-coated (3000 rpm, 30 s) on KVG 10 MHz QCM devices with gold electrodes (from Quartz Crystal Technology GmbH) and calcined at 300 °C and 400 °C. Toluene was used as an adsorptive and the measurements were performed at 25 °C. The BET surface area was calculated using the cross-sectional area of toluene of 0.314 nm2.
Dynamic light scattering (DLS) was performed using a Malvern Zetasizer-Nano equipped with a 4 mW He–Ne laser (633 nm) and an avalanche photodiode detector. The scattering data were weighted based on the particle number.
Acknowledgements
The authors are grateful to the German Research Foundation (DFG, grants No. FA 839/1-1 and SM 199/6-1), the Grant Agency of the Czech Republic (grant No. 104/08/0435-1) and the Academy of Sciences of the Czech Republic (grant KAN100400702) for the financial support. The authors thank Dr Steffen Schmidt (LMU) for TEM and SEM measurements.Notes and references
- C. Sanchez, C. Boissiere, D. Grosso, C. Laberty and L. Nicole, Chem. Mater., 2008, 20, 682 CrossRef CAS.
- B. M. Smarsly and D. Fattakhova-Rohlfing, in Solution processing of inorganic materials, ed. D. B. Mitzi, John Wiley & Sons, Hoboken, New Jersey, 2009, pp. 283 Search PubMed.
- P. D. Yang, D. Y. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Nature, 1998, 396, 152 CrossRef CAS.
- D. Grosso, C. Boissiere, B. Smarsly, T. Brezesinski, N. Pinna, P. A. Albouy, H. Amenitsch, M. Antonietti and C. Sanchez, Nat. Mater., 2004, 3, 787 CrossRef CAS.
- J. Lee, M. C. Orilall, S. C. Warren, M. Kamperman, F. J. Disalvo and U. Wiesner, Nat. Mater., 2008, 7, 222 CrossRef CAS.
- D. Grosso, C. Boissiere, L. Nicole and C. Sanchez, J. Sol-Gel Sci. Technol., 2006, 40, 141 CrossRef CAS.
- C. J. Brinker and G. W. Scherer, Sol–gel science: the physics and chemistry of sol–gel processing, Academic Press, San Diego, 1989 Search PubMed.
- T. J. Barton, L. M. Bull, W. G. Klemperer, D. A. Loy, B. McEnaney, M. Misono, P. A. Monson, G. Pez, G. W. Scherer, J. C. Vartuli and O. M. Yaghi, Chem. Mater., 1999, 11, 2633 CrossRef CAS.
- J. H. Ba, J. Polleux, M. Antonietti and M. Niederberger, Adv. Mater., 2005, 17, 2509 CrossRef CAS.
- T. Brezesinski, J. Wang, J. Polleux, B. Dunn and S. H. Tolbert, J. Am. Chem. Soc., 2009, 131, 1802 CrossRef CAS.
- V. Müller, M. Rasp, G. Štefanić, J. Ba, S. Günther, J. Rathousky, M. Niederberger and D. Fattakhova-Rohlfing, Chem. Mater., 2009, 21, 5229 CrossRef CAS.
- V. Müller, M. Rasp, J. Rathousky, B. Schutz, M. Niederberger and D. Fattakhova-Rohlfing, Small, 2010, 6, 633 CrossRef.
- D. Fattakhova-Rohlfing, J. M. Szeifert, Q. Q. Yu, V. Kalousek, J. Rathousky and T. Bein, Chem. Mater., 2009, 21, 2410 CrossRef CAS.
- J. M. Szeifert, J. M. Feckl, D. Fattakhova-Rohlfing, Y. Liu, V. Kalousek, J. Rathousky and T. Bein, J. Am. Chem. Soc., 2010, 132, 12605 CrossRef CAS.
- Y. J. Liu, J. M. Szeifert, J. M. Feckl, B. Mandlmeier, J. Rathousky, O. Hayden, D. Fattakhova-Rohlfing and T. Bein, ACS Nano, 2010, 4, 5373 CrossRef CAS.
- M. Batzill and U. Diebold, Prog. Surf. Sci., 2005, 79, 47 CrossRef CAS.
- D. Kohl, J. Phys. D: Appl. Phys., 2001, 34, R125 CrossRef CAS.
- M. Tiemann, Chem.–Eur. J., 2007, 13, 8376 CrossRef CAS.
- R. C. Hayward, P. Alberius-Henning, B. F. Chmelka and G. D. Stucky, Microporous Mesoporous Mater., 2001, 44–45, 619 CrossRef CAS.
- R. W. J. Scott, N. Coombs and G. A. Ozin, J. Mater. Chem., 2003, 13, 969 RSC.
- K. G. Severin, T. M. Abdel-Fattah and T. J. Pinnavaia, Chem. Commun., 1998, 1471 RSC.
