Magnetic field effects on photodecomposition of methylene blue over ZnO particles†
Supawan
Joonwichien
*,
Eiji
Yamasue
,
Hideyuki
Okumura
and
Keiichi N.
Ishihara
Department of Socio-Environmental Energy Science, Graduate School of Energy Science, Kyoto University, Kyoto, 6068501, Japan. E-mail: supawan@namihei.mtl.kyoto-u.ac.jp; Fax: +81 (0) 75 753-5488; Tel: +81 (0) 75 7535476
First published on 14th September 2011
Abstract
The magnetic field effects (MFEs) on the photocatalytic degradation of methylene blue (MB) solution over ZnO particles have been studied. A positive MFE was clearly confirmed, while the MFE is decreased with increasing the interval time (settling time) between the preparation of MB solution and the start of photodegradation. It is suggested that one of the key factors of the MFEs is the amount of dissolved oxygen in the MB solution.
The study of magnetic field effects (MFEs) on the kinetics of photochemical reactions have been considered1,2 not only in homogeneous systems3 but also in heterogeneous systems.4,5 It is said that one of the key factors of MFE proposed is the existence of radicals and radical ions.6,7 Therefore it is reasonable to consider similar MFEs in other photocatalytic systems. However, the MFEs investigated on the heterogeneous photocatalytic reaction are limited only to the titanium dioxide (TiO2) catalyst system.8,9,10 Thus we report the MFEs on the kinetics of photocatalytic degradation of methylene blue (MB) over zinc oxide (ZnO).
As the characteristics of the photocatalyst, powdery ZnO (wurtzite form, 99.9% purity, BET surface area of 3.8 m2 g−1, less than 5 μm, Wako Pure Chemical Ind. Ltd.) is used. The MB solution with the concentration of 0.02 mmol L−1 is prepared using granular MB solid powders (Nacalai Tesque, Inc.) dissolved into distilled water, followed by manual shaking for 5 min. After settling in the dark for a certain period of time (which is hereafter called “settling time”), 0.005 g of photocatalyst powder is immediately mixed with the MB solution in a small reaction cell made of transparent quartz (1 cm × 1 cm × 4.4 cm, about 4 mL of inner volume) with a plastic cap in order to prevent evaporation. The pH of 6.72 ± 0.04 is observed for MB solution and it showed unchanged during the experiment. The concentration of dissolved oxygen is monitored by a multi-function meter equipped with dissolved oxygen (DO) electrode (OE-270AA, DKK-TOA Corp.). When the settling time is 5 min, the DO is 7.57 ± 0.04 mg L−1. The experimental setup is schematically shown in Fig. 1. The mixture of MB solution and the powder in the quartz cell is irradiated from the bottom by ultraviolet (UV) LED lamp (OMRON ZUV-L8V, Kyoto, Japan) with the center light wavelength of 365 nm and with the intensity of 600 mW cm−2. The distance from the top surface of LED light source to the cell bottom is set to be 10 mm. The UV-Vis-NIR spectrometer (Perkin-Elmer lambda 900) is used to monitor the MB concentration, by comparison with the pre-determined calibration curves. Since the slope of calibration curve decreases with temperature from 15 to 35 °C (The maximum increase of temperature due to light irradiation is 6 °C), another calibration curve for the slope and the temperature is also determined.
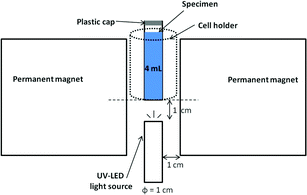 | ||
| Fig. 1 Schematic diagram of the experimental apparatus. | ||
To measure the decomposition of the MB solution, the reaction cell is directly put into the spectrometer at regular time intervals and the absorption spectrum is measured compared with the blank test. As for the magnetic field, an external static magnetic field intensity of 0.3, 0.5, or 0.7 tesla (T) is applied by sandwiching the cell, where the other experimental configuration and photodegradation conditions are the same as those in the absence of an external magnetic field. All the experiments are performed at least three times to confirm reproducibility. All the results of photocatalytic degradation are shown using an averaged value with an error bar, where the concentration is normalized by the initial concentration.
Fig. 2 shows the photodegradation of MB solution with settling time of 5 min as a function of UV irradiation time. It is reproducibly found that the positive MFE clearly exists on the photodegradation of the MB solution, and the MFE is monotonously increased with the increase in the magnetic field applied.
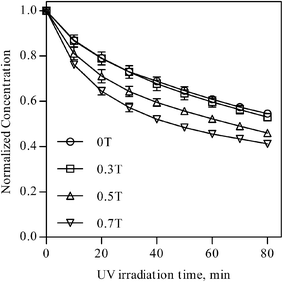 | ||
| Fig. 2 Effect of variation of the magnetic field on the photodegradation rate of MB for ZnO powders. | ||
In order to clarify the mechanism of MFEs, the settling time is varied from 5 min to 1 h, 3 h and 24 h. The results for various settling times are shown in Fig. 3. It is quite interesting to find that the MFEs are decreased with increasing the settling time. Also the photodegradation abilities under 0 T are decreased with increasing the setting time.
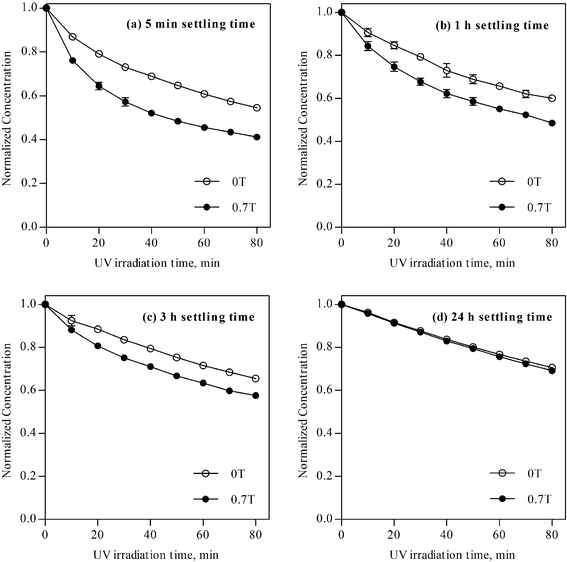 | ||
| Fig. 3 Photodegradation of MB using ZnO for settling time of (a) 5 min (selected data from Fig. 2), (b) 1 h, (c) 3 h, and (d) 24 h. | ||
Firstly, the relationship between the photodegradation ability at under 0 T and the initial DO concentration (prior to UV irradiation) will be considered. Fig. 4 shows the relationship between the amounts of DO in the initial MB solution and the settling time. It is found that the DO level decreases with increasing the settling time. Namely, through the shaking process, supersaturated small air bubbles (containing 21 vol% of oxygen gas) are introduced into the solution, which may react with photo-generated electrons (possibly indirectly) on UV irradiation resulting in production of superoxide anions on and/or near the photocatalyst surface. It has been reported that the dissolved molecular oxygen is strongly electrophilic and the dissolved oxygen acts as a scavenger of an electron producing the superoxide ion (˙O2-), thereby reducing the electron-hole recombination rate.11,12 Then, the higher the DO concentration is, the more effective the photodegradation becomes in general, although the amount of oxygen molecules chemically or physically adsorbed on the ZnