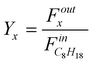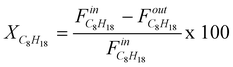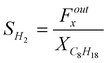Study of the main reactions involved in reforming of exhaust gas recirculation (REGR) in gasoline engines
S. Rijo
Gomes
a,
N.
Bion
a,
G.
Blanchard
b,
S.
Rousseau
b,
D.
Duprez
a and
F.
Epron
*a
aLaboratoire de Catalyse en Chimie Organique, UMR 6503, CNRS/University of Poitiers, 4 rue Michel Brunet, B27, Poitiers Cedex, 86022, France. Tel: +33 5 49 45 48 32; Fax: +33 5 49 45 37 41; E-mail: florence.epron@univ-poitiers.fr
bPSA Peugeot Citroën, Centre Technique de Vélizy A, DTI/DRIA/DSTF/PCEA, Route de Gisy, Case courrier VV1415, Vélizy Villacoublay Cedex, 78943, France
First published on 18th July 2011
Abstract
In a previous paper, it was demonstrated that a 1 wt% Rh catalyst supported on modified ZrO2 exhibits a high activity and stability in REGR conditions. The purpose of the present study was to investigate the main reactions involved in REGR conditions in the presence of this catalyst, namely steam reforming (SR), dry reforming (DR), and the reactions producing methane, i.e. methanation, decomposition and hydrogenolysis. Isooctane (C8H18) was used as a molecule representative of gasoline, and a mixture of N2, H2O, CO2 and O2 as model exhaust gas. A reaction scheme was proposed for REGR conditions. Based on the study of individual reactions (SR, DR and methane formation) at 450 °C, 520 °C and 580 °C, the complex system can be described following three consecutive steps: reforming reactions, yielding H2 and CO2 as primary products; reverse water gas shift (RWGS), yielding CO as secondary product; and finally methanation reactions, specially with CO, yielding CH4 as tertiary product.
1. Introduction
The exhaust gas recirculation (EGR) technique was developed in the 1970's in order to reduce NOx emissions1–3 by decreasing (i) the flame temperature and (ii) the oxygen concentration, by dilution effect, in the combustion chamber. Presently, EGR systems are used in a majority of Diesel engines but their use is limited to 15 to 20% of EGR, which are the maximum admitted in gasoline engines. A higher dilution is damaging for the quality of the combustion and even causes problems of ignition.2,3 The Reformed Exhaust Gas Recirculation (REGR), consisting in reforming the fuel with exhaust gas to produce hydrogen in the EGR loop, was developed in the 1980's.4–9 It improves the overall engine efficiency, decreases the emissions of NOx and hydrocarbons and reduces the variation of cylinder pressure.10–13 The REGR reaction involves a complex system of chemical reactions, in which primary reactions lead to the decomposition of the hydrocarbon, while many secondary reactions may also take place between the different products and the reactants. The main catalytic reactions are steam reforming (1), water gas shift (3), dry reforming (4), partial oxidation (5) and total oxidation (6). Steam reforming (1) is normally written with CO as product, but the hydrocarbon can be also steam reformed directly to CO2 and H2(2).14–16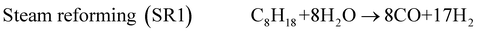 | (1) |
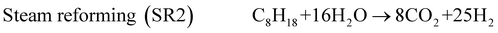 | (2) |
 | (3) |
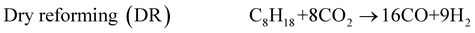 | (4) |
 | (5) |
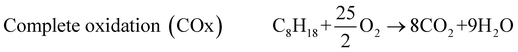 | (6) |
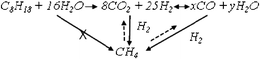
Methane is a typical product associated with the reforming reactions, and may result from the secondary reaction between H2 and CO, (7) and/or (8), or H2 and CO2(9) or directly from hydrocarbon decomposition (10) or hydrogenolysis (11).4
 | (7) |
 | (8) |
 | (9) |
 | (10) |
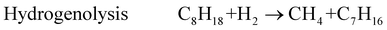 | (11) |
In a previous study,17 we have shown that a Rh(1 wt%) catalyst supported on doped zirconia is very active for hydrogen production in EGR conditions with isooctane as model molecule. It was demonstrated that, in the reaction conditions used, a slight deactivation occurred, attributed to metal sintering and to the poisoning of the metal-support interface. The carbon deposition was negligible. The H2 yield was very close to that predicted by the thermodynamic equilibrium.