- L. M. Qi, J. M. Ma, H. M. Cheng and Z. G. Zhao, Langmuir, 1998, 14, 2579 CrossRef CAS.
- T. Brezesinski, A. Fischer, K. Iimura, C. Sanchez, D. Grosso, M. Antonietti and B. M. Smarsly, Adv. Funct. Mater., 2006, 16, 1433 CrossRef CAS.
- T. Wagner, C. D. Kohl, M. Froba and M. Tiemann, Sensors, 2006, 6, 318 CrossRef CAS.
- J. K. Shon, H. Kim, S. S. Kong, S. H. Hwang, T. H. Han, J. M. Kim, C. Pak, S. Doo and H. Chang, J. Mater. Chem., 2009, 19, 6727 RSC.
- P. D. Yang, D. Y. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Chem. Mater., 1999, 11, 2813 CrossRef CAS.
- C. Velasquez, F. Rojas, J. M. Esparza, A. Ortiz and A. Campero, J. Phys. Chem. B, 2006, 110, 11832 CrossRef CAS.
- F. L. Chen and M. L. Liu, Chem. Commun., 1999, 1829 RSC.
- N. Ulagappan and C. N. R. Rao, Chem. Commun., 1996, 1685 RSC.
- H. Miyata, M. Itoh, M. Watanabe and T. Noma, Chem. Mater., 2003, 15, 1334 CrossRef CAS.
- G. J. Li and S. Kawi, Talanta, 1998, 45, 759 CrossRef CAS.
- C. Velasquez, F. Rojas, M. L. Ojeda, A. Ortiz and A. Campero, Nanotechnology, 2005, 16, 1278 CrossRef CAS.
- A. Thomas, H. Schlaad, B. Smarsly and M. Antonietti, Langmuir, 2003, 19, 4455 CrossRef CAS.
- B. Yuliarto, H. S. Zhou, T. Yamada, I. Honma and K. Asai, Chem. Lett., 2003, 32, 510 CrossRef CAS.
- V. N. Urade and H. W. Hillhouse, J. Phys. Chem. B, 2005, 109, 10538 CrossRef CAS.
- J. H. Pan, S. Y. Chai, C. Lee, S. E. Park and W. I. Lee, J. Phys. Chem. C, 2007, 111, 5582 CrossRef CAS.
- S. F. Shao, M. Dimitrov, N. Guan and R. Kohn, J. Mater. Chem., 2009, 19, 8411 RSC.
- M. J. Hampdensmith, T. A. Wark and C. J. Brinker, Coord. Chem. Rev., 1992, 112, 81 CrossRef CAS.
- C. A. Kawaguti, S. H. Pulcinelli, C. V. Santilli and A. F. Craievich, J. Sol-Gel Sci. Technol., 2006, 37, 213 CrossRef CAS.
- V. Briois, S. Belin, M. Z. Chalaca, R. H. A. Santos, C. V. Santilli and S. H. Pulcinelli, Chem. Mater., 2004, 16, 3885 CrossRef CAS.
- L. Broussous, C. V. Santilli, S. H. Pulcinelli and A. F. Craievich, J. Phys. Chem. B, 2002, 106, 2855 CrossRef CAS.
- L. R. B. dos Santos, A. F. Craievich, C. V. Santilli and S. H. Pulcinelli, J. Appl. Crystallogr., 2000, 33, 609 CrossRef CAS.
- J. S. Johnson and K. A. Kraus, J. Am. Chem. Soc., 1959, 81, 1569 CrossRef CAS.
- J. S. Johnson and K. A. Kraus, J. Phys. Chem., 1959, 63, 440 CrossRef CAS.
- M. J. Hampdensmith, T. A. Wark, A. Rheingold and J. C. Huffman, Can. J. Chem., 1991, 69, 121 CAS.
- E. L. Crepaldi, G. Soler-Illia, D. Grosso, F. Cagnol, F. Ribot and C. Sanchez, J. Am. Chem. Soc., 2003, 125, 9770 CrossRef CAS.
- H. P. Klug and L. E. Alexander, X-Ray Diffraction Procedures, John Wiley & Sons, New York, 1974 Search PubMed.
- J. J. Laskowski, J. Young, R. Gray, R. Acheson and S. D. Forder, Anal. Chim. Acta, 1994, 286, 9 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Change in pH of precursor solution during aging process, adsorption isotherms of toluene on SnO2 films, TGA of the coating solutions and HRTEM/SAED images of the calcined mesoporous films. See DOI: 10.1039/c0nr00872a |
| ‡ Present address: Department of Chemistry, University of Hamburg, Martin-Luther-King-Platz 6, 20146 Hamburg, Germany. E-mail: mailto:fried@chemie.uni-hamburg.de. |
| This journal is © The Royal Society of Chemistry 2011 |
