Radiopaque iodinated nano-emulsions for preclinical X-ray imaging†
François
Hallouard
a,
Nicolas
Anton
*a,
Guy
Zuber
a,
Philippe
Choquet
b,
Xiang
Li
a,
Youri
Arntz
c,
Gaëlle
Aubertin
b,
André
Constantinesco
b and
Thierry F.
Vandamme
a
aUniversity of Strasbourg, Faculty of Pharmacy; CNRS 7199, Laboratoire de Conception et Application de Molécules Bioactives, équipe de Pharmacie Biogalénique, 74 route du Rhin, F-67400, Illkirch, France. E-mail: nanton@unistra.fr; Fax: +33 3 6885 4306; Tel: +33 3 6885 4213
bService de Biophysique et Médecine Nucléaire, CHRU Strasbourg, Hôpital de Hautepierre; IMFS CNRS 3240, Université de Strasbourg, Laboratoire de biomécanique, 1 avenue Molière, F-67098, Strasbourg, France
cUniversity of Strasbourg, Faculty of Pharmacy; CNRS 7213, Laboratoire de Biophotonique et Pharmacologie, équipe de biophotonique, 74 route du Rhin, F-67400, Illkirch, France
First published on 25th August 2011
Abstract
The context of the this research is the development of nanoparticulate systems exhibiting long circulation times in the blood pool, loaded with X-ray contrasting compounds, to be used as blood pool contrast agents in computed tomography. This study presents an original, new and simple formulation of radiopaque nano-emulsions composed of iodinated oil, formed by a spontaneous emulsification method. As a result, extremely monodisperse, iodinated nano-droplets were generated, ranging in size from 20 to 190 nm, presenting an iodine concentration of around 85 mg I/mL, and coated with a polyethylene glycol shell which ensured their stealth properties against the immune system in the blood stream. In vivo assays demonstrated a significant contrast effect, along with a long residence in the blood pool. This study highlights novel nano-formulations used as efficient contrast agents for preclinical X-ray imaging applications, along with simple and efficient alternatives for the generation of iodinated nano-emulsions.
1 Introduction
Micro-computed tomography (micro-CT), is a very powerful and non-invasive tool used to establish high-resolution images with isotropic voxels in relatively short scan times. This technology is particularly suitable for visualizing and differentiating bones and related inorganic constituents from the organic (soft tissue). It is possible to extend the scope of micro-CT application to soft tissue by using X-ray opaque, exogeneous compounds to provide contrast by their selective biodistribution.Radiopacity is achieved using atoms with high atomic number. Most contrasting agents are composed of iodine because this specie offers a good compromise between contrasting power, safety and cost.1 Contrast agents used for human applications are hydrosoluble, organic, iodinated molecules. Even if these contrast agents are cleared very quickly from large and small animals, they are effective in humans since clinical CT scanners for human are very fast (e.g. five seconds to acquire 15 cm thick volume). However, their use appear limited with micro-CT systems since they tend to be very slow in comparison (e.g. ten minutes to scan a mouse). Hence the need to have long circulating agents.2
We recently reviewed in detail1 the different strategies for the formulation of blood pool contrast agents as well as the potential and methods for improving their biocompatibility and pharmacokinetic properties. We then presented an overview of the toxicology of these nanoparticulate contrast agents. Formulations are mainly constituted of liposomes,3,4chylomicrons,5 micelles,6dendrimers7 or polymeric nanoparticles.8,9
The general methods used to confer long-circulating properties to contrast agents involve the control of nanoparticle stability and surface properties.10–17 Although the results are promising, it is worth noting the complexity of the chemistry involved in such formulations, which greatly limits their transposition in this specific field, posing problems even when it comes to potential industrial scale-ups and commercialization (only 2 are available).
Associated with the use of biocompatible excipients, nano-emulsions are prime candidates for our imaging aims and specifications. Not only are these dispersions extremely homogeneous, but they also present very useful stability properties and can remain stable for months.18
Nano-emulsion generating processes are generally divided into two groups. The first one comprises “high-energy” processes based on the use of specific devices (high pressure homogenizers, sonifiers, rotor/stator apparatus and so on), supplying enough energy to increase the water/oil interface in order to attain nanometric range droplet sizes. Due to the simplicity of the process, such methods are the most widespread in nano-emulsion formulation. Nevertheless, they are limited by significant inherent drawbacks such as very low energy yields which present a potential problem for industrial scale-up, or difficult conditions of formulation, likely to induce the degradation of fragile molecules during the encapsulation process.