The aim of the present paper is to evaluate the impact of steam and dry reforming reactions on the product distribution and more especially on the hydrogen yield obtained in REGR conditions. For that purpose, the steam and dry reforming as well as the reforming of the exhaust gas recirculation reactions will be carried out by varying the contact time in order to establish the reaction pathways. As methane is a key product to optimize the hydrogen yield, the reactions responsible for methane formation as well as those leading to its transformation will be also studied. Finally, a thermodynamic study will be carried out in order to separate the kinetic limitations from the thermodynamic ones. This work will help adjusting the experimental conditions in order to optimize the hydrogen yield in the REGR conditions.
2. Experimental
2.1 Catalyst
The catalyst used in the present study is a Rh(1 wt%) supported on zirconia doped with La2O3, Nd2O3 and Y2O3. Its preparation, as well as its characteristics, were described in detail in ref. 17. This catalyst presents a BET surface area of 62 m2/g and a rhodium dispersion of 60%.2.2 Catalytic test
The model feed composition of exhaust gas recirculation (with gasoline) was given by PSA Peugeot Citroën Group. It contains 2.2 vol.% of isooctane, 13 vol.% of CO2, 12 vol.% of H2O, 1 vol.% of O2 and 71.3 vol.% of N2 (REGR conditions). A blank test in REGR conditions showed that without catalyst all the oxygen is consumed, and it was demonstrated that C8H18 oxidation reactions occur mainly in the homogeneous gas phase with a C8H18 conversion of around 6% (≈ 3% for partial oxidation (5) and ≈ 3% for total oxidation (6)). The total gas flow rate was 250 Ncm3 min−1. Anhydrous isooctane (C8H18) was supplied by Carlo Erba (99.5%); CO2, O2 and N2 were provided by Air Liquide. Ultra pure water was used.The reforming of exhaust gas recirculation (REGR) reaction was carried out in a quartz tubular continuous flow reactor. The experimental procedure is given in detail in ref. 17. The catalyst was activated under the REGR conditions, i.e. in the presence of all the reactants, by heating with a ramp of 10 °C min−1, from 130 °C to the reaction temperature.
Steam and dry reforming reactions were studied at different temperatures in order to identify the individual contributions of H2O and CO2 in the reforming of exhaust gas recirculation, using various catalyst masses. Three reaction conditions were used:
-steam reforming (SR): in the presence of all the reactants of REGR conditions except CO2 and O2, replaced by nitrogen gas. The inlet partial pressures of C8H18 (2.2%) and H2O (12%) were maintained constant;
-dry reforming (DR): in the presence of all the reactants of REGR conditions except H2O and O2, replaced by nitrogen gas. The inlet partial pressure of C8H18 (2.2%) and CO2 (13%) were maintained constant;
-reforming of exhaust gas reforming (REGR): corresponding to the REGR conditions given at the beginning of this paragraph.
These reactions conditions are reported in Table 1(a).
| Inlet feed composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reactions | C8H18 | H2O | CO2 | O2 | CH4 | CO | H2 | N2 | |
| (a) | REGR | 2.2 | 12.0 | 13.5 | 1.0 | — | — | — | 71.3 |
| SR | 2.2 | 12.0 | — | — | — | — | — | 85.8 | |
| DR | 2.2 | — | 13.5 | — | — | — | — | 84.3 | |
| (b) | MetCO | — | — | — | — | — | 8.0 | 14.0 | 78.0 |
| MetCO2 | — | — | 13.5 | — | — | — | 14.0 | 72.5 | |
| HYD | 2.2 | — | — | — | — | — | 14.0 | 83.8 | |
| DECOMP | 2.2 | — | — | — | — | — | — | 97.8 | |
| (c) | CH4 REGR | — | 12 | 13.5 | 1.0 | 2.2 | — | — | 71.3 |
| CH4 SR | — | 12 | — | — | 2.2 | — | — | 85.8 | |
| CH4 DR | — | — | 13.5 | — | 2.2 | — | — | 84.3 | |
This study was carried out by using a fixed flow rate of the feed and changing the amount of the catalyst in the range of 15 mg – 300 mg at 450 °C, 520 °C and 580 °C. Each experimental run used a fresh catalyst and was carried out for 2 h time-on-stream for each catalyst mass and temperature. The experimental points were collected at 50 min of reaction, which minimizes the effect of catalyst deactivation, which always occurs.17
The catalyst performances are characterized by the product yield:
Where 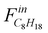 is the molar flow rate of isooctane at the inlet of the reactor and Foutx the molar flow rate of the product x at the oulet.
is the molar flow rate of isooctane at the inlet of the reactor and Foutx the molar flow rate of the product x at the oulet.
