Functionalisation of heteroaromatic N-oxides using organic superbase catalyst†
Yuta
Araki
,
Koji
Kobayashi
,
Misato
Yonemoto
and
Yoshinori
Kondo
*
Graduate School of Pharmaceutical Sciences, Tohoku University, Aramaki Aza Aoba 6-3, Aoba-ku, Sendai 980-8578, Japan. E-mail: ykondo@mail.pharm.tohoku.ac.jp; Fax: +81-22-795-6804; Tel: +81-22-795-6804
First published on 17th November 2010
Abstract
Functionalisation of quinoline N-oxide was investigated using phophazene base as a catalyst; alkynylation and heteroarylation at 2-position were successfully achieved via nucleophilic addition–elimination process.
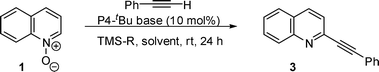
The chemistry of heteroaromatic N-oxides have received much attention due to their usefulness as synthetic intermediates and their biological or functional importance.1 Various methodologies have been developed for introducing carbon functionalities into adjacent position of N-oxide and the use of organometallic nucleophiles has been considered to be one of the most important processes for the selective functionalisation.2 Recently, transition metal catalyzed coupling reactions have been widely used for the C–H functionalisation of heteroaromatics, and heteroaromatic N-oxides have been also successfully transformed at adjacent position of N-oxide.3 Transition-metal free processes are regarded to be attractive in medicinal oriented synthesis, and the reactions of organolithium, or organomagnesium nucleophiles with heteroaromatic N-oxide have also attracted recent significant attention.4Organosilicon nucleophiles have been also utilized for the introduction of allyl or cyano moiety,5 but no example for the introduction of alkynyl or aryl moiety using alkynylsilanes or arylsilanes has been reported. In connection with our recent interest on the development of phosphazene base catalyzed reactions,6 we focused our study on the use of alkynylsilane and heteroarylsilane for functionalising heteroaromatic N-oxides. By extending the concept, we finally developed a new simple process for introducing carbon functionality via organocatalytic deprotonative addition of nucleophile in the presence of phosphazene catalyst and organosilicon additive (Fig. 1).7
 | ||
| Fig. 1 Reaction of quinoline N-oxide with nucleophiles in the presence of organic superbase catalyst. | ||
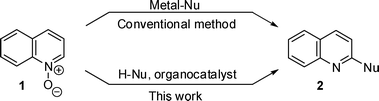
The addition–elimination reaction of quinoline N-oxide with trimethylsilylphenylacetylene was first investigated and the reaction proceeded smoothly in the presence of 10 mol% P4-tBu in DMF to give 2-phenylethynylquinoline (3) in 87% yield (Scheme 1). As other alkynylsilanes, trimethylsilyl-4-methoxyphenylacetylene and trimethylsilyl-4-chlorophenylacetylene was also used for the reaction and the reaction with the latter alkynylsilane was found to proceed somewhat slower (Scheme 1).
 | ||
| Scheme 1 Reaction of quinoline N-oxide with alkynylsilanes. | ||
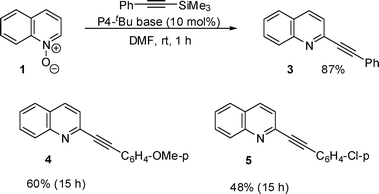
The reaction of quinoline N-oxide with 2-trimethylsilylbenzothiazole was then examined and the reaction proceeded smoothly under the similar reaction conditions at room temperature. The use of cyclopentylmethyl ether as a solvent gave the best result and the desired heterobiaryl (6) was obtained in 77% yield (Scheme 2).
 | ||
| Scheme 2 Reaction of quinoline N-oxide with 2-TMS-benzothiazole. | ||
Plausible reaction pathway for this catalytic nucleophile introduction using organosilicon compounds is shown in Fig. 2. First, activation of organosilicon compounds by P4-tBu forms a reactive carbanion, which attacks the 2-position of quinoline N-oxide. The oxygen anion was silylated and P4-tBu or related base deprotonates 2-hydrogen of the adduct to eliminate trimethylsilanoxide to form 2-functionalised quinoline. The phosphazenium silanoxide reacts with silicon nucleophile to generate the phosphazenium carbanion complex which attacks quinoline N-oxide for the following catalytic cycle.
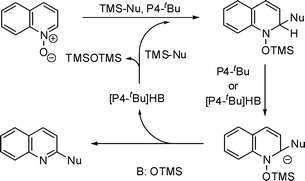 | ||
| Fig. 2 Plausible mechanism for functionalisation of quinoline N-oxide using silylated nucleophiles. | ||
Our next interest was then focused on the development of a method for introducing carbon functionality via the deprotonative addition of nucleophile in the presence of phosphazene catalyst and organosilicon additive. First, the reaction of quinoline N-oxide and phenylacetylene was examined using 10 mol% P4-tBu as a catalyst in the presence of an organosilicon additive. When trimethylsilyldiethylamine was used as an additive, the reaction proceeded in poor yields (Table 1, entries 1, 2). On the other hand, when trimethylsilylpropyne (TMSP) was used as an additive, the yields were dramatically improved (Table 1, entries 3,4). Effect of solvent was examined using DMF and toluene, but no significant difference was observed.
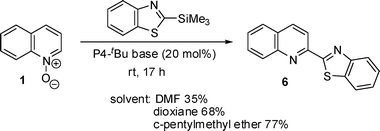
The deprotonative catalytic system was then applied for other nucleophiles using quinoline N-oxide. Terminal alkynes with methoxyphenyl and 2-pyridyl moieties were employed for the reaction and the alkynylation proceeded smoothly (Scheme 3). Quinoline N-oxide was also reacted with benzothiazole in the presence of 20 mol% P4-tBu and TMSP using c-pentylmethyl ether as a solvent and benzothiazolylquinoline (6) was obtained in 60% yield.
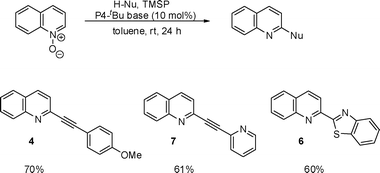 | ||
| Scheme 3 Reaction of quinoline N-oxide with H-nucleophiles using TMSP. | ||
Plausible reaction pathway for this catalytic nucleophile introduction is shown in Fig. 3. First, deprotonation of nucleophile by P4-tBu forms a reactive phosphazenium carbanion complex, which attacks the 2-position of quinoline N-oxide. The oxygen anion was then silylated by the silicon additive reagent and another reactive phosphazenium anion deprotonates 2-hydrogen of the adduct to eliminate trimethylsilanoxide. The phosphazenium silanoxide reacts with silicon additive reagent in the presence of nucleophile generates the phosphazenium carbanion complex again. At this moment the exact reason for the effectiveness of TMSP on the catalytic activity is not clear, but the stability of the released anion from a silicon additive is considered to be an important factor. Further systematic experiments for clarifying the mechanism are needed.
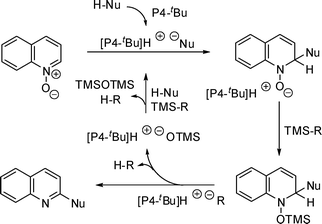 | ||
| Fig. 3 Plausible mechanism for functionalisation of quinoline N-oxide. | ||
When quinoline N-oxide was reacted in the absence of electrophile with trimethylsilyldiethylamine, dimerization reaction occurred to give biquinoline N-oxide (8) in 40% yield (Scheme 4). This type of dimerization reaction has been reported by the treatment of quinoline N-oxide with organometallic deprotonating agents.8 In our case, it can be understood that quinoline N-oxide was deprotonated to produce a carbanion which attacked another quinoline N-oxide to give the biquinoline N-oxide. This kind of dimerization was first achieved under organocatalytic reaction conditions by the present work.
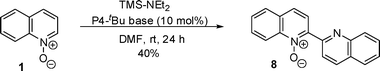 | ||
| Scheme 4 Dimerization of quinoline N-oxide. | ||
In summary, phosphazene base catalyzed reactions are found to be effective for selective functionalisation of quinoline N-oxide and silylated and non-silylated nucleophiles were successfully used for the introduction of carbon functionalities. Further optimization of reaction conditions and expansion of the scope of the catalytic reactions are under investigation.
Acknowledgements
This work was partly supported by the Grant-in Aid for Scientific Research on Priority Areas “Advanced Molecular Transformations of Carbon Resources” (No.19020005) and “Synergistic Effects for Creation of Functional Molecules” (No.20036007) and the Grants (No.19390002) from the Ministry of Education, Science, Sports and Culture, Japan.Notes and references
- (a) S. Youssif, ARKIVOC, 2001, 242; (b) A. Albini, S. Pietra, Heterocyclic N-oxides, CRC Press, Boca Raton, 1991 Search PubMed; (c) A. Albini, Synthesis, 1993, 263 CrossRef CAS.
- (a) N. Nishiwaki, S. Minakata, M. Komatsu and Y. Ohshiro, Chem. Lett., 1989, 773 CAS; (b) T. Kato and H. Yamanaka, J. Org. Chem., 1965, 30, 910 CAS; (c) T. Kato, H. Yamanaka, T. Adachi and H. Hiranuma, J. Org. Chem., 1967, 32, 3788 CrossRef CAS; (d) R. M. Kellogg and T. J. Van Bergen, J. Org. Chem., 1971, 36, 1705 CrossRef CAS; (e) D. L. Comins and A. H. Abdullah, J. Org. Chem., 1982, 47, 4315 CrossRef CAS; (f) D. L. Comins and J. D. Brown, Tetrahedron Lett., 1986, 27, 2219 CrossRef CAS.
- (a) J.-P. Leclerc and K. Fagnou, Angew. Chem., Int. Ed., 2006, 45, 7781 CrossRef CAS; (b) L.-C. Campeau, D. R. Stuart, J.-P. Leclerc, M. Bertrand-Laperle, E. Villemure, H.-Y. Sun, S. Lasserre, N. Guimond, M. Lecavallier and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 3291 CrossRef.
- (a) H. Andersson, F. Almqvist and R. Olsson, Org. Lett., 2007, 9, 1335 CrossRef CAS; (b) H. Andersson, X. Wang, M. Björklund, R. Olsson and F. Almqvist, Tetrahedron Lett., 2007, 48, 6941 CrossRef CAS; (c) H. Andersson, M. Gustafsson, R. Olsson and F. Almqvist, Tetrahedron Lett., 2008, 49, 6901 CrossRef CAS; (d) A. M. Prokhorov, M. Makosza and O. N. Chupakhin, Tetrahedron Lett., 2009, 50, 1444 CrossRef.
- (a) H. Vorbrüggen and K. Krolikiewicz, Tetrahedron Lett., 1983, 24, 889 CrossRef; (b) H. Vorbrüggen and K. Krolikiewicz, Synthesis, 1983, 316 CrossRef; (c) H. Vorbrüggen, Acc. Chem. Res., 1995, 28, 509 CrossRef.
- (a) T. Imahori, C. Hori and Y. Kondo, Adv. Synth. Catal., 2004, 346, 1090 CrossRef CAS; (b) M. Ueno, C. Hori, K. Suzawa, M. Ebisawa and Y. Kondo, Eur. J. Org. Chem., 2005, 1965 CrossRef CAS; (c) K. Kobayashi, M. Ueno and Y. Kondo, Chem. Commun., 2006, 3128 RSC; (d) M. Ueno, A. E. H. Wheatley and Y. Kondo, Chem. Commun., 2006, 3549 RSC; (e) K. Suzawa, M. Ueno, A. E. H. Wheatley and Y. Kondo, Chem. Commun., 2006, 4850 RSC; (f) K. Kobayashi, M. Ueno, H. Naka and Y. Kondo, Chem. Commun., 2008, 3780 RSC; (g) M. Terada, C. Kanazawa and M. Yamanaka, Heterocycles, 2007, 74, 819 CrossRef CAS; (h) C. Kanazawa and M. Terada, Tetrahedron Lett., 2007, 48, 933 CrossRef CAS; (i) C. Kanazawa and M. Terada, Chem.–Asian J., 2009, 4, 1668 CrossRef CAS; (j) C. Kanazawa, K. Goto and M. Terada, Chem. Commun., 2009, 5248 RSC; (k) C. Kanazawa, A. Ito and M. Terada, Synlett, 2009, 4, 638.
- (a) M. Ueno, M. Yonemoto, M. Hashimoto, A. E. H. Wheatley, H. Naka and Y. Kondo, Chem. Commun., 2007, 2264 RSC; (b) Y. Hirono, K. Kobayashi, M. Yonemoto and Y. Kondo, Chem. Commun. 10.1039/C0CC03106B.
- (a) Y. Tagawa, K. Hama, Y. Goto and M. Hamana, Heterocycles, 1992, 34, 2243 CrossRef CAS; (b) Y. Tagawa, K. Hama, Y. Goto and M. Hamana, Heterocycles, 1995, 40, 809 CrossRef CAS; (c) I. S. Kovalev, V. L. Rusinov and O. N. Chupakhin, Chem. Heterocycl. Compd., 2009, 45, 176 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectral data for synthesized compounds. See DOI: 10.1039/c0ob00740d |
| This journal is © The Royal Society of Chemistry 2011 |