In this study, we have chosen to focus on the second group of nano-emulsion generating methods using “low-energy” processes. The principle is very simple, based on spontaneous emulsification mechanisms described elsewhere.19 It consists in bringing into contact two liquid phases, both at thermodynamic equilibrium. One is an organic phase solubilizing hydrophilic compounds (surfactants, solventsetc.) and the other is an aqueous medium. When mixed, the system is in a state of non-equilibrium, resulting in very rapid diffusion of the surfactants and/or solvents (hydrophilic species) from the organic to the aqueous phase. This then generates nano-emulsion droplets. It is worth noting that, unlike microemulsions, nano-emulsions are not thermodynamically stable, but present great kinetic stability.20 Nano-emulsion droplets are similar to Brownian particles in that they are stable for months, destabilized only by the very slow Ostwald ripening.18 To summarize, the formulation of nano-emulsions by means of this method is extremely straightforward, simply involving the mixing of two liquids with no need for energy or specific devices. It is a solvent-free process requiring a low amount of surfactant, and results in very stable nanodispersion. The simplicity of the process makes it suitable for quick formulation just prior to administration. It would be easy, for instance, to mix two ampoules containing these two phases. This premise is the basis of the present study. Here we present an original formulation of nano-emulsions based on a novel approach, involving extremely simple spontaneous emulsification of iodinated amphiphiles. The low-energy nano-emulsification process is used, with an iodinated oil as the hydrophobic phase. Oil iodination is performed following the one-step Wijs reaction, in which the oil molecules with non-conjugate double bonds take up the amount of iodine chloride in a short time, forming iodochloro compounds. In addition to the simplicity of the whole process, this study aims to highlight another salient point: the particular location of the iodine atoms, right in the middle of each hydrophobic chain. So here is a solution that potentially influences the physicochemical properties of the iodinated compounds and is very simple to use. Despite these advantages, it is hardly dealt with in the literature, where it is more common to read about iodine binding at the extremities of the molecule. In this work, we therefore wanted to point out the potential and efficiency of this chemical configuration. After in-depth chemical characterization and studying the influence of the formulation parameters on the nano-emulsion features and stability, an in vivo evaluation of this iodinated nanoparticle contrast agent is performed in mice.
2 Materials and methods
2.1 Materials
The chemicals used in this study were purchased from commercial sources, in accordance with European Pharmacopoeia, and were used without further purification. Iodine monochloride (98%), Na2S2O3 (99%), cyclohexane (99.9%) and sodium hydroxide (99%) were obtained from Sigma (Saint Louis, USA). Potassium iodide (99%) was purchased from Fluka (Saint Louis, USA), NaH2PO4-2H2O (98%) from Merck (Darmstadt, Germany) and dichloromethane (99.95%) from Carlo Erba (Val-de-Reuil, France). Nonionic surfactant from BASF (Ludwigshafen, Germany), i.e. Cremophor ELP, batch 29054609T0, was kindly provided by Laserson (Etampes, France) and used as received. This nonionic highly polyethoxylated surfactant is a polyoxiethylated-35 castor oil with a number of ethylene oxide groups given around 35, that is to say with a molecular weight around 1500 g·mol−1. This surfactant is be a mixture of different oligomers of molecular weights following a Poisson-like distribution centered on the one of announced by the manufacturer. These amphiphiles exhibit a hydrophilic-lipophilic balance, HLB, around ∼12–14, and create the PEG layer surrounding the nano-emulsions droplets from their hydrophilic PEG moiety. Finally, the hydrophobic phase, i.e. Labrafil M 1944 CS® (Oleoyl Macrogolglycerides), batch 0807541, was obtained from Gattefossé (Saint-Priest, France).2.2 Methods
The hydrodynamic diameters and PDI of the nano-droplets were obtained by dynamic light scattering (DLS) using a Malvern NanoZS instrument (Orsay, France). The helium/neon laser, 4 mW, operated at 633 nm, with the scatter angle fixed at 173° and the temperature maintained at 25 °C. PDI is a measure of the broadness of size distribution derived from the cumulant analysis of DLS data according to ISO 13321![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1996; for a single Gaussian population with standard deviation, σ, and mean size, xPCS, thus PDI = σ2/x2PCS is the relative variance of the distribution. In other words, it shows the quality of the dispersion. Values ≤0.1 reflect very good monodispersity and quality of the nanoparticulate suspensions. Zeta potential measurements were performed with the same apparatus. Measurements were performed three times for each point.
1996; for a single Gaussian population with standard deviation, σ, and mean size, xPCS, thus PDI = σ2/x2PCS is the relative variance of the distribution. In other words, it shows the quality of the dispersion. Values ≤0.1 reflect very good monodispersity and quality of the nanoparticulate suspensions. Zeta potential measurements were performed with the same apparatus. Measurements were performed three times for each point.
The viscosity of iodinated oil was measured with a rotational viscometer (DV I+, Brookfield, Boston, Massachusetts, USA) at 25 °C, whereas for nano-emulsions, it was determined by means of a capillary Ostwald viscometer, at 37 °C.
The reproducibility of the nano-emulsion formulations was studied by repeating the experiments five times. The pH, osmolarity, size, zeta potential and PDI were determined for each sample.
AFM measurements were performed using a commercial microscope (the Solver Pro-M, from NT-MDT Inc., Moscow). Sample preparation protocol was following: 100 μL of a diluted iodinated nano-emulsion suspensions (oil concentration ∼0.3 wt.%) was deposited on the freshly cleaved mica substrate, followed by the addition of 10 μL of a MgCl2 stock solution to obtain a final concentration of 10 nM. The measurements were performed 10 min after sample preparation to allow the interactions between oil droplets and mica. All measurements were conducted in liquid phase (MilliQ water) by using the tapping mode. The cantilevers used were NSG03 type (NT-MDT Inc., Moscow) with a typical spring constant of 1.7 N/m, a resonance frequency of 32 kHz in liquid, and a tip curvature radius of 10 nm. Images were acquired with a resolution of 512 × 512 and a scan rate of 2 Hz.
In vitro measurements were performed on iodinated oil and nano-emulsions. An in vivo evaluation of the products was carried out using a nude mice model (weight ca. around 20 g), anaesthetized with isoflurane during administration of the product and throughout the CT scans. 200 μL of nano-emulsions were intravenously injected in the tail-vein, and CT scans were performed at 5, 75 and 210 min after administration. The whole experiment were repeated with five different mice.
The experiments were performed in accordance with the Committee on Animal Research and Ethics of the University of Lyon-1.