The isooctane, oxygen, carbon dioxide and water conversions, in % (example for isooctane):
The selectivity to H2, CO2, CO, CH4 (example for H2):
The selectivities are given in mol of product per mol of C8H18 converted. This avoids a complex normalization procedure to 100%. Reactions (1) to (6) show that the maximum selectivities are 25 for H2 (eqn (2)), 8 for CO2 (eqn (2) or (6)) and 16 for CO (eqn (4)).
3. Results and discussion
Fig. 1 shows the evolution of C8H18 conversion with the ratio of weight of catalyst to C8H18 flow rate (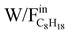 ) in the range of 1.0 – 21.0 gcat/mol C8H18 0/h at reaction temperatures of 450 °C, 520 °C and 580 °C.
) in the range of 1.0 – 21.0 gcat/mol C8H18 0/h at reaction temperatures of 450 °C, 520 °C and 580 °C.
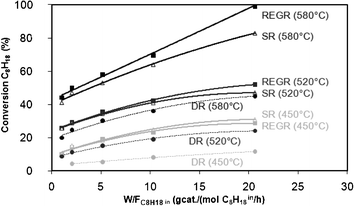 | ||
Fig. 1 Isooctane conversion as a function of contact time (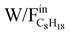 ) at 450 ºC, 520 ºC and 580 ºC for REGR, SR and DR conditions. ) at 450 ºC, 520 ºC and 580 ºC for REGR, SR and DR conditions. | ||
From this figure, it can be seen that the increase in the contact time (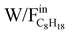 ) results in an increase in C8H18 conversion. Moreover, the conversion reaches a plateau for the highest W/F values in the majority of reaction conditions: at 450 °C (REGR, SR, DR), 520 °C (REGR, SR, DR) and 580 °C (DR). This indicates that C8H18 conversion depends on the catalyst mass and approaches the equilibrium conversion for the highest
) results in an increase in C8H18 conversion. Moreover, the conversion reaches a plateau for the highest W/F values in the majority of reaction conditions: at 450 °C (REGR, SR, DR), 520 °C (REGR, SR, DR) and 580 °C (DR). This indicates that C8H18 conversion depends on the catalyst mass and approaches the equilibrium conversion for the highest 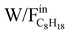 . On the contrary, this phenomenon is not observed at 580 °C for the SR and REGR conditions: a linear correlation between the conversion and the contact time was observed in the whole W/F range, and a C8H18 conversion of 100% was achieved for a
. On the contrary, this phenomenon is not observed at 580 °C for the SR and REGR conditions: a linear correlation between the conversion and the contact time was observed in the whole W/F range, and a C8H18 conversion of 100% was achieved for a 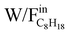 of 21 gcat/mol C8H18 0/h in REGR conditions.
of 21 gcat/mol C8H18 0/h in REGR conditions.
At low contact times, REGR and SR conditions lead to similar C8H18 conversion whatever the temperature, which means that the presence of CO2 and O2 (in REGR conditions) has no impact on the catalyst activity at the beginning of the reaction. Consequently, the reaction between C8H18 and H2O (steam reforming) occurs in the same way in the presence or not of CO2 and O2. However, when the contact time increases, especially at 580 °C, the C8H18 conversion increases in a larger extent in the presence of CO2, O2 and H2O together, compared to the conversion obtained by steam reforming. Remembering that C8H18 oxidation reactions occur mainly in the homogeneous gas phase with a total consumption of oxygen and a C8H18 conversion of around 6% (see paragraph 2.2), this result indicates that the presence of CO2 has a positive effect on the isooctane conversion, but only at 580 °C and when the W/F value increases. At lower temperatures, the dry reforming reaction is probably kinetically limited and the steam reforming reaction is the major reaction contributing to isooctane consumption.
Finally, Fig. 1 shows that, whatever the temperature, the conversion reached in the dry reforming (DR) condition is always lower than those obtained by SR and REGR conditions, and this trend is all the more pronounced as the W/F is important.
In the following, the three reaction conditions (SR, DR and REGR) will be studied in more detail.
