Supramolecular design for polymer/titanium oxo-cluster hybrids: an open door to new organic–inorganic dynamers†
Fabien
Périneau
abcd,
Sandrine
Pensec
ab,
Clément
Sanchez
cd,
Costantino
Creton
e,
Laurence
Rozes
cd and
Laurent
Bouteiller
*ab
aUPMC Univ Paris 06, UMR 7610, Chimie des Polymères, F-75005, Paris, France
bCNRS, UMR 7610, Chimie des Polymères, F-75005, Paris, France. E-mail: laurent.bouteiller@upmc.fr
cUPMC Univ Paris 06, UMR 7574, Chimie de la Matière Condensée de Paris, F-75005, Paris, France
dCNRS, UMR 7574, Collège de France, Chimie de la Matière Condensée de Paris, F-75005, Paris, France
ePhysico-chimie des Polymères et des Milieux Dispersés, UMR 7615, UPMC-CNRS-ESPCI, 10 rue Vauquelin, 75231, Paris Cedex 05, France
First published on 3rd October 2011
Abstract
Hybrid materials from organic polymers and inorganic solids often possess enhanced properties compared to their isolated constituents, if intimate mixing is achieved. We report a new approach combining inorganic nanobuilding blocks and supramolecular interactions, exemplified by a titanium oxo-cluster bearing four hydrogen bond acceptors and a telechelic PDMS with two hydrogen bond donors. With these simple components, we demonstrate that well chosen supramolecular interactions can be used to obtain new hybrid dynamers having the intrinsic properties of the inorganic component (such as photochromism) and improved mechanical properties, due to the supramolecular cross-linking of the polymer chains.
Hybrid materials combining organic polymers and inorganic solids are remarkably versatile systems, which often display enhanced properties compared to their isolated components.1 In order to further improve their properties, the key is to control their nanostructure. Therefore, a nanobuilding block (NBB) methodology was recently developed to reach such nanostructured hybrids.2 This approach consists in functionalizing molecularly defined inorganic clusters with organic moieties able to initiate a polymerization, to copolymerize with an organic monomer, or to be grafted on a preformed polymer. The covalent grafting of the NBBs on the polymer matrix may limit phase separation and afford materials with a homogeneous distribution at the 10-nanometre scale.
In order to push the NBB concept further and to introduce some flexibility in the design, we propose to investigate the feasibility of a supramolecular approach. Indeed, supramolecular chemistry has been successfully used in polymer science,3 where the reversibility brought by weak (but controlled) interactions can improve processing, can yield self-healing materials,4 and can afford pathways to thermodynamically stable materials. Moreover, the potential of coupling inorganic components by well defined supramolecular synthons has been well demonstrated in the case of metallic nanoparticles,5 and polyoxometallates.6 Of course, supramolecular chemistry has also been widely used to enhance interactions between the components of organic polymers/inorganic hybrid materials.7 However, these studies were restricted to relatively coarse inorganic components or to unspecific supramolecular interactions. Our aim is therefore to investigate the potential of functionalizing well-defined inorganic clusters with highly specific supramolecular synthons in order to form new polymer/inorganic NBB hybrid materials: hybrid dynamers.
The design of our hybrid supramolecular network is schematized in Fig. 1. It consists in the association of two complementary elementary units viahydrogen bonding. In order to avoid phase separation, each unit must be capable of hetero-association with its counterpart while preventing any homo-association. We chose the following system: a structurally well-defined titanium oxo-cluster NBB bearing hydrogen bond acceptors and a telechelic polydimethylsiloxane with hydrogen bond donor chain ends. The functional titanium oxo-cluster was synthesized from the known Ti16O16(OEt)32 (labelled “Ti16”). The structure of Ti16 oxo-cluster is based on an inorganic oxo-core composed of 16 TiO6 octahedra surrounded by 32 ethoxy ligands. Half of these ligands bridge two titanium atoms while the other 16 ligands, the terminal ligands, are linked to only one titanium atom. These terminal ethoxy groups can be selectively exchanged by transalcoholysis or transesterification reactions in organic solvents.8 The post-modification of Ti16 leads to functional NBBs which can be further employed in the build up of hybrid materials.9 In our case, the terminal ethoxy groups of Ti16 were partially exchanged by 1-(2-hydroxyethyl)-2-pyrrolidone in toluene at 50 °C for 72 h (see Fig. 2a). 1H NMR analyses show that 4 of the 32 ethoxy groups of the Ti16 were exchanged by pyrrolidone ligands (Fig. S1†).10 Additionally, 17O NMR analyses were performed to assess the integrity of the oxo-core of Ti16–pyrrolidone. Here, the Ti16 precursor was prepared from conventional Ti(OR)4 alkoxide and 17O enriched water. Consequently, the organic ligands keep their low natural abundance and the NMR spectrum only displays resonances relative to the 17O of the oxometallic core. The resonances observed on the spectrum (Fig. S3†) can be attributed as follows: μ2-O (753 ppm), μ3-O (567 ppm, 564 ppm, 562 ppm, 558 ppm) and μ4-O (385 ppm) and prove that the core of the cluster is not affected by the exchange of the peripheral ligands.
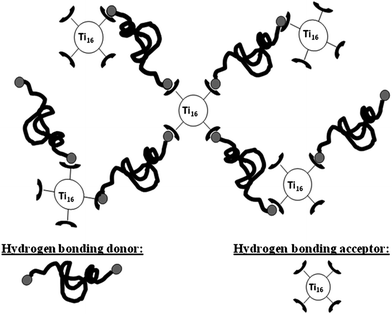 | ||
| Fig. 1 Schematic representation of the hybrid supramolecular network composed of titanium oxo-clusters and PDMS. | ||
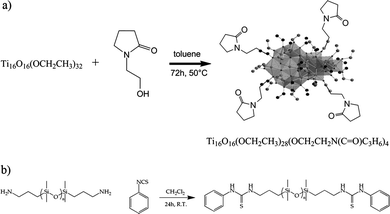 | ||
| Fig. 2 Synthesis of Ti16–pyrrolidone (a) and thiourea terminated PDMS (PDMS1 and PDMS2) (b). | ||
The carbonyl functions of the pyrrolidone ligands are simple, yet efficient hydrogen bond acceptors. The complementary unit to be placed on the polymer therefore needs to bear a strong hydrogen bond donor. This group must fulfill two other requirements: (i) it should not be able to exchange with the cluster ligands; and (ii) it should be a weak hydrogen bond acceptor, to avoid competing with the desired hetero-association. The thiourea synthon was chosen because it is known to be a strong hydrogen bond donor together with a weak hydrogen bond acceptor (Fig. S6†).11 Two samples of thiourea terminated polydimethylsiloxanes were prepared from telechelic aminopropyl terminated polydimethylsiloxanes of different molar masses by reaction with phenyl isothiocyanate (Fig. 2b). 1H NMR analyses confirm the modification of the aminopropyl terminated PDMS as proved by the presence at 7.65 ppm and at 6.11 ppm of singlets corresponding to the N–H protons (Fig. S4†). This is correlated with the disappearance at 1 ppm of the NH2 protons (Fig. S4†). The molar mass of the polymers was determined by 1H NMR and SEC to be about 8000 g mol−1 and 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 for PDMS1 and PDMS2, respectively. 29Si NMR showed evidence of only two kinds of silicon atoms in the PDMS, that is, terminal silicon atoms and silicon atoms from the backbone of the macromolecular chain (Fig. S5†).
000 g mol−1 for PDMS1 and PDMS2, respectively. 29Si NMR showed evidence of only two kinds of silicon atoms in the PDMS, that is, terminal silicon atoms and silicon atoms from the backbone of the macromolecular chain (Fig. S5†).
The hybrid supramolecular network was prepared by mixing equimolar amounts of Ti16–pyrrolidone and the thiourea-modified PDMS1 in CH2Cl2. Upon evaporation of the solvent, the pale yellow liquid became a transparent gel as shown in Fig. 3. This qualitative observation reveals two important features. First, the dispersion of the cluster in the polymer matrix is homogeneous, as proved by the following blank experiment: mixing under the same conditions the non-functional cluster Ti16 and a non-functional PDMS (trimethyl-terminated) yields a precipitate.12 Second, the gelation of the sample is characteristic of multi-topic interactions between the constituents.13 The gel formation probably results from the association, viahydrogen bonding, of the N–H groups of the PDMS with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the cluster, eventually leading to a three-dimensional hybrid network. Indeed, hydrogen bonding is demonstrated by FTIR analysis in the region of the N–H functions of thiourea groups, as shown in Fig. 4. The spectrum of thiourea terminated PDMS1 in CHCl3 shows two bands located at 3410 and 3393 cm−1, which can be attributed to free N–H groups adjacent to the aliphatic and aromatic groups, respectively.14 The absence of a band at 3200–3300 cm−1 confirms the lack of hydrogen bonding in dilute solution. However, in bulk, the spectrum of the same polymer shows an additional large band at 3250 cm−1, which corresponds to hydrogen bonded N–H groups (with the thiocarbonyl and/or the oxygen atoms of the siloxane chains as acceptors). The bulk spectrum of the hybrid supramolecular network HSN1 also shows a strong band at 3300 cm−1. Moreover, the disappearance of the band at 3400 cm−1 proves that hydrogen bonding is enhanced when Ti16–pyrrolidone is introduced in the polymer. Therefore, Ti16–pyrrolidone is equipped with stronger hydrogen bond acceptors than the polymer.