3 Results
3.1 Iodinated nano-emulsions
The principle of generating nano-emulsions, illustrated in Fig. 1, shows the simplicity of the process. Once the organic and aqueous phases were in contact with each other, the hydrophilic species solubilized in oil (i.e.nonionic surfactants) underwent a very rapid diffusion towards water. This resulted in a demixing of the lipophilic molecules in the form of nanometric-scaled droplets, immediately stabilized by the surfactants. The morphology of the resulting droplets (bottom part of Fig. 1) was simply an iodinated oily core surrounded by a nonionic surfactant layer, thus developing a “hairy” surface due to the PEG moiety of the amphiphiles.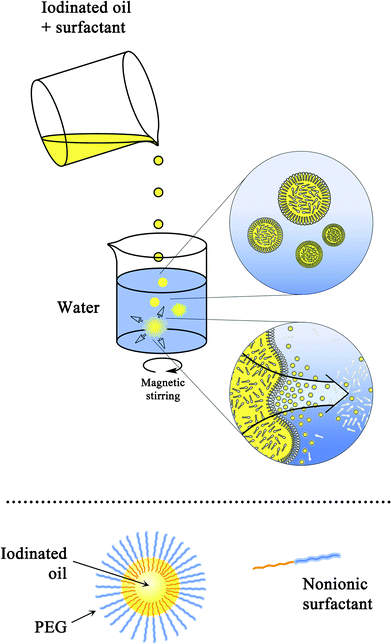 | ||
| Fig. 1 Diagram of the nano-emulsion formulation process (top), and morphology of the nano-droplets formed (bottom). | ||
In order to grasp and optimize the formulation of nano-emulsions, it was necessary to study the influence of the formulation parameters on the resulting nano-emulsion properties. This is illustrated in Fig. 2, by showing the relationship between both SOR and size, and SOR and PDI. The nano-emulsification of iodinated and non-iodinated Labrafil M 1944 CS® was also compared, using the same surfactant (Cremophor ELP®) and aqueous phase. This is reported in Fig. 2.
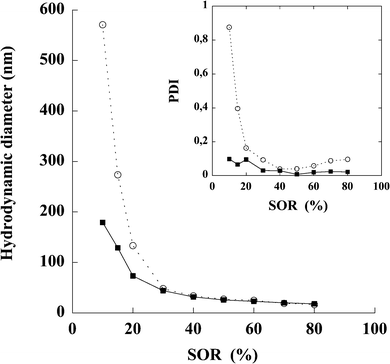 | ||
| Fig. 2 Nano-emulsions formulated with non-iodinated oil (open circles) and iodinated oil (filled squares). Surfactant = Cremophor ELP®, oil = (native or iodinated) Labrafil M 1944 CS®. Hydrodynamic diameters are plotted against the surfactant oil weight ratio (SOR). Inset: The corresponding polydispersity indices are plotted vs.SOR. | ||
The formulation chosen to be used as the blood pool contrast agent was a compromise between the quality of the dispersion (i.e. low size and PDI) and amount of surfactant. The formulation parameters were SOR = 15% and SOWR = 40%, and the result of its characterization is reported in Table 1, showing a significant reproducibility of the experiments. The very low PDI values indicate the extreme monodispersity of the nano-emulsion. Zeta potentials measured on iodinated and non-iodinated nano-emulsions disclose a significant negative surface charge as well as similar values for both systems. This is finally coherent with the chemical nature of the compounds used (oil can contain free fatty acid) and with the fact that chemical bounding of iodine has not influence on the surface charge.
Further characterization of these nano-emulsions (SOR = 15%) were conducted using the AFM microscopy (atomic force microscopy) in liquid conditions and tapping mode for less invasive imaging. Fig. 3 presents a typical topographical image of nano-emulsion droplets deposed on atomic flat mica surface. The oil droplets appear regular in shape and size, which is actually coherent with the log-normal distribution and low PDI values disclosed by the dynamic light scattering measurements (≤0.1, see Fig. 4). As well, the droplet size given by the AFM picture is coherent with the DLS results (dh = (120 ± 2) nm). The images were acquired in low force mode to minimize the morphological distortion of the sample, we observed a surface shadowing effect induced by the interactions between the tip with a soft sample. Section analysis show a typical diameter around (150 ± 20) nm
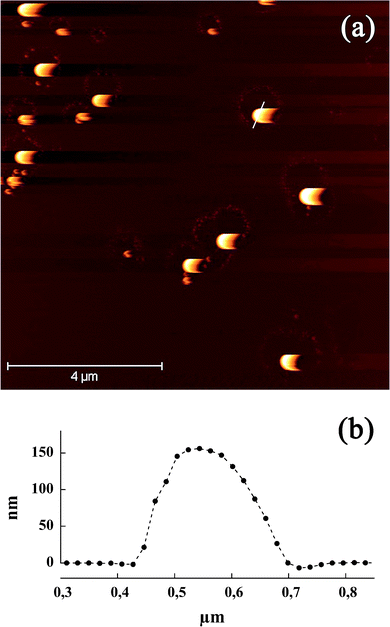 | ||
| Fig. 3 (a) AFM tapping mode images in liquid phase of iodinated nano-emulsions (SOR = 15%). Nano-emulsion droplets were deposed on mica substrate. (b) Section of a nano-emulsion droplet corresponding to the white line in (a). | ||
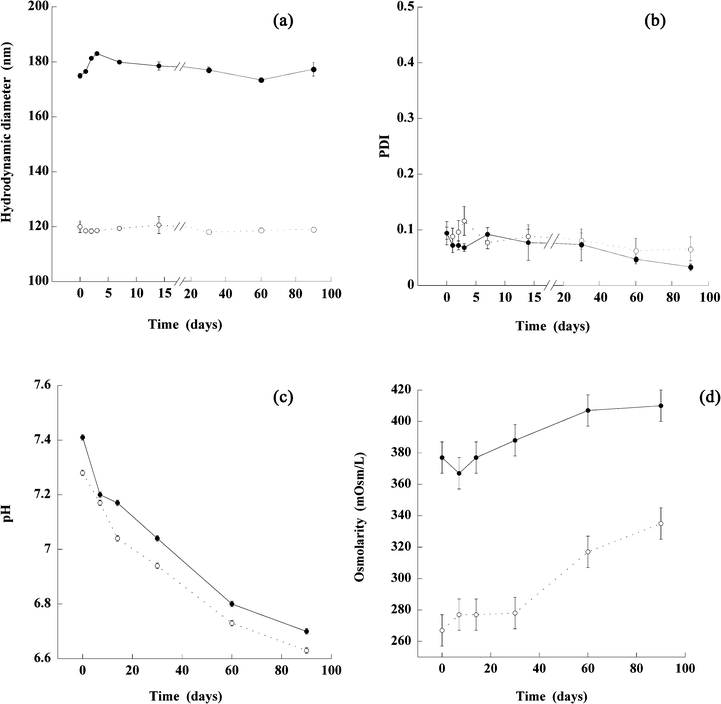 | ||
| Fig. 4 In vitro stability at (4 ± 2) °C for 3 months of nano-emulsions formulated with iodized/chlorinated Labrafil M 1944 CS®, Cremophor ELP® (non-ionic surfactant) using phosphate buffer as the aqueous phase. Nano-emulsion 1 (filled circles) was formulated with a SOR of 10% and a phosphate buffer with an osmolarity of 330 mOsm/L and nano-emulsion 2 (open circles) was formulated with a SOR of 15% and a phosphate buffer with an osmolarity of 270 mOsm/L. The size (a) and PDI (b) of both emulsions were measured at 0, 1, 2, 3, 7, 14, 30, 60 and 90 days and pH (c) and osmolarity (d) were measured at 0, 7, 14, 30, 60 and 90 days. | ||
3.2 Stability study
An in vitro stability study was performed for 90 days at (4 ± 2) °C, on two representative iodinated nano-emulsions sized 120 and 180 nm, formulated with controlled pH and osmolarity-compatible with the parenteral administration route. This study includes the follow-up of the hydrodynamic diameter, PDI, pH and osmolarity, and the results are reported in Fig. 4.The samples exhibit a great stability in time, which is totally coherent with the expected behavior for nano-emulsions. The sizes are centered around the initial one, along with low and constant PDI values lower than 0.1. However, in both formulations we observed similarly fall of the pH and osmolarity: A slow decrease of the pH values appeared, reaching about 6.8 pH at 90 days, along with an increase in osmolarity around 40 mOsm/L, after having been stable for one month. These results reveal a slight, gradual, regular release from the nano-emulsion droplets, of a substance impacting on the pH, and thus also resulting in an increase in osmolarity. This may be a leakage of iodine.
3.3 In vitro evaluation
In this section, the in vitro X-ray attenuation of our iodinated nano-emulsion and of commercial nano-emulsions (Fenestra VC®), were evaluated by establishing a calibration curve. A commercial hydrophilic contrast agent, XenetiX®, was used as a reference, at a concentration of 300 mg I/mL. The results are shown in Fig. 5 and the corresponding values reported in Table 2.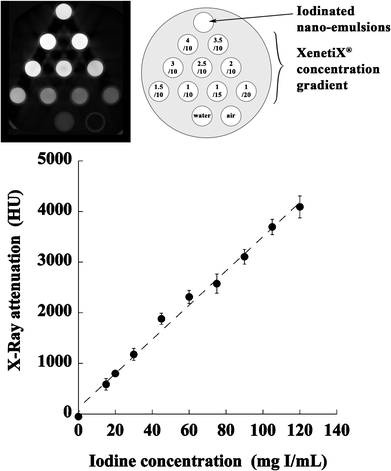 | ||
| Fig. 5 In vitro X-Ray visibility of the iodinated nano-emulsions (120 nm), water, air, and various dilutions of a commercial hydrophilic contrast agent, XenetiX® at 300 mg I/mL. (Top) raw data from the microCT apparatus, and a diagram of the dilutions. (Bottom) resulting calibration curve established with XenetiX®. | ||
| Opacification (HU) | Iodine concentration (mg I/mL) | |
|---|---|---|
| Water | −50.75 ± 109.24 | — |
| Air | −1007.04 ± 114.86 | — |
| Iodinated nano-emulsion | 3083.28 ± 130.03 | 85.52 ± 0.51 |
| Fenestra VC® | 2003.55 ± 120.95 | 55.63 ± 0.24 |
These results confirm the significant attenuation induced by iodinated nano-emulsions, providing values even higher than 53%, more than for the commercial products (Fenestra VC® at around 55 mg I/mL,22 which we confirmed, see Table 2). The higher attenuation is due to higher iodine content. The stability of iodinated nano-emulsions in fetal bovine serum was evaluated and reported in Table 3. The samples appear very stable and homogeneous up to 12 h, and the bigger nano-emulsion (SOR = 15%) shows the first signs of destabilization (slight precipitation) around 24 h, whereas the smaller nano-emulsion remains very stable.
| 5 min | 12 h | 1 day | |
|---|---|---|---|
| Iodinated NE SOR 15% | ++ | ++ | − |
| Iodinated NE SOR 30% | ++ | ++ | ++ |
3.4 In vivo evaluation of iodinated nano-emulsions
The selected iodinated nano-emulsions described above (SOR = 15% and SOWR = 40%, sized 120 nm see Table 1) were intravenously injected in mice. The results presented in Fig. 6 and Fig. 7, show pictures focused respectively on the heart and kidney, acquired 75 min after injecting the nano-emulsions. A clear vascular contrast is obtained, providing clear images of the blood pool and thus proving the potentials of such iodinated nano-emulsions as blood pool contrast agents for preclinical imaging by disclosing their significant X-ray attenuation power.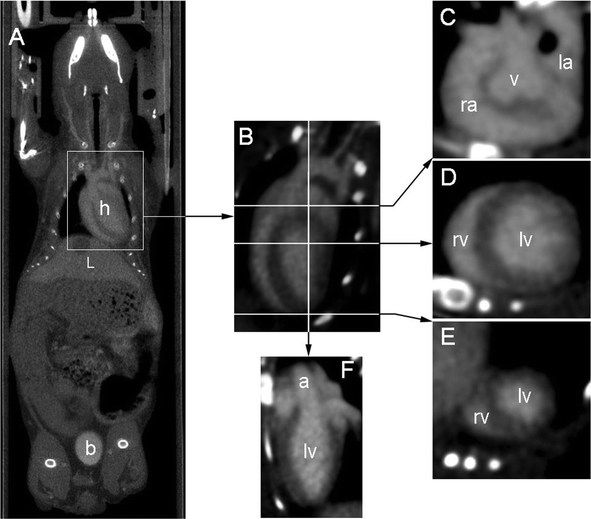 | ||
| Fig. 6 Images illustrating the contrast enhancement obtained 75 min after IV injection. (A) Whole body coronal view crossing heart (h), liver (L) and bladder (b), showing the dual elimination route. (B) Four cavity slice through the heart (obtained after reorientation), lines indicate positions of corresponding transverse slices (see below). (C) Transverse slice through the base of the heart: right atrium (ra), aortic valve plane (v), left atrium (la). (D) Transverse slice through the middle of the heart : right ventricle (rv) and left ventricle (lv). (E) Transverse slice through the apex of the heart. (F) Slice through the left ventricle showing the beginning of the aortic cross (a). | ||
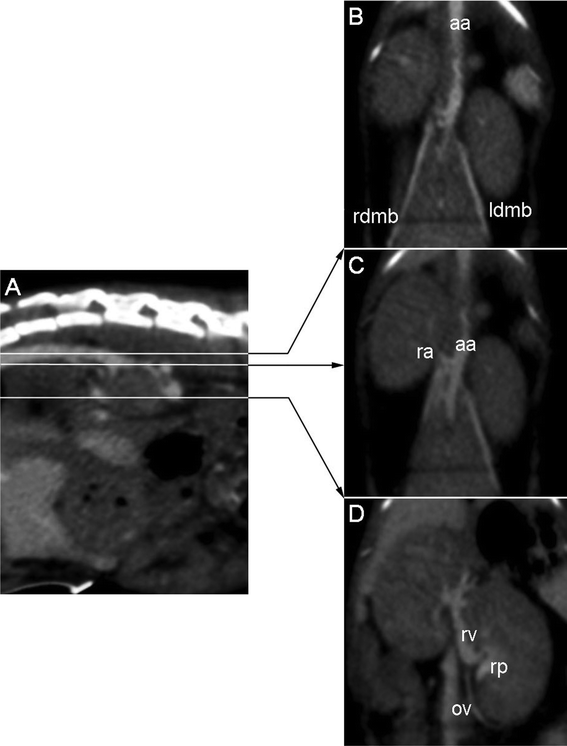 | ||
| Fig. 7 Anatomical details of kidney vascularization at 75 min after IV injection: (A) Sagittal slice through the lombar spine, lines indicate positions of corresponding coronal slices. (B) Coronal slice through the dorsal part of the kidneys, showing the abdominal course of the aorta (aa) and the right and left dorsal muscular branches (rdmd and ldmb respectively). (C) Coronal slice 1.5 mm lower than (B) showing the right renal artery (ra). (D) Coronal slice 5 mm lower than (C) showing the venous side of kidney vascularization: left renal vein (rv) and left ovarian vein (ov), as well as the contrasted urine in the renal pelvis (rp). | ||
Fig. 8 reports the temporal evolution profile of X-ray attenuation in the heart and liver, through three representative acquisitions to the point when the signal is no longer detectable. The mean attenuation is calculated using a cylindrical volume (region of interest) placed inside the left ventricle and the hepatic parenchyma (in yellow on the figure).