3.1 Steam reforming (SR)
Fig. 2 shows the evolution of selectivities to various products (H2, CO, CO2 and CH4) as a function of the C8H18 conversion obtained for different contact times at 450 °C, 520 °C and 580 °C, for SR conditions.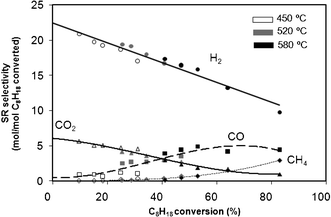 | ||
| Fig. 2 Selectivity as a function of isooctane conversion at 450 °C, 520 °C and 580 °C for SR conditions. Data obtained at different contact times. | ||
From this figure it can be seen that the selectivity does not depend directly on the temperature: whatever the temperature and the considered product, the selectivity as a function of the conversion follows the same evolution. H2 appears to be a primary product, since its selectivity decreases with contact times. CH4 is at least a secondary product, its selectivity increasing with the contact time with no CH4 being produced for low values of conversion. This result indicates that CH4 is probably not produced directly by C8H18 decomposition (reaction (10)) or C8H18 hydrogenolysis (reaction (11)). The reactions yielding methane will be discussed in detail in paragraph 3.4.1.
Surprisingly, CO2 seems to be a primary product and CO a secondary product. The selectivities to H2 and CO2 are around 23 and 6 mol/mol, respectively, for a C8H18 conversion extrapolated at 0%. It means that, for SR conditions, the major reaction is probably the following (2): C8H18 + 16H2O → 8CO2 + 25H2 which leads to selectivities close to those observed experimentally (25 and 8 for H2 and CO2, respectively).
From these results, the following reaction scheme can be proposed, considering that CO is a secondary product and comes from reverse water gas shift reaction (RWGS) and CH4 from CO2 or CO:
3.2 Dry reforming (DR)
Fig. 3 shows the evolution of selectivities to various products (H2, CO and CH4) as a function of the C8H18 conversion obtained for different contact times at 450 °C, 520 °C and 580 °C, for DR conditions.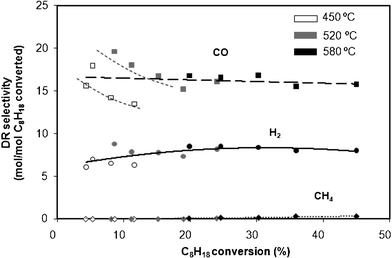 | ||
| Fig. 3 Selectivity as a function of isooctane conversion at 450 °C, 520 °C and 580 °C for DR conditions. Data obtained at different contact times. | ||
This figure shows that at 450 °C and 520 °C, the selectivity to CO decreases with C8H18 conversion whereas at 580 °C it is independent of the conversion. Consequently, in these conditions, selectivity to CO seems to depend directly on the temperature. By contrast, the selectivity to H2 shows to be independent of both the conversion and the temperature. In average, the selectivities to H2 and to CO are around 7 and 17 mol/mol, respectively, which are close to what is given by the reaction (4): C8H18 + 8CO2 → 16CO + 9H2. The Reverse Water Gas Shift (RWGS) reaction may also occur, slightly increasing the selectivity to CO and decreasing the one to H2. However, the amount of H2O, calculated from the O atomic balance, is always close to zero.
The reaction scheme proposed for the dry reforming reaction in the present reaction conditions is the following:
| C8H18 + 8CO2 → 16CO + 9H2 |
| H2 + CO2 ↔ CO + H2O |
3.3 Reforming of exhaust gas recirculation (REGR)
Fig. 4 shows the evolution of selectivities to various products (H2, CO and CH4) as a function of the C8H18 conversion obtained for different contact times at 450 °C, 520 °C and 580 °C, for REGR conditions.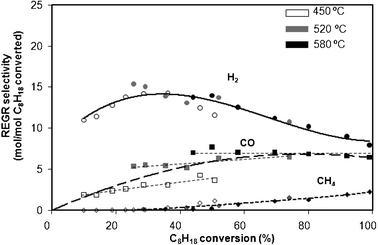 | ||
| Fig. 4 Selectivity as a function of isooctane conversion at 450 °C, 520 °C and 580 °C for REGR conditions. Data obtained at different contact times. | ||
On the one hand, the selectivity to H2 decreases from C8H18 conversions higher than ∼30% at 520 °C and 580 °C, and presents values between those obtained in SR and DR conditions, as expected. Note that, at these temperatures, it is not experimentally possible to obtain conversions lower than roughly 30% by decreasing the mass of catalyst. At 450 °C, conversions as low as 10% can be reached and an optimum in selectivity to H2 can be seen: it increases up to 30% of C8H18 conversion, and then decreases. This type of evolution was not observed for SR (C8H18 + H2O) and DR (C8H18 + CO2) at 450 °C. However, the simultaneous presence of H2O and CO2 could be at the origin of this phenomenon. The presence of O2, or more precisely, the possibility of a total oxidation, could be another possible explanation: for low C8H18 conversion, if total oxidation predominates the selectivity to H2 will converge to zero. Although blank experiments reveal that the whole O2 is consumed in homogeneous gas phase, the hypothesis that a part of O2 reaches the catalyst surface is not excluded. The presence of an optimal selectivity to H2 will be discussed more in detail in the next section.
On the other hand, the selectivity to CO increases up to 50% of C8H18 conversion, and then is constant, as observed for SR and DR conditions. As far as the selectivity to CH4 is concerned, it increases continuously from 50% of C8H18 conversion. Thus, for REGR conditions, CO and CH4 are not primary products, as it was established for the experiments in SR conditions. It can be inferred that the reaction scheme deduced from the experiments performed on SR and DR conditions can be extrapolated for REGR conditions. As the inlet gas contains a large amount of CO2, it is not possible to determine its net production. CO2 is very probably a primary product as observed in SR.
A comparison of SR, DR and REGR in terms of H2 yield, obtained for different contact times at 450 °C, 520 °C and 580 °C, can be made from Fig. 5. It can be seen that the SR presents the most favorable conditions for H2 production up to 80% of C8H18 conversion. Above this value the presence of CO2 and O2 (REGR conditions) becomes beneficial. For DR conditions, the absence of H2O induces a drastic decrease in the H2 yield, and a decrease in C8H18 conversion in the same W/F range.
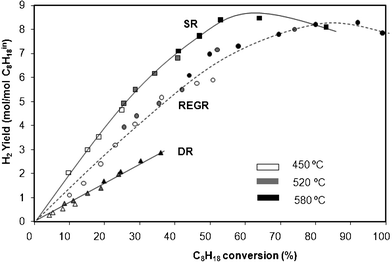 | ||
| Fig. 5 H2 selectivity as a function of isooctane conversion at 450 °C, 520 °C and 580 °C for SR, DR and REGR conditions. Data obtained at different contact times. | ||
3.4 Reactions involving methane production or consumption
One of the major problems associated with reforming reactions is the formation of methane. H2 and CH4 are most of the time thermodynamically and kinetically correlated: if CH4 formation increases significantly, normally the H2 production decreases (and vice-versa). Consequently, for this type of systems, it is important to find a catalyst that prevents CH4 formation or is very active for methane conversion. It has been previously shown that methane is probably not a primary product in REGR conditions. One of the aims of the present study is to find the reactions leading to the formation or consumption of methane in the REGR conditions.| Conversion (%) | Yield (mmol/min/gcat) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C8H18 | H2O | CO2 | CO | H2a | H2O | CO2 | CO | H2 | CH4 | ||
| a Negative value of conversion correspond to formation. | |||||||||||
| REGR | Thermo | 100 | 64 | 16 | — | — | 10.2 | 11.7 | 4.2 | ||
| Exp. | 68 | 54 | 7 | — | — | 7.7 | 12.1 | 1.2 | |||
| MetCO | Thermo | — | — | — | 45 | 39 | 0.7 | 1.0 | 1.7 | ||
| Exp. | — | — | — | 40 | 33 | 0.3 | 1.1 | 1.4 | |||
| MetCO2 | Thermo | — | — | 36 | — | 42 | 3.8 | 3.3 | 0.2 | ||
| Exp. | — | — | 35 | — | 38 | 3.6 | 3.4 | 0.2 | |||
| HYD | Thermo | 91 | — | — | — | 100 | 11.7 | ||||
| Exp. | 8 | — | — | — | −4 | 0.2 | |||||
| DECOMP | Thermo | 100 | — | — | — | — | 2.2 | ||||
| Exp. | 2.8 | — | — | — | — | 0 | |||||
For REGR conditions, the thermodynamic equilibrium predicts a H2 yield of 11.7 mmol/min/gcat. Experimentally, this value is easily reached and even exceeded (12.1 mmol/min/gcat). Furthermore, a maximal conversion of 68% is reached for C8H18 instead of the 100% predicted by thermodynamics. The high H2 yield can be explained by the kinetic inhibition of methane formation: it was observed a maximum of 1.2 compared to 4.2 mmol CH4/min/gcat predicted by thermodynamics.
For MetCO conditions, one can observe that the amount of CH4 produced (1.4 mmol/min/gcat) is almost the same as that obtained in REGR conditions, but lower than the thermodynamic prediction (1.7 mmol/min/gcat). This result suggests that (i) it is possible that the CH4 produced during REGR comes from CO and (ii) the MetCO is kinetically limited in the applied conditions.
For MetCO2 conditions, the product distribution obtained is similar to that predicted at the thermodynamic equilibrium. However, the quantity of methane formed (0.2 mmol/min/gcat) is low compared to REGR conditions. Hence, probably a small amount of CH4 is formed from CO2, but not all the CH4, because this reaction is thermodynamically limited in the applied conditions.
