Hierarchical polymer assemblies constructed by the mutual template effect of cationic polymer complex and anionic supramolecular nanofiber†
Kouta
Sugikawa
ab,
Munenori
Numata
*c,
Daiki
Kinoshita
c,
Kenji
Kaneko
d,
Kazuki
Sada
ab,
Atsushi
Asano
e,
Shu
Seki
e and
Seiji
Shinkai
*fg
aDepartment of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, Fukuoka 812-8581, Japan
bPresent Address: Division of Chemistry, Graduate School of Science, Hokkaido University, Sapporo 060-0810, Japan
cGraduate School of Life and Environmental Science, Kyoto Prefectural University, Shimogamo, Sakyo-ku, Kyoto 606-8522, Japan. E-mail: numata@kpu.ac.jp
dDepartment of Material Science and Engineering, Graduate School of Engineering, Kyushu University, Moto-oka 744, Nishi-ku, Fukuoka 819-0395, Japan
eDepartment of Applied Chemistry, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
fInstitute of Systems, Information Technologies and Nanotechnologies (ISIT), 203-1, Motooka, Nishi-ku, Fukuoka 819-0385, Japan. E-mail: shinkai_center@mail.cstm.kyushu-u.ac.jp
gDepartment of Nanoscience, Faculty of Engineering, Sojo University, 4-22-1, Ikeda, Kumamoto 860-0082, Japan
First published on 3rd November 2010
Abstract
Creation of higher-ordered polymeric architectures composed of alternative assemblies of single-walled carbon nanotubes (SWNTs) and fibrous porphyrin J-aggregates can be easily achieved utilizing the cationic semi-artificial polysaccharide which can act not only as a tubular host for SWNTs but also as a one-dimensional template for porphyrin molecules. This new class of hierarchical polymer assembly is formed, for the first time, by the mutual template effect of two components, i.e., the cationic SWNT complexes and the anionic porphyrin supramolecular nanofibers. In the present system, the self-assembling behaviors of the SWNT complexes as well as the final properties of the SWNT nanoarchitectures are strongly affected by the packing mode of porphyrin molecules on the cationic semi-artificial polysaccharide. Furthermore, we have confirmed that the light energy captured by the porphyrin J-aggregates is effectively transferred to SWNTs.
Introduction
Creation of supramolecular nanoarchitectures using polymers as building blocks has been a difficult challenge for chemists because of its complexity but still been an important target because of its potential that they are directly useful for designing functional nanomaterials.1–6 Unlike the well-established self-assembly systems of programmed molecules, however, there is no established general strategy on how functional polymers can be built up toward well-ordered nanoarchitectures exhibiting the desired functions. To overcome the difficulty mainly arising from the strong cohesive nature of polymers, we have so far employed a supramolecular approach that β-1,3-glucans, helix-forming natural polysaccharides, can wrap an individual polymer such as single-walled carbon nanotubes (SWNTs) or polythiophenes, leading to the creation of supramolecular one-dimensional (1-D) complexes.7 Advantageously, when a semi-artificial β-1,3-glucan bearing a molecular recognition site is used as a wrapping component instead of natural one, the surface of the resultant complex acquires the potential self-assembling capability toward further organization of the complexes. In fact, we have successfully demonstrated that β-1,3-glucans modified with cationic trimethylammonium