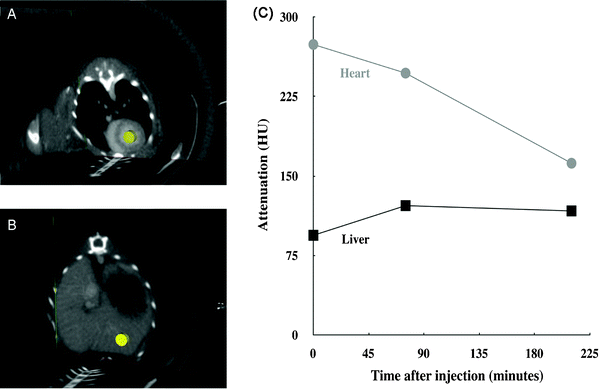 | ||
| Fig. 8 Follow-up of the attenuation, measured in Hounsfield Units (HU), for the left ventricle and liver after IV injection of the contrast agent. (A) Three-slice view showing the cylindrical region of interest (ROI) in yellow, in the left ventricle where all the measurements were taken. (B) Three-slice view showing the cylindrical ROI in the hepatic parenchyma where all the measurements were taken. The chosen ROI did not contain large vessels. (C) The attenuation over time curve showing the mean for each ROI, with administration of the IV injection at the origin of the time axis. As references, the values of the X-ray attenuation in these two ROI were also measured before the IV injection: 5 and 15 HU, respectively for heart and liver. | ||
4 Discussion
The main challenge for this study is to achieve a simple and efficient chemical iodination, with a negligible impact on both the physico-chemical properties of the oil and the emulsification process.In the literature, the solution to this problem has generally been to graft iodine atoms to the extremities of the oily molecules using multi-step chemistry,22 in order to avoid the potential change in their solubility and their interaction with the different compounds of the formulation. Iodinated triglycerides (ITG) were formulated with this aim in mind, and used as the iodinated hydrophobic phase in the formulation of blood pool contrast agents. As regards the application of nano-emulsions as blood pool contrast agents, only a few formulations are proposed, commercialized and registered, such as for example Fenestra VC® and Fenestra LC®.5 These products are chylomicron-like in design and either include a specific PEG coating (for blood pool contrast agents) or not (for hepatocyte-selective contrast agents). The oily core of the nano-droplet comprises ITG, synthesized using multi-step chemistry, binding the tri-iodobenzene functions to the hydrophobic extremity of medium chain triglycerides. The nano-emulsions are thus generated using a high-energy process.
The current study proposes a simpler and more efficient alternative, combining (i) one-step chemistry, and (ii) a quick, spontaneous nano-emulsification process. The chemical characterization confirms complete iodination of the unsaturated sites by Wijs reaction. In this study, the hydrophobe used as the oil core for nano-emulsion droplets was selected by means of the two following criteria: (i) good compatibility with the low-energy spontaneous nano-emulsification method (before and after iodination), and (ii) the presence of non-conjugate double bonds in its chemical structure to facilitate iodine incorporation. Labrafil M 1944 CS®, a mixture of PEGylated fatty acids, totally suits these requirements, allowing full iodination following Wijs reaction and thus producing the iodinated oily compounds illustrated in Fig. 9.
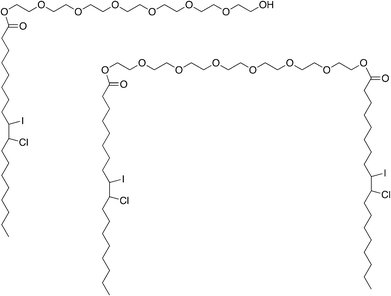 | ||
| Fig. 9 Structure of the two predominant molecules in the iodinated oil. These two predominant molecules correspond to oleoyl macrogol-6-glycerides iodinated/chlored by the Wijs reaction. Iodine and chlorine are linked at oleic acid double bound during this complete reaction. | ||
According to the European Pharmacopoeia, Labrafil M 1944 CS® iodine value is between 24 and 38 g expecting a theoretical maximum range of iodine between 19 to 27.5 wt%. Now as seen before, iodinated Labrafil M 1944 CS® contains 24.53% of iodine and according to the 1H NMR and 13C NMR, it does not have any double-bound. Thus, we can conclude that Wijs reaction is complete.
Although the iodine is in the middle of each oily chain, its impact on the nano-emulsion formulation process, as shown in Fig. 2, appears to be relatively reduced. The spontaneous emulsification process is not affected by the new properties of the oil and seems, in fact, to be more efficient, giving rise to smaller droplets with a thinner size distribution. This may be due to the slight modifications in the oil hydrophobicity caused by the presence of iodine, which in turn results in slight changes in the affinities between the oil and the surfactants, a critical point of the nano-emulsification process.19 These appear to be minor variations, probably due to the length of the fatty chains, which counterbalance the chemical perturbation. The lower the oil proportion in the oil/surfactant mixture (i.e. the higher the SOR), the lower the resulting nano-emulsion droplet size and PDI. Since the affinities between the oil and the surfactants during the emulsification process impact on the transfer velocity of the diffusing molecules, closely linked to the droplet size, the iodinated oily molecules can be said to show an enhanced hydrophobicity compared to native ones. This then results in inducing a faster diffusion of the surfactants during the emulsification process, resulting in smaller nano-emulsion droplets. However, the formulation of nano-emulsions is not conditioned by a specific organization of lipids (as is the case for liposome formulation for instance) and this explains the robustness of the process against such chemical modifications.
The stability of the nano-suspensions formed, presented in Fig. 4, shows how compatible the results are with the target applications. Furthermore, the simplicity of the formulation process renders this technology suitable for extemporaneously prepared applications. In other words, users will easily be able to formulate the nano-emulsification on their own, by simply mixing the two pre-sterilized and appropriately packaged phases (organic and aqueous), and then following the appropriate protocol. It will thus be possible to create nano-emulsions just before use. Finally, as shown in Fig. 4, the formulated nano-emulsions remain very stable for the first month of storage, the pH overall is ≥7.0 and the osmolarity remains stable. This concretely means that once formulated by users, the iodinated suspensions remain stable for a reasonable period and may be used for performing experiments.
To summarize, in comparison to the other types of iodinated contrast agents these low-energy iodinated nano-emulsions offered many advantages, but also, they can be complementary and considered as alternatives to metal nanoparticle (e.g.gold or bismuth nanoparticles23–27) contrast agents. However, the great advantage of the nano-emulsified forms remains in the stability of the oil droplets as it is shown in Fig. 4 of the manuscript. Indeed, the stability of nano-emulsions is governed by the thermodynamic properties of the system inducing a strong colloidal repulsion of the droplets, whereas it is often a problem for the other types of nanoparticulate contrast agents, needing specific surface modification in order to increase the colloidal stability. Other advantages lies in the very simple spontaneous nano-emulsion formulations, the very simple chemistry followed for the iodine incorporation. Likewise, due to the use of nonionic PEGylated surfactants, no specific step of chemical surface functionalization is needed to obtain the aimed stealth properties, as it is often the case in the formulation of blood pool contrast agents. Finally, all of these properties are basic advantages of the nano-emulsified systems, however, the high iodine loading even increase the interest of using such a system as blood pool contrast agent.
The in vitro experiments shown in Fig. 5 reveal iodinated nano-emulsions to be sufficiently loaded with iodine to meet the requirements of their target application as blood pool contrast agents. In the literature, the iodine concentrations of blood pool contrast agents are shown to range from 22 to ∼100 mg/mL,3,5,8,9,28–31 thus placing the present iodinated nano-emulsions in the upper range of existing systems. A further significant advantage of the present formulation is the combination of its simplicity (spontaneous emulsification) with its high iodine concentration, where other spontaneously formed systems (micelles or polymeric nanoparticles) only have a reduced amount of iodine once in suspension.8,9,28 Regarding now the nano-emulsion behavior in presence of blood serum, the results disclosed in Table 3, giving the suspension stable up to 12 h is fully compatible with use as blood pool contrast agent, since nano-emulsions are gradually eliminated from the body within a time lower than 12 h.
The in vivo evaluation (Fig. 6, Fig. 7 and Fig. 8) confirms that, once injected (i.e. once diluted in the blood pool), the iodinated nano-emulsions still show significant X-ray attenuation and thus a notable vascular contrast. Fig. 6 (A) clearly highlights the vascularization of the heart (h), liver (L) (even showing a reverse contrast of the gallbladder), arterial and venous system. Fig. 6 (B) to (F) show the delineation of the cardiac muscles, emphasizing the right atrium (ra), aortic valve plane (v), left atrium (la), right and left ventricle (rv and lv), and aorta (a). The first indications of the elimination mechanism also appear in this figure through the concomitant presence of contrasting materials in the bladder (b) and liver (L). This result indicates that one of the elimination routes of these 120 nm nano-emulsions is through the kidneys. This is coherent in as far as the glomerule pore diameters are in the same size range, from 50 to 100 nm,32 enabling glomerular filtration of this nano-emulsion. This is confirmed by imaging the kidney vascularization, as illustrated in Fig. 7, clearly showing the aorta (aa) right and left dorsal muscular branches (rdmd and ldmb respectively), the right renal artery (ra), and finally the venous side of kidney vascularization: the left renal vein (rv) and left ovarian vein (ov), as well as the contrasted urine in the renal pelvis (rp).
As expected, the temporal in vivo follow-up of the X-ray attenuation presented in Fig. 8 confirms the gradual decrease of the iodinated nano-emulsion concentration in the blood pool. The persistence of the signal for up to 4 h shows the long-circulating properties of the nano-droplets, confirming their suitability for use as a tool for preclinical imaging of the vascular system of small laboratory animals. This result is comparable to those reported in the literature, obtained using blood pool contrast agents of various types, e.g. liposomes, chylomicrons, micelles.3,5,28,29,31 Finally, the attenuation observed in the liver slightly increases between the first and the second acquisition, but appears globally constant throughout the experiments. This result indicates an undeniable hepatic uptake, but, given the decrease of the X-ray attenuation in the heart, it could suggest the involvement of a different elimination route. This actually confirms the observation in Fig. 6 with the marked contrast in the bladder (b) indicating the elimination of nano-emulsions via the kidneys by glomerular filtration, along with a slighter contrast in the liver (L). Such a concomitant elimination of nano-emulsions could simply be related to their size distribution. Although the low polydispersity index (PDI = 0.066) indicates a thin log-normal distribution centered on 120 nm, a large share of the droplets range in the size of the glomerule pore (see above) and the bigger emulsion droplets will be captured by the reticuloendothelial system.
5 Conclusion
This study presents an original, new formulation of radiopaque nano-emulsions composed of iodinated oil, formed by a spontaneous emulsification method. The advantages of this method are mainly the simplicity of the chemical reaction of oil iodination (Wijs reaction) and the generation of nano-emulsions, along with significant results in terms of in vivo contrasting and stealth properties. Furthermore, this work elucidates the link between the formulation parameters and the properties of the nano-emulsions obtained. When injected in nude mice, the iodinated nano-emulsions, formulated by spontaneous emulsification, provide a significant contrast of the blood pool, along with stealth properties (X-ray attenuation visible up to 4 h) making them suitable for use as a blood pool contrast agent.References
- F. Hallouard, N. Anton, P. Choquet, A. Constantinesco and T. Vandamme, Biomaterials, 2010, 31, 6249–6268 CrossRef CAS.
- M. Bourin, P. Jolliet and F. Ballereau, Clin. Pharmacokinet., 1997, 32(3), 180–193 CrossRef CAS.
- S. J. Mukundan, K. Ghaghada, C. Badea, C. Kao, L. Hedlund, J. Provenzale, G. Johnson, E. Chen, R. Bellamkonda and A. Annapragada, Am. J. Roentgenol., 2006, 186, 300–307 CrossRef.
- D. B. Elrod, R. Partha, D. Danila, S. W. Casscells and J. L. Conyers, Nanomedicine: NBM, 2009, 5, 4245 Search PubMed.
- J. Weichert, F. Lee Jr., M. Longino, S. Chosy and R. Counsell, Acad. Radiol., 1998, 5, 16–19 CrossRef.
- V. Torchilin, Colloids Surf., B, 1999, 16, 305–319 CrossRef CAS.
- A. Yordanov, A. Lodder, E. Woller, M. Cloninger, N. Patronas, D. Milenic and M. Brechbiel, Nano Lett., 2002, 2(6), 595–599 CrossRef CAS.
- A. Galperin, D. Margel, J. Baniel, G. Dank, H. Biton and S. Margel, Biomaterials, 2007, 28(30), 4461–4468 CrossRef CAS.
- W. Ho Kong, W. Jae Lee, Z. Yun Cui, K. Hyun Bae, T. Gwan Park, J. Hoon Kim, K. Park and S. Won Seo, Biomaterials, 2007, 28(36), 5555–5561 CrossRef.
- V. Torchilin and V. Trubetskoy, Adv. Drug Delivery Rev., 1995, 16, 141–155 CrossRef CAS.
- D. Lasic, F. Martin, A. Gabizon, S. Huang and D. Papahadjopoulos, Biochim. Biophys. Acta, Biomembr., 1991, 1070, 187–192 CrossRef CAS.
- A. Chonn, S. Semple and P. Cullis, J. Biol. Chem., 1992, 267, 18759–18765 CAS.
- E. Ranucci, G. Spagnoli, L. Sartore and P. Ferutti, Macromol. Chem. Phys., 1994, 195, 3469–3479 CrossRef CAS.
- L. Sartore, E. Ranucci, P. Ferutti, P. Caliceti, O. Schiavon and F. Veronese, J. Bioact. Compat. Polym., 1994, 9, 411–427 CrossRef CAS.
- M. Woodle, C. Engbers and S. Zalipsky, Bioconjugate Chem., 1994, 5, 493–496 CrossRef CAS.
- O. Monfardini, C. Schiavon, P. Caliceti, M. Morpurgo, J. Harris and F. Veronese, Bioconjugate Chem., 1995, 6, 62–69 CrossRef.
- H. Takeuchi, H. Kojima, H. Yamamoto and Y. Kawashima, J. Controlled Release, 2001, 75, 83–91 CrossRef CAS.
- N. Anton, J.-P. Benoit and P. Saulnier, J. Controlled Release, 2008, 128(3), 185–199 CrossRef CAS.
- N. Anton and T. Vandamme, Int. J. Pharm., 2009, 377, 142–147 CrossRef CAS.
- N. Anton and T. Vandamme, Pharm. Res., 2010 Search PubMed in press, year.
- J. Wijs, Anal. Bioanal. Chem., 1898, 37(5), 277–283 CAS.
- J. Weichert, M. Longino, D. Bakan, M. Spigarelli, T. Chou, S. Schwendner and R. Counsell, J. Med. Chem., 1995, 38, 636–646 CrossRef CAS.
- R. Popovtzer, A. Agrawal, N. Kotov, A. Popovtzer, J. Balter, T. Carey and R. Kopelman, Nano Lett., 2008, 8, 4593–4596 CrossRef CAS.
- W. Eck, A. Nicholson, H. Zentgraf, W. Semmler and S. Bartling, Nano Lett., 2010, 10, 2318–2322 CrossRef CAS.
- R. Menk, E. Schültke, C. Hall, F. Arfelli, A. Astolfo, L. Rigon, A. Round, K. Ataelmannan, S. MacDonald and B. Juurlink, Nanomedicine: NBM., 2011 Search PubMed in press, year.
- O. Rabin, J. Perez, J. Grimm, G. Wojtkiewicz and R. Weissleder, Nat. Mater., 2006, 5, 118–122 CrossRef CAS.
- D. Pan, T. Williams, A. Senpan, J. Allen, M. Scott, P. Gaffney, S. Wickline and G. Lanza, J. Am. Chem. Soc., 2009, 131, 15522–15527 CrossRef CAS.
- V. Torchilin, M. Frank-Kamenetsky and G. Wolf, Acad. Radiol., 1999, 6(1), 61–65 CrossRef CAS.
- C. Y. Kao, E. A. Hoffman, K. C. Beck, R. V. Bellamkonda and A. V. Annapragada, Acad. Radiol., 2003, 10, 475–483 CrossRef.
- Y. Fu, D. Nitecki, D. Maltby, G. Simon, K. Berejnoi, H.-J. Raatschen, B. Yeh, D. Shames and R. Brasch, Bioconjugate Chem., 2006, 17(4), 1043–1056 CrossRef CAS.
- T. Henning, A. Weber, J. Bauer, R. Meier, J. Carlsen, E. Sutton, S. Prevrhal, S. Ziegler, H. Feussner, H. Daldrup-Link and E. Rummeny, Acad. Radiol., 2008, 15(3), 342–349 CrossRef.
- M. Vaubourdolle, Toxicologie, Sciences mathmatiques, physiques et chimiques, Groupe Liaisons, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1ra00048a |
| This journal is © The Royal Society of Chemistry 2011 |