The HYD conditions show that it is thermodynamically possible to produce a great amount of CH4 (11.7 mmol/min/gcat) from H2 and C8H18. Nevertheless, one can deduce from the results presented in Table 2 that this reaction is strongly kinetically inhibited: the CH4 formation (0.2 mmol/min/gcat) is extremely low compared to the value calculated at the thermodynamic equilibrium.
For DECOMP conditions, it is thermodynamically possible to completely convert isooctane to produce 2.2 mmol/min/gcat of methane, and also ethylene (2.5 mmol/min/gcat) and propylene (1.2 mmol/min/gcat), as major products. However, only 2.8% of isooctane were converted in the experimental conditions and methane formation was not observed (Table 2), the only products being isobutene and traces of C7–C8 molecules.
In conclusion, the studies on the reactions potentially responsible for methane formation showed that most of the methane probably comes from the reaction between CO and H2 thus confirming that methane is a secondary product, as proposed in paragraph 3.1. The formation of CH4 directly from C8H18 or by methanation of CO2 is strongly kinetically and thermodynamically limited, respectively.
-CH4 REGR conditions, for which C8H18 was totally replaced by CH4 (same inlet partial pressure);
-steam reforming (CH4 SR) conditions, corresponding to the reaction between CH4 and H2O only;
-dry reforming (CH4 DR) conditions, corresponding to the reaction between CH4 and CO2 only.
Reactions conditions are summarized in Table 1(c).
The results obtained in these three experimental conditions, reported in Table 3, show that high conversions of CH4 are obtained whatever the reaction conditions (REGR, SR and DR) without kinetic limitations. The experimental values are close to the thermodynamic predictions. Thus, CH4 is very reactive at 580 °C confirming that any deviation from the thermodynamic equilibrium is due to CH4 formation and not to CH4 reactivity.
| Conversion (%) | Yield (mmol/min/gcat) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | H2O | CO2a | CO | H2O | CO2 | H2 | CO | ||
| a Negative values of conversion correspond to formation. | |||||||||
| CH4 REGR | Thermo | 100 | 4 | −2 | 4 | — | — | 3.5 | — |
| Exp. | 98 | 6 | −2 | 6 | — | — | 3.6 | — | |
| CH4 SR | Thermo | 99 | 32 | — | 32 | — | 1.1 | 6.0 | — |
| Exp. | 95 | 30 | — | 30 | — | 1.1 | 6.0 | — | |
| CH4 DR | Thermo | 98 | — | 27 | — | 1.1 | — | 2.1 | 1.1 |
| Exp. | 93 | — | 25 | — | 1.0 | — | 2.1 | 1.0 | |
3.5 Thermodynamic study
A thermodynamic study was performed in order to help to understand two particular behaviors observed by varying the C8H18 conversion:-the SR conditions lead to the highest hydrogen yields up to C8H18 conversions of 80% at 580 °C, but for higher conversions, the best results are obtained in REGR conditions,
-in REGR conditions, for C8H18 conversions higher than roughly 30%, the selectivity to H2 decreases with the conversion. At lower conversions, only obtained at 450 °C, the selectivity to H2 increases with the conversion (up to 27% of conversion): an optimal selectivity to H2 is evidenced at a medium conversion.
The calculations were based on the minimization of the Gibbs free energy, by taking into account the possibility of obtaining various C8H18 conversions, as it was observed experimentally, whereas the “classical” calculations give 100% of C8H18 conversion. For that purpose, the calculations were made considering 100%, 80%, 60%, 40%, 20% and 5% of C8H18 conversions, simulated by 1.0, 0.8, 0.6, 0.4, 0.2 and 0.05 mol of C8H18 at the inlet flow, respectively, as described in ref. 17. The inlet partial pressure of H2O, O2 and CO2 and the total molar flow inlet were maintained constant. Consequently, the decrease in the C8H18 molar flow was compensated by changing nitrogen proportion. In thermodynamic calculations, the only way to take into account kinetic limitations is to exclude one product from the distribution. Since it was experimentally shown that the CH4 yield is much lower than that given by calculation at the thermodynamic equilibrium, the distribution of products at the equilibrium was calculated considering the absence or the presence of CH4 as reaction product.