groups (CUR-N+) or anionic sulfonate groups (CUR-SO3−) helically wrap SWNTs and endow the self-assembling ability toward SWNTs, resulting in the construction of the highly-ordered SWNT nanoarchitecture through the electrostatic interactions between the cationic and the anionic complexes.8 Along this line aiming at the creation of highly-ordered polymer assemblies by the novel strategy, we herein show a new polymer-assembling system using the programmed polymeric molecules, where the well-established supramolecular porphyrin nanofiber acts as a complementary 1-D building block for a polymer assembly, resulting in a new class of polymer-small molecule nanocomposites (Fig. 1). Importantly, it is expected for the present system that the dynamic association/dissociation ability of the porphyrin-based supramolecular nanofibers leads to the creation of various polymer assemblies through their structural conversion, which could not be realized only by covalent-bond-based polymer components.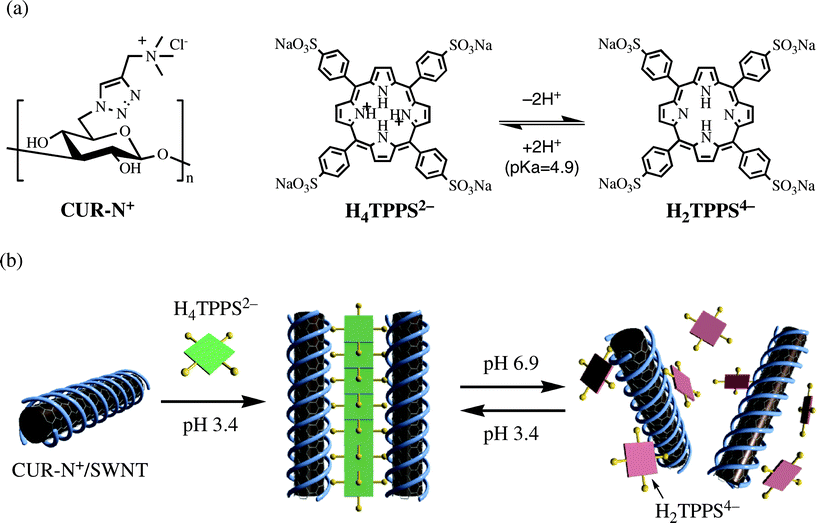 | ||
| Fig. 1 (a) Structures of CUR-N+ and the diacid (H4TPPS2−) and free-base (H2TPPS4−) forms of the tetrakis(4-sulfonatophenyl)porphyrin, and (b) schematic illustration of the present concept. | ||
It has been known that tetrakis(4-sulfonatophenyl)porphyrin (H2TPPS4−) generates the di-acid form (H4TPPS2−) under the acidic conditions, i.e., below pH = 3.0, where pKa value for H2TPPS4− is 4.9, and therefore tends to self-assemble into the well-regulated fibrous structure (J-aggregate) through electrostatic interactions.9–12 As the resultant supramolecular nanofiber structure carries anionic charges arising from the sulfonate groups, it would be useful as a supramolecular 1-D building block to form a supramolecular polymeric architecture with CUR-N+ or its SWNT complex. It thus occurred to us that the self-assembly of H4TPPS2− molecules and the cationic CUR-N+/SWNT complexes would lead to highly-ordered self-organization of SWNTs, where the H4TPPS2− J-aggregates act as a supramolecular adhesive agent for the cationic complexes. If the H4TPPS2− J-aggregates could act as a photosensitizing component, the resultant polymeric architecture may exhibit the excellent light-harvesting abilities, transferring the light energy to SWNT.5,13,14 In addition, one can expect for this system that a structural change in the H4TPPS2− aggregate by external stimuli may induce a drastic change in the SWNTs orientation mode; that is, the resultant composite would acquire the dynamic properties arising from a structural charge in the supramolecular nanofibers, resulting in the formation of “soft” polyion complexes.
Results and discussion
Self-assembly of H4TPPS2− molecules into their J-aggregate proceeds only under the acidic conditions, i.e., below pH = 3.0, where the electrostatic interactions among the protonated central pyrrole groups and the peripheral sulfonate groups play a crucial role.9–11 Thus, as a preliminary experimental condition, H4TPPS2− solution was mixed with aqueous solution containing only CUR-N+ without SWNTs, adjusting the pH to 3.4 with AcOH/AcONa buffering solution. Although this pH value is slightly higher than the critical pH for H4TPPS2− molecules to form the J-aggregate, we expected that H4TPPS2− molecules would be self-organized into the J-aggregate with the aid of CUR-N+ template. To clarify the template effect of CUR-N+ on the formation