O groups of the cluster, eventually leading to a three-dimensional hybrid network. Indeed, hydrogen bonding is demonstrated by FTIR analysis in the region of the N–H functions of thiourea groups, as shown in Fig. 4. The spectrum of thiourea terminated PDMS1 in CHCl3 shows two bands located at 3410 and 3393 cm−1, which can be attributed to free N–H groups adjacent to the aliphatic and aromatic groups, respectively.14 The absence of a band at 3200–3300 cm−1 confirms the lack of hydrogen bonding in dilute solution. However, in bulk, the spectrum of the same polymer shows an additional large band at 3250 cm−1, which corresponds to hydrogen bonded N–H groups (with the thiocarbonyl and/or the oxygen atoms of the siloxane chains as acceptors). The bulk spectrum of the hybrid supramolecular network HSN1 also shows a strong band at 3300 cm−1. Moreover, the disappearance of the band at 3400 cm−1 proves that hydrogen bonding is enhanced when Ti16–pyrrolidone is introduced in the polymer. Therefore, Ti16–pyrrolidone is equipped with stronger hydrogen bond acceptors than the polymer.
 | ||
| Fig. 3 Qualitative comparison between the hybrid supramolecular network HSN1 (left) and the thiourea-modified PDMS1 (right). | ||
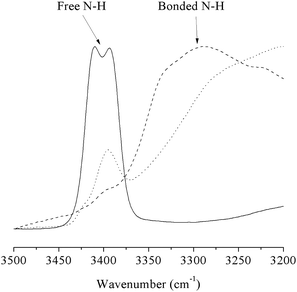 | ||
| Fig. 4 FTIR spectra showing N–H absorption bands of thiourea-modified PDMS1 in CHCl3 (straight line) or in bulk (dotted line), and the hybrid supramolecular network HSN1 in bulk (dashed line). | ||
Similarly, adding equimolar amounts of Ti16–pyrrolidone to the thiourea-modified PDMS2 increased significantly the viscosity of the material. The fact that a very viscous oil is obtained with the higher molar mass polymer (HSN2), whereas a gel is obtained for HSN1 is probably due to the lower concentration of hydrogen bonding groups in HSN2. It may also be the consequence of a larger proportion of supramolecular macrocycles.
Therefore, introducing hydrogen bonding groups on both the cluster and polymer matrix seems to be a reliable way to homogeneously disperse the cluster into the polymer matrix and therefore impart the material with the intrinsic properties of the cluster. For example, titanium oxo-based hybrids are known to present a photochromic behavior when exposed to UV light.15 Indeed, a stable darkening of samples has been assigned to an efficient trapping of photo-excited electrons as small polarons (Ti3+) due to sample irradiation by UV photons and the concomitant rapid scavenging of the photo-excited holes by the organic domains. Thus, a hybrid supramolecular network HSN1 sample was placed between two quartz plates and exposed to UV light. It went from pale yellow to grey-blue in a few minutes, and after irradiation, the color faded in the presence of air in a few hours (Fig. 5). This experiment could be repeated several times, showing the reversibility of the photochromic phenomenon.
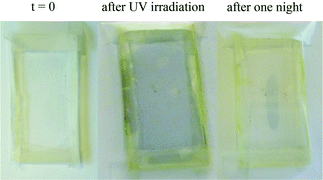 | ||
| Fig. 5 Photochromic behaviour of the hybrid supramolecular network HSN1. Left: initial sample; middle: sample right after UV irradiation; right: sample one night after UV irradiation. | ||
We have reported the synthesis of a titanium oxo-cluster bearing four hydrogen bond acceptor groups and telechelic polydimethylsiloxanes with two hydrogen bond donors. With these particularly simple components, we demonstrate that well chosen supramolecular interactions can be used to obtain homogeneous hybrid materials having the intrinsic properties of the inorganic component (such as photochromism) and improved mechanical properties, due to the supramolecular cross-linking of the polymer chains. The ability of the NBBs to be post-modified and functionalized in a controlled manner allows the easy tuning of the reversible crosslinking density and leads to hybrid dynamers with tunable mechanical properties and specific properties inherent to the nature of the metallic oxo-core.
Support from the French Agence Nationale de la Recherche (ANR) in the frame of its programme in Nanosciences and Nanotechnologies (project MECHYBRIDES ANR-06-NANO-017) is acknowledged.
Notes and references
- (a) G. J. A. A. Soler-Illia and O. Azzaroni, Chem. Soc. Rev., 2011, 40, 1107 RSC; (b) B. Lebeau and P. Innocenzi, Chem. Soc. Rev., 2011, 40, 886 RSC; (c) A. Leonard, P. Dandoy, E. Danloy, G. Leroux, C. F. Meunier, J. C. Rooke and B.-L. Su, Chem. Soc. Rev., 2011, 40, 860 RSC; (d) C. Sanchez, P. Belleville, M. Popall and L. Nicole, Chem. Soc. Rev., 2011, 40, 696 RSC; (e) L. Nicole, L. Rozes and C. Sanchez, Adv. Mater., 2010, 22, 3208 CrossRef CAS; (f) A. Zamboulis, N. Moitra, J. J. E. Moreau, X. Cattoen and M. Wong Chi Man, J. Mater. Chem., 2010, 20, 9322 RSC; (g) K. M. L. Taylor-Pashow, J. Della Rocca, R. C. Huxford and W. Lin, Chem. Commun., 2010, 46, 5832 RSC.
- (a) C. Sanchez, G. Soler-Illia, F. Ribot, T. Lalot, C. R. Mayer and V. Cabuil, Chem. Mater., 2001, 13, 3061 CrossRef CAS; (b) U. Schubert, Chem. Mater., 2001, 13, 3487 CrossRef CAS; (c) G. Kickelbick, Prog. Polym. Sci., 2003, 28, 83 CrossRef CAS; (d) E. T. Kopesky, G. H. McKinley and R. E. Cohen, Polymer, 2006, 47, 299 CrossRef CAS; (e) L. Rozes, N. Steunou, G. Fornasieri and C. Sanchez, Monatsh. Chem., 2006, 137, 501 CrossRef CAS; (f) L. Rozes and C. Sanchez, Chem. Soc. Rev., 2011, 40, 1006 RSC.
- (a) L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071 CrossRef CAS; (b) J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814 CrossRef CAS; (c) A. Ciferri, Supramolecular Polymers, Marcel Dekker Inc, New York, 2005 Search PubMed; (d) W. H. Binder and R. Zirbs, Adv. Polym. Sci., 2007, 207, 1 CrossRef CAS; (e) L. Bouteiller, Adv. Polym. Sci., 2007, 207, 79 CrossRef CAS; (f) J.-M. Lehn, Aust. J. Chem., 2010, 63, 611 CrossRef CAS.
- (a) P. Cordier, F. Tournilhac, C. Soulie-Ziakovic and L. Leibler, Nature, 2008, 451, 977 CrossRef CAS; (b) J. A. Syrett, C. R. Becer and D. M. Haddleton, Polym. Chem., 2010, 1, 978 RSC.
- (a) R. Zirbs, F. Kienberger, P. Hinterdorfer and W. H. Binder, Langmuir, 2005, 21, 8414 CrossRef CAS; (b) S. S. Kinge, M. Crego-Calama and D. N. Reinhoudt, Langmuir, 2007, 23, 8772 CrossRef CAS.
- (a) C. Allain, S. Favette, L.-M. Chamoreau, J. Vaissermann, L. Ruhlmann and B. Hasenknopf, Eur. J. Inorg. Chem., 2008, 3433 CrossRef CAS; (b) M. Carraro, A. Sartorel, G. Scorrano, C. Maccato, M. H. Dickman, U. Kortz and M. Bonchio, Angew. Chem., Int. Ed., 2008, 47, 7275 CrossRef CAS.
- (a) Q. R. Huang, W. Volksen, E. Huang, M. Toney, C. W. Frank and R. D. Miller, Chem. Mater., 2002, 14, 3676 CrossRef CAS; (b) W. Loos, S. Verbrugghe, E. J. Goethals, F. E. Du Prez, I. V. Bakeeva and V. P. Zubov, Macromol. Chem. Phys., 2003, 204, 98 CrossRef CAS; (c) T. Ogoshi and Y. Chujo, Macromolecules, 2003, 36, 654 CrossRef CAS; (d) J. De Girolamo, P. Reiss and A. Pron, J. Phys. Chem. C, 2007, 111, 14681 CrossRef CAS; (e) Y.-C. Yen, S.-W. Kuo, C.-F. Huang, J.-K. Chen and F.-C. Chang, J. Phys. Chem. B, 2008, 112, 10821 CrossRef CAS; (f) P. Imin, F. Chen and A. Adronov, Polym. Chem., 2011, 2, 411 RSC.
- G. Fornasieri, L. Rozes, S. Le Calvé, B. Alonso, D. Massiot, M. Rager, M. Evain, K. Boubekeur and C. Sanchez, J. Am. Chem. Soc., 2005, 127, 4869 CrossRef CAS.
- (a) S. Trabelsi, A. Janke, R. Hassler, N. E. Zafeiropoulos, G. Fornasieri, S. Bocchini, L. Rozes, M. Stamm, J. F. Gerard and C. Sanchez, Macromolecules, 2005, 38, 6068 CrossRef CAS; (b) G. Soler-Illia, L. Rozes, M. K. Boggiano, C. Sanchez, C. O. Turrin, A. M. Caminade and J. P. Majoral, Angew. Chem., Int. Ed., 2000, 39, 4250 CrossRef; (c) L. Rozes, G. Fornasieri, S. Trabelsi, C. Creton, N. E. Zafeiropoulos, M. Stamm and C. Sanchez, Prog. Solid State Chem., 2005, 33, 127 CrossRef CAS; (d) F. Périneau, S. Pensec, C. Sassoye, F. Ribot, L. Van Lokeren, R. Willem, L. Bouteiller, C. Sanchez and L. Rozes, J. Mater. Chem., 2011, 21, 4470 RSC; (e) F. Périneau, G. J. Hu, L. Rozes, F. Ribot, C. Sanchez, C. Creton, L. Bouteiller and S. Pensec, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2636 CrossRef.
- This is based on the relative integration of the peaks between 5.2 ppm and 4 ppm (methylene protons labeled 1 and 3 in Fig. S1†) and peaks between 3.8 ppm and 3 ppm (methylene protons labeled 4 and 5 in Fig. S1†).
- T. Pinault, B. Andrioletti and L. Bouteiller, Beilstein J. Org. Chem., 2010, 6, 869 CrossRef CAS.
- The transparency of the HSN1 gel lasted for at least 4 months of storage, although the samples (either as a film between glass slides or as bulk material in a vial) were not protected from ambient moisture. This good hydrolytic stability can probably be attributed to the strong hydrophobicity of the polysiloxane matrix.
- Although this simple qualitative test proves the gel-like behaviour of the sample, a full rheological analysis would be useful to probe the dynamics of the system.
- (a) A. R. Katritzky, H. J. Keogh, K. Lempert and J. Puskas, Tetrahedron, 1969, 25, 2265 CrossRef CAS; (b) Y. Mido, T. Yamanaka and R. Awata, Bull. Chem. Soc. Jpn., 1977, 50, 27 CrossRef CAS; (c) V. Simic, L. Bouteiller and M. Jalabert, J. Am. Chem. Soc., 2003, 125, 13148 CrossRef CAS.
- (a) O. Kameneva, A. I. Kuznestov, L. A. Smirnova, L. Rozes, C. Sanchez, A. Alexandrov, N. Bityurin, K. Chhor and A. Kanaev, J. Mater. Chem., 2005, 15, 3380 RSC; (b) A. I. Kuznetsov, O. Kameneva, N. Bityurin, L. Rozes, C. Sanchez and A. Kanaev, Phys. Chem. Chem. Phys., 2009, 11, 1248 RSC; (c) M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin, C. Sanchez and G. Ferey, J. Am. Chem. Soc., 2009, 131, 10857 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and 1H, 13C, 17O, 29Si NMR spectra. See DOI: 10.1039/c1py00341k |
| This journal is © The Royal Society of Chemistry 2011 |