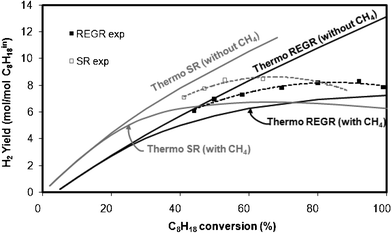 | ||
| Fig. 6 Evolution of the H2 yield at the thermodynamic equilibrium (—) and comparison with experimental evolution (- - -) of H2 yields for REGR (in black) and SR (in grey) at 580 °C as a function of C8H18 conversion. Data obtained at different contact times. | ||
Looking at the curves obtained taking or not into account CH4 in the thermodynamic calculations, one can see that whatever the reaction conditions, SR or REGR, the highest hydrogen yields are obtained, as expected, without production of methane. The experimental H2 yields obtained at various values of hydrocarbon conversion by varying the contact time (same Fig. 6) are between the limits predicted by thermodynamic calculation with and without methane, for both REGR and SR conditions. For example, in REGR conditions, 100% of isooctane conversion is required to obtain the maximum of H2 yield (7.25 mol/mol C8H18in) by calculation taking into account the formation of methane, whereas the calculation excluding CH4 formation shows that a C8H18 conversion of 51% is sufficient to produce the same amount of H2. Experimentally, 7.25 mol H2/mol C8H18 0 are detected with ∼60% of C8H18 conversion and CH4 is formed in low amount. There is no doubt that the presence of methane as equilibrium product has a considerable influence on thermodynamic H2 yield limit. In order to reasonably use this type of calculation (without methane as product), the experimental CH4 yield has to be much lower than that calculated at the thermodynamic equilibrium taking into account methane formation. Fig. 7 shows the evolution of the CH4 yield calculated at the thermodynamic equilibrium with CH4 as product compared to the experimental values collected during REGR and SR conditions, as a function of C8H18 conversion. As expected, CH4 yields are always lower than those calculated at the thermodynamic equilibrium.
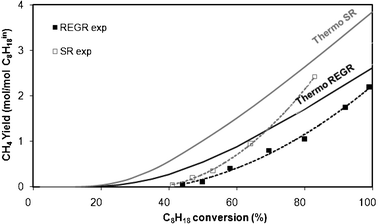 | ||
| Fig. 7 CH4 yield at the thermodynamic equilibrium (—) and experimental CH4 (- - -) yield as a function of C8H18 conversion. CH4 experimental data were obtained for different contact times in REGR (in black) and SR (in grey) conditions. | ||
Finally, the curves obtained by calculation at the thermodynamic equilibrium by varying the C8H18 conversion (Fig. 6) are relevant to predict that, at low conversion, the SR conditions give higher H2 yields than REGR, whereas at conversions higher than roughly 75%, the opposite is observed, in agreement with the experimental results.
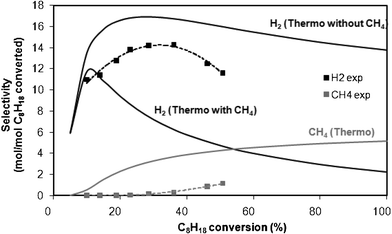 | ||
| Fig. 8 Evolution of the selectivity to H2 (in black) and CH4 (in grey) at the thermodynamic equilibrium (—) and in experimental conditions (- - -) during REGR at 450 °C as a function of C8H18 conversion. Data obtained at different contact times. | ||
The results show that the selectivity to H2 predicted by the thermodynamic calculation (with or without methane), presents also a maximum. Experimentally, the formation of methane is negligible. Thus, if one compares the experimental results with the calculated curve without CH4, one can observe an optimum at the same C8H18 conversion (30%). Consequently the maximum of selectivity to H2 observed at medium conversion can be attributed to a thermodynamic effect.
4. Conclusions
It was previously demonstrated that Rh(1 wt%) supported on ZrO2(73.8 wt%)-La2O3-Nd2O3-Y2O3(26.2 wt%) is a catalyst that exhibits high activity and stability for H2 production in REGR conditions.17 The aim of the present work was to study the reaction scheme of non-elementary steps in the presence of this catalyst. It can be concluded that the REGR reaction occurs in 3 steps:Step 1:
| C8H18 + 16H2O → 8CO2 + 25H2 |
| C8H18 + 8CO2 → 16CO + 9H2 |
Oxidation reaction is negligible, except for low isooctane conversions:
Step 2:
| CO2 + H2 ↔ CO + H2O |
Step 3:
| 2CO + 2H2 ↔ CH4 + CO2 |
Step 1 corresponds to the direct reaction between C8H18 and the co-reactants, i.e. water (SR reaction) or carbon dioxide (DR). However, for low C8H18 conversions, i.e. at low contact time, the DR reaction is negligible. For the SR reaction, H2 and CO2 are primary products. Step 2 corresponds to the RWGS that gives CO, a secondary product. Methane is formed only in step 3 as a tertiary product. Different reactions were studied with the purpose of determining those responsible for methane formation. It was shown that a great amount of CH4 comes from the methanation reaction between CO and H2. The formation of CH4 directly from C8H18 or by the reaction between H2 and CO2 is strongly kinetically and thermodynamically limited, respectively. It was also demonstrated that CH4 is extremely reactive, which proves the possibility of transforming the methane produced in REGR conditions.