of the H4TPPS2− J-aggregate, UV-vis spectroscopic measurements were performed with gradually changing the [trimethylammonium (in CUR-N+)]/[sulfonate (in H4TPPS2−)] ratio. As shown in Fig. 2a, UV-vis spectrum of H4TPPS2− in the absence of CUR-N+ shows characteristic peaks at 435 nm (Soret-band) and 645 nm (Q-band) arising from monomeric H4TPPS2−.2,10 Upon addition of CUR-N+, however, both Soret- and Q-bands were significantly red-shifted to 490 nm and 710 nm, respectively, which is indicative of the formation of the J-aggregate (Fig. 2a).10,15,16 Furthermore, an intense split-type band of induced circular dichroism (ICD) in the J-aggregate region was observed (Fig. 2c). This result suggests that the effective interaction between H4TPPS2− molecule and CUR-N+ template facilitates the formation of the H4TPPS2− J-aggregate. These spectral changes are almost saturated at [trimethyl ammonium]/[sulfonate] = 0.5, indicating that two sulfonate groups in an individual H4TPPS2− molecule are neutralized by two trimethylammonium groups of CUR-N+. In this case, two other sulfonate groups can still take part in the intermolecular interaction with adjacent H4TPPS2− molecules, so that the J-aggregate can be created with the aid of CUR-N+ template.17 Further increase in the cationic component, i.e., in the [trimethylammonium]/[sulfonate] ratio from 1.0 to 3.0, somewhat broad peak assignable to H4TPPS2− H-aggregate was newly observed at 410 nm, with decreasing the peak intensity attributable to the J-aggregate. These results suggest that all sulfonate groups in the H4TPPS2− molecule interact with trimethylammonium groups, where H4TPPS2− molecules are no longer self-assembled into the J-aggregate due to the lack of the intermolecular electrostatic interactions.18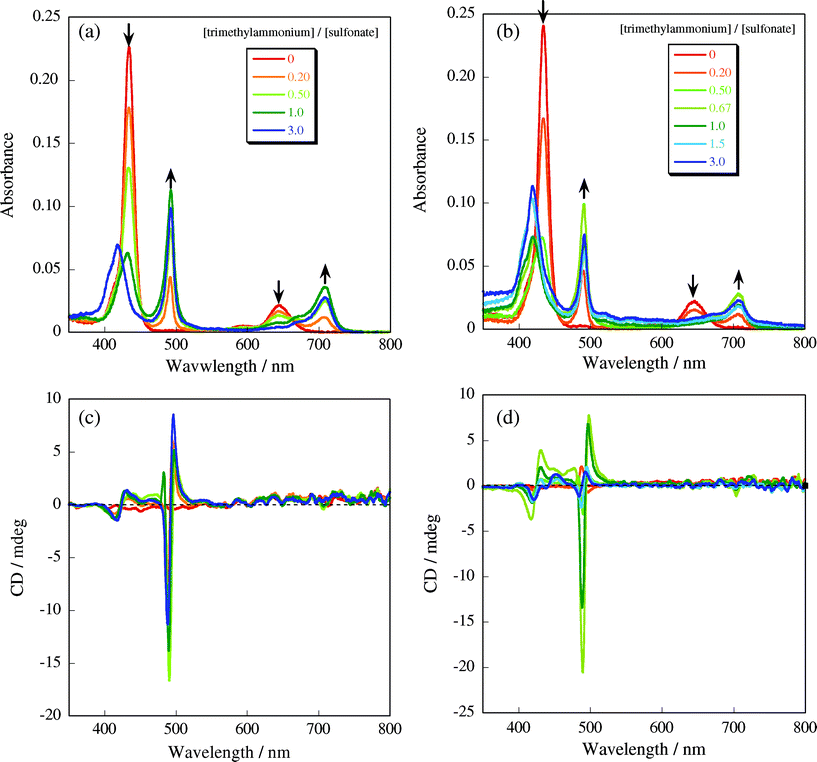 | ||
| Fig. 2 UV-vis and CD spectral changes of H4TPPS2− upon addition of only CUR-N+ (a) and (c), and CUR-N+/SWNT complex (b) and (d), respectively: 25 °C, 1.0 cm cell. | ||
We then mixed CUR-N+/SWNT complex with H4TPPS2−, expecting that the H4TPPS2− J-aggregate would form on the CUR-N+/SWNT complex, which would also lead to the self-organization of SWNTs due to the electrostatic interaction. When the [trimethylammonium]/[sulfonate] ratio was increased from 0.0 to 0.67, the Soret- and Q-bands of monomeric H4TPPS2− gradually decreased and new peaks assignable to the J-aggregate appeared at 490 nm and 710 nm, as shown in Fig. 2b, as in the case of CUR-N+ template system. As a reference experiment, we have confirmed that there is no specific interaction between SWNTs and H4TPPS2− under the same conditions as those for CUR-N+ or CUR-N+/SWNT template system; that is, when SWNTs was mixed with H4TPPS2− solution, no significant change in UV-vis spectra of H4TPPS2− was observed. CD spectral changes strongly support the view that H4TPPS2− molecules form the J-aggregate on the CUR-N+/SWNT complex, as shown in Fig. 2d, being affected by chiral CUR-N+. The split-type of ICD signals appears at 490 nm and 498 nm and the cross section wavelength of the peaks is consistent with the absorption maximum of the H4TPPS2− J-aggregate. Beside, the CD intensity also reached to the maximum when the [trimethylammonium]/[sulfonate] ratio was at 0.67, implying that H4TPPS2− molecules self-assemble on the CUR-N+/SWNT complex through the electrostatic interaction, leading to the creation of the J-aggregate structure.
The charge neutralization of cationic CUR-N+ by anionic H4TPPS2− would cause the drastic morphological changes from each component as observed in our previous systems.8,19 Taking the conditions for UV-vis and CD spectroscopic data into account, we firstly carried out TEM (transmission electron microscopic) observation for H4TPPS2−assemblies supported by CUR-N+, adjusting the [trimethylammonium]/[sulfonate] ratio to 0.50. It is well accepted that H4TPPS2− itself forms rod-like structure under the acidic conditions. H4TPPS2− in the presence of CUR-N+, however, self-assembled to sheet-like structure with 80–300 nm in length in which H4TPPS2− rod-like structure and CUR-N+ would be piled up alternately (Fig. S1a ESI†). From this result, we presumed the self-organization of the CUR-N+/SWNT complex with the aid of the H4TPPS2− J-aggregate structure. Expectedly, the H4TPPS2− J-aggregate formation on the CUR-N+/SWNT complex ([trimethylammonium]/[sulfonate] = 0.50) leads to the creation of the sheet-like nanostructure with the width about 20 nm, as shown in Fig. 3a, which corresponds to the aggregate of several tens CUR-N+/SWNT complexes. In addition, the length of the sheet-like structure can be estimated to be ca.1 μm, the value of which is almost consistent with the average length of used SWNTs (0.6–1.2 μm, Fig. S2a ESI†), indicating that the CUR-N+/SWNT complex acts as a 1-D building block. Importantly, the magnified TEM image revealed the presence of the highly-ordered fibrous assemblies within the sheet-like structure, as shown in Fig. 3b, indicating that the CUR-N+/SWNT complexes are regularly piled up with the aid of the anionic H4TPPS2− J-aggregates. The direct evidence that the sheet-like structure contains the H4TPPS2− J-aggregates was obtained from EDX (energy dispersive X-ray spectroscopy) analyses: as shown in Fig. 3c, characteristic peaks assignable to nitrogen and sulfur atoms were clearly detected at 0.39 keV and 2.30 keV, respectively. It should be emphasized here that no sheet-like structure can be seen from H4TPPS2− without CUR-N+/SWNT complex under the same conditions (Fig. S1b ESI†).16 This fact suggests that the assistance of the CUR-N+/SWNT complex as a cationic template is indispensable for the H4TPPS2− J-aggregate formation. Spectral changes as shown in Fig. 2a–2d indicate that, at the given pH condition, i.e., pH = 3.4, most H4TPPS2− molecules exist their monomeric state. Once upon addition of CUR-N+ or CUR-N+/SWNT complex, the equilibrium between H4TPPS2− monomer and its J-aggregate is shifted to the J-aggregate formation, which further promotes the assembly of the CUR-N+/SWNT complex toward the sheet-like structure. The present sheet-like structure is thus formed, for the first time, by the mutual template effect of two components. When the [trimethylammonium]/[sulfonate] ratio was changed from 1.3 to 3.0, where the J-aggregate no longer act as the template for the assembly of the CUR-N+/SWNT complexes, the sheet-like structure could not be observed (Fig. S2b ESI†).
![TEM image of the sheet-like structure ([trimethylammonium]/[sulfonate] = 0.50) (a) and its magnified image (b). EDX spectrum of the sheet-like structure (c).](/image/article/2011/OB/c0ob00407c/c0ob00407c-f3.gif) | ||
| Fig. 3 TEM image of the sheet-like structure ([trimethylammonium]/[sulfonate] = 0.50) (a) and its magnified image (b). EDX spectrum of the sheet-like structure (c). | ||
In order to reconfirm that the H4TPPS2− J-aggregate is indispensable for the alignment of CUR-N+/SWNT complexes, the solution containing the sheet-like structures ([trimethylammonium]/[sulfonate] = 0.5) was neutralized by NaOH aqueous solution to dissociate the J-aggregate structure supported by the intermolecular NH+-SO3− electrostatic interaction. Upon addition of NaOH aqueous solution (1.0 M), the solution pH was increased from 3.4 to 6.9, which would lead to the dissociation of the J-aggregate into monomeric H2TPPS4−. UV-vis and CD spectral changes also support this view (Fig. 4a and 4b); that is, the absorption peaks assignable to the H4TPPS2− J-aggregate was dramatically decreased with increasing pH values, and a new peak assignable to monomeric H2TPPS4− appeared at 414 nm. The split-type ICD also disappeared during this treatment, suggesting that H2TPPS4− molecules no longer interact with the cationic CUR-N+/SWNT complex. TEM observation provided the direct image that the sheet-like structures consisting of CUR-N+/SWNT complexes are transformed to the individual fibrous assemblies with several nanometer widths, where H4TPPS2− molecules would be adsorbed onto the complex surfaces in a random fashion (Fig. 4c). Interestingly, when the pH value of the solution mixture was restored to 3.4, the sheet-like nanostructure was reconstructed (Fig. 4d) and the original absorption spectral peaks assignable to the J-aggregate emerged again. In this case, dissociated H4TPPS2− molecules were not entirely self-assembled to the initial J-aggregate as shown in the UV-vis spectra. This is due to the adsorption of some H4TPPS2− molecules onto the complex surfaces in a random fashion, which suppress the reformation of the J-aggregate. These results clearly suggest that the intermolecular interactions among H2TPPS4− molecules, i.e., J-aggregate formation and dissociation of H2TPPS4− molecules, are controllable on the complex surface, which eventually governs the self-assembling properties of the polymer complex.
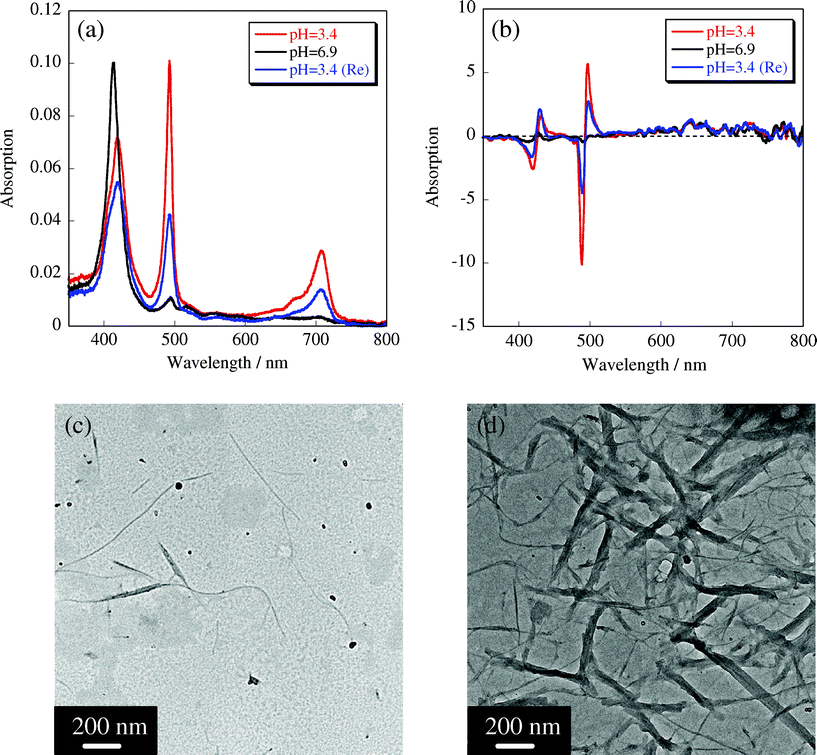 | ||
| Fig. 4 Reversible UV-vis and CD spectral changes of the sheet-like structure (a) and (b); pH = 3.4 (red line), pH = 6.8 (black line) and after restoring to pH = 3.4 (blue line). TEM images of the dissociated structure at pH 6.9 (c) and the reconstructed sheet-like structures at pH 3.4 (d). | ||
Finally, we measured the fluorescence spectrum of the H4TPPS2− J-aggregate in the presence of CUR-N+/SWNT complex, expecting that the reliable evidence for the inner structure of the sheet like-structure was obtained; that is, in the present system, CUR-N+/SWNT complex would be surrounded by an H4TPPS2− J-aggregate in the sheet-like structure, so that the photo energy captured by the J-aggregate would be effectively transferred to SWNTs. Accordingly, upon excitation at 490 nm, the fluorescence intensity of the H4TPPS2− J-aggregate drastically decreased with increasing CUR-N+/SWNT complex concentrations, as shown in Fig. 5. The effective quenching of the fluorescence supports the view that CUR-N+/SWNT complex and the H4TPPS2− J-aggregate is piled-up alternatively to form the sheet-like structure.20
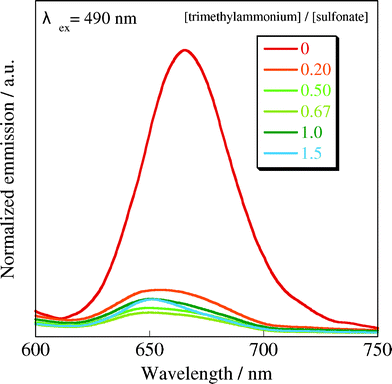 | ||
| Fig. 5 Fluorescence spectral change of H4TPPS2− upon addition of CUR-N+/SWNT: 25 °C, 1.0 cm cell, excitation at 490 nm. | ||
In addition to the steady-state fluorescence, the fluorescence decay of H4TPPS2− at 660 nm was investigated by time-resolved means, which are expected to support the excited-state interactions between the H4TPPS2− J-aggregate and SWNT. A fluorescence lifetime of 0.45 ns was registered for H4TPPS2− in the presence of CUR-N+/SWNT complex. As a reference sample, lifetime of 0.76 ns was observed for H4TPPS2− on CUR-N+ template without SWNT. In this case, the H4TPPS2− J-aggregate would be aligned on CUR-N+ template to create the sheet-like structure as shown in Fig. S1,† and therefore some deactivation of the excited sate arising from the interaction between H4TPPS2− and CUR-N+ would occur in the sheet-like structure. Along this line, the further short-lived component observed in the presence of CUR-N+/SWNT complex would be mainly attributed to the electron transfer from H4TPPS2− to SWNT (Fig. 6a and 6b).21 The related photoinduced electron transfer in the porphyrin/carbon nanotube hybrid systems has been reported by several research groups, where π-radical cation of porphyrin was detected as a transitional species.22 Together with the similarity of the present system in comparing with the related porphyrin/carbon nanotube hybrid systems, we believe that the present system would lead to the development of novel photonic nanomaterials, where the light-harvesting properties would be tunable at a molecular level, dominating the final composite size, mechanical strength, electronic properties, and others.
![(a) Fluorescence decay kinetics for H4TPPS2− observed at 660 nm in the presence of CUR-N+/SWNT complex (red line) and CUR-N+ (blue line) upon excitation at 355 nm, (b) transient fluorescence spectra in the presence of CUR-N+/SWNT complex at 0–0.1 ns (violet line) and 0.5–0.8 ns (blue line) after pulse exposure: [trimethylammonium]/[sulfonate] ratio of the samples was adjusted to 1.0.](/image/article/2011/OB/c0ob00407c/c0ob00407c-f6.gif) | ||
| Fig. 6 (a) Fluorescence decay kinetics for H4TPPS2− observed at 660 nm in the presence of CUR-N+/SWNT complex (red line) and CUR-N+ (blue line) upon excitation at 355 nm, (b) transient fluorescence spectra in the presence of CUR-N+/SWNT complex at 0–0.1 ns (violet line) and 0.5–0.8 ns (blue line) after pulse exposure: [trimethylammonium]/[sulfonate] ratio of the samples was adjusted to 1.0. | ||
Conclusion
In conclusion, we have demonstrated that the cationic β-1,3-glucan/SWNT complex can be successfully organized with the support of the anionic supramolecular H4TPPS2− nanofiber structures. This self-organized nanostructures were dissociated and reconstructed in a reversible manner by the pH change, which indicates that the supramolecular nanofiber could act as a complementary 1-D building block for the construction of higher-ordered polymer assemblies. Therefore, the final assembling architecture is strongly affected by the [trimethylammonium]/[sulfonate] ratio, accompanying a morphological change in the H4TPPS2− fibrous assembly. It is worthy to emphasize as a novel concept in a molecular assembly that in the present system, the CUR-N+/SWNT complex acts as a 1-D template for H4TPPS2− molecules, promoting the self-organization of H4TPPS2− even under somewhat weak acidic conditions. Simultaneously, this self-assembling event of H4TPPS2− on the polymer template induces the higher-ordered polymeric assemblies in a mutual supporting manner, acting as a supramolecular glue for the polymer complex. The mutual template effects of the 1-D complex and the supramolecular nanofiber structures characterize the present system. Additionally, the supramolecular nanofiber structures, surrounding the cationic SWNT complex, play a role of light-harvesting chromophores upon photo-irradiation: in the polymeric assemblies, thus the photo energy transfers from the peripheral H4TPPS2− J-aggregate to central SWNTs. Throughout the present system, we can provide a novel concept to create hierarchical polymer assemblies assisted by the supramolecular nanofiber structure as an adhesive agent.Experimental
General
UV-vis spectroscopic studies were performed by using a SHIMADZU UV-3100 and a Jasco V-580 spectrophotometer, respectively. CD spectra were obtained using a Jasco V-710 spectrometer. Fluorescence spectra were measured using a Perkin–Elmer LS-55 luminescence spectrometer. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were acquired by using a JEOL TEM-2010 (accelerate voltage 120 kV) and a TECNAI-20, FEI (accelerate voltage 200 kV), respectively. The sample solution was placed on a copper TEM grid with a holey carbon support film. The TEM grid was dried under reduced pressure for 6 h before TEM observation. Energy dispersive X-ray spectroscopy (EDX) spectra were obtained using a TECNAI-20, FEI.Materials
Curdlan (MW = 1,000,000) was obtained from Wako Chemicals. SWNTs produced by the Hipco (high-pressure decomposition of carbon monoxide) process were purchased from Carbon Nanotechnologies, Inc. and metallic catalysts were purified according to the literature. Tetrakis(4-sulfonatophenyl) porphyrin (H2TPPS4−) was obtained from Aldrich. The synthesis of CUR-N+ was carried out according to the method previously reported by us.7Sample preparation for measurements
Acknowledgements
The authors thank Mitusi Sugar Co., Japan, for providing the schizophyllan samples. This work was partially supported by the MEXT, Grant-in-Aid for Scientific Research on Innovative Areas “Emergence in Chemistry”. M. N. thanks Mitsubishi Chemical Corporation Fund, Izumi Science and Technology Foundation, Kansai Research Foundation for Technology Promotion, Tokuyama Science Foundation, Iketani Science Technology Foundation and the Sumitomo Foundation, for financial supports.Notes and References
- H. Goto, K. Akagi and H. Shirakawa, Synth. Met., 1997, 84, 373–374 CrossRef CAS.
- T. M. Long and T. M. Swager, J. Am. Chem. Soc., 2002, 124, 3826–3827 CrossRef CAS.
- N. Akima, Y. Iwasa, S. Brown, A. M. Barbour, J. Cao, J. L. Musfeldt, H. Matsui, N. Toyota, M. Shiraishi, H. Shimoda and O. Zhou, Adv. Mater., 2006, 18, 1166–1169 CrossRef CAS.
- H. Xin and A. T. Woolley, J. Am. Chem. Soc., 2003, 125, 8710–8711 CrossRef CAS.
- (a) T. Hasobe, S. Fukuzumi and P. V. Kamat, J. Am. Chem. Soc., 2005, 127, 11884–11885 CrossRef CAS; (b) D. M. Guldi, G. M. A. Rahman, F. Zerbetto and M. Prato, Acc. Chem. Res., 2005, 38, 871–878 CrossRef CAS; (c) T. Umeyama, M. Fujita, N. Tezuka, N. Kadota, Y. Matano, K. Yoshida, S. Isoda and H. Imahori, J. Phys. Chem. C, 2007, 111, 11484–11493 CrossRef CAS.
- (a) Y. Kubo, Y. Kitada, R. Wakabayashi, T. Kishida, M. Ayabe, K. Kaneko, M. Takeuchi and S. Shinkai, Angew. Chem., Int. Ed., 2006, 45, 1548–1553 CrossRef CAS; (b) M. Ikeda, Y. Furusho, K. Okoshi, S. Tanahara, K. Maeda, S. Nishino, T. Mori and E. Yashima, Angew. Chem., Int. Ed., 2006, 45, 6491–6495 CrossRef CAS; (c) T. Fukushima, K. Asaka, A. Kosaka and T. Aida, Angew. Chem., Int. Ed., 2005, 44, 2410–2413 CrossRef CAS.
- (a) M. Numata, M. Asai, K. Kaneko, A.-H. Bae, T. Hasegawa, K. Sakurai and S. Shinkai, J. Am. Chem. Soc., 2005, 127, 5875–5884 CrossRef CAS; (b) Y. Kaneko and J.-I. Kadokawa, Chem. Rec., 2005, 5, 36–46 CrossRef CAS; (c) T. Sanji, N. Kato and M. Tanaka, Org. Lett., 2006, 8, 235–238 CrossRef CAS.
- M. Numata, K. Sugikawa, K. Kaneko and S. Shinkai, Chem.–Eur. J., 2008, 14, 2398–2404 CrossRef CAS.
- A. D. Schwab, D. E. Smith, C. S. Rich, E. R. Young, W. F. Smith and J. C. De Paula, J. Phys. Chem. B, 2003, 107, 11339–11345 CrossRef CAS.
- O. Ohno, Y. Kaizu and H. Kobayashi, J. Chem. Phys., 1993, 99, 4128–4139 CrossRef CAS.
- C. Escudero, J. Crusats, I. Díez-Pérez, Z. El-Hachemi and J. M. Ribó, Angew. Chem., Int. Ed., 2006, 45, 8032–8035 CrossRef CAS.
- K. Hosomizu, M. Oodoi, T. Umeyama, Y. Matano, K. Yoshida, S. Isoda, M. Isosomppi, N. V. Tkachenko, H. Lemmetyinen and H. Imahori, J. Phys. Chem. B, 2008, 112, 16517–16524 CrossRef CAS.
- P. W. Bohn, Annu. Rev. Phys. Chem., 1993, 44, 37–60 CrossRef CAS.
- R. F. Service, Science, 1996, 271, 920–922 CAS.
- N. C. Maiti, S. Mazumdar and N. Periasamy, J. Porphyrins Phthalocyanines, 1998, 2, 369–376 CrossRef CAS.
- Generally, H4TPPS2− can form a stable J-aggregate below pH = 3.0. To avoid decomposition of glycosil bonds, we dared adjust the solution pH to somewhat higher pH values. It should be emphasized that H4TPPS2− formed stable J-aggregates even at higher pH, being assisted by the CUR-N+ template.
- Upon addition of H4TPPS2−, CUR-N+ fibrous structures are aligned in the same direction to create a bundle structure, in which the H4TPPS2− J-aggregate acts as a “glue” for CUR-N+ assembly: see Fig. S1.
- L. Na and T.-S. Yang, Talanta, 1994, 41, 1657–1662 CrossRef CAS.
- K. Sugikawa, M. Numata, K. Kaneko, K. Sada and S. Shinkai, Langmuir, 2008, 24, 13270–13275 CrossRef CAS.
- When the pH value of the solution containing the sheet-like structures ([trimethylammonium]/[sulfonate] = 0.5) was increased to be 7.2, the fluorescence intensity of the quenched H4TPPS2− was recovered by 78% comparing free H4TPPS2− (Fig. S3 ESI†). This means that the photoinduced electron transfer would be controllable by changing the pH values, which directly influence the organization and the dissociation of H4TPPS2− around the CUR-N+/SWNT complex.
- (a) S. Seki, Y. Koizumi, T. Kawaguchi, H. Habara and S. Tagawa, J. Am. Chem. Soc., 2004, 126, 3521–3528 CrossRef CAS; (b) Y. Koizumi, S. Seki, S. Tsukuda, S. Sakamoto and S. Tagawa, J. Am. Chem. Soc., 2006, 128, 9036–9037 CrossRef CAS.
- (a) D. M. Guldi, G. M. A. Rahman, M. Prato, N. Jux, S. Qin and W. Ford, Angew. Chem., Int. Ed., 2005, 44, 2015–2018 CrossRef CAS; (b) A. Satake, Y. Miyajima and Y. Kobuke, Chem. Mater., 2005, 17, 716–724 CrossRef CAS; (c) S. Campidelli, C. Sooambar, E. L. Diz, C. Ehli, D. M. Guldi and M. Prato, J. Am. Chem. Soc., 2006, 128, 12544–12552 CrossRef CAS; (d) T. Hasobe, S. Fukuzumi and P. V. Kamat, J. Am. Chem. Soc., 2005, 127, 11884–11885 CrossRef CAS; (e) D. S. Hecht, R. J. A. Ramirez, M. Briman, E. Artukovic, K. S. Chichak, J. F. Stoddart and G. Grüner, Nano Lett., 2006, 6, 2031–2036 CrossRef CAS; (f) M. Alvaro, P. Atienzar, P. Cruz, J. L. Delgado, V. Troiani, H. Garcia, F. Langa, A. Palker and L. Echegoyen, J. Am. Chem. Soc., 2006, 128, 6626–6635 CrossRef CAS; (g) H. Li, B. Zhou, Y. Lin, L. Gu, W. Wang, K. A. S. Fernando, S. Kumar, L. F. Allard and Y.-P. Sun, J. Am. Chem. Soc., 2004, 126, 1014–1015 CrossRef CAS; (h) D. Baskaran, J. W. Mays, X. P. Zhang and M. S. Bratcher, J. Am. Chem. Soc., 2005, 127, 6916–6917 CrossRef CAS; (i) E. Maligaspe, A. S. D. Sandanayaka, T. Hasobe, O. Ito and F. D'Souza, J. Am. Chem. Soc., 2010, 132, 8158–8164 CrossRef CAS; (j) R. Chitta, A. S. D. Sandanayaka, A. L. Schumacher, L. D'Souza, Y. Araki, O. Ito and F. D'Souza, J. Phys. Chem. C, 2007, 111, 6947–6955 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images of CUR-N+/H4TPPS2− composites ([trimethylammonium]/[sulfonate] = 0.5) and CUR-N+/SWNT/H4TPPS2− composites ([trimethylammonium]/[sulfonate] = 3.0). See DOI: 10.1039/c0ob00407c |
| This journal is © The Royal Society of Chemistry 2011 |