The highest H2 yield is obtained in SR conditions up to 80% of C8H18 conversions; but, for higher conversions, the presence of CO2 and O2 in the feed (REGR conditions) has a beneficial effect on hydrogen production. Thermodynamic calculations taking into account the experimental conversions and the low amount of methane produced enabled us to demonstrate that this phenomenon is not a kinetic effect but a thermodynamic one. The thermodynamic calculation explains the presence of a maximum of selectivity to H2 reached at medium conversion at 450 °C.
Acknowledgements
ADEME is gratefully acknowledged for financial support (PREDIT Program n° 06 06 C0137).References
- N. Ladommatos, S. Abdelhalim and H. Zhao, Appl. Therm. Eng., 1998, 18, 963 CrossRef CAS.
- G.H. Abd-Alla, Energy Convers. Manage., 2002, 43, 1027 CrossRef CAS.
- M. Zheng, G.T. Reader and J.G. Hawley, Energy Convers. Manage., 2004, 45, 883 CrossRef CAS.
- Y. Jamal, T. Wagner and M. L. Wyszynski, Int. J. Hydrogen Energy, 1996, 21, 507 CrossRef CAS.
- A. Tsolakis, A. Megaritis and M. L. Wyszynski, Fuel, 2004, 83, 1837 CrossRef CAS.
- S. Peucheret, M. L. Wyszynski, R. S. Lehrle, S. Golunski and H. Xu, Int. J. Hydrogen Energy, 2005, 30, 1583 CrossRef CAS.
- M.L. Wyszynski and Y. Jamal, Int. J. Hydrogen Energy, 1994, 7, 557 Search PubMed.
- A. Tsolakis, A. Megaritis and D. Yap, Energy, 2008, 33, 462 CrossRef CAS.
- A. Tsolakis, A. Megaritis, M.L. Wyszynski and K. Theinnoi, Energy, 2007, 32, 2072 CrossRef CAS.
- P. Leung, A. Tsolakis, J. Rodríguez-Fernández and S. Golunski, Energy Environ. Sci., 2010, 3, 780 CAS.
- S. Golunski, Energy Environ. Sci., 2010, 3, 1918 CAS.
- E. Ambroise, C. Courson, A. Kiennemann, A.C. Roger, O. Pajot, E. Samson and G. Blanchard, Top. Catal., 2009, 52, 2101 CrossRef CAS.
- E. Ambroise, C. Courson, A.C. Roger, A. Kiennemann, G. Blanchard, S. Rousseau, X. Carrier, E. Marceau, C. La Fontaine and F. Villain, Catal. Today, 2010, 154, 133 CrossRef CAS.
- J. Xu and G. F. Froment, AIChE J., 1989, 35, 88 CrossRef CAS.
- J. Xu and G. F. Froment, AIChE J., 1989, 35, 97 CrossRef CAS.
- M. Pacheco, J. Sira and J. Kopasz, Appl. Catal., A, 2003, 250, 161 CrossRef CAS.
- S. Rijo Gomes, N. Bion, G. Blanchard, S. Rousseau, V. Bellière-Baca, V. Harlé, D. Duprez and F. Epron, Appl. Catal., B, 2011, 102, 44 CrossRef.
- E. Guglielminotti, E. Gianello, F. Pinna, G. Strukul, S. Martinengo and L. Zanderighi, J. Catal., 1994, 146, 422 CrossRef CAS.
- L. Villegas, N. Guilhaume, H. Provendier, C. Daniel, F. Masset and C. Mirodatos, Appl. Catal., A, 2005, 281, 75 CrossRef CAS.
- A. Birot, F. Epron, C. Descorme and D. Duprez, Appl. Catal., B, 2008, 79, 17 CrossRef CAS.
- B. Coq, R. Dutartre, F. Figueras and T. Tazi, J. Catal., 1990, 122, 438 CrossRef CAS.
- L. Pirault-Roy, D. Teshner, Z. Paal and M. Guérin, Appl. Catal., A, 2003, 245, 15 CrossRef CAS.
- T.A. Westrich, X. Chen and J.W. Schwank, Appl. Catal., A, 2010, 386, 83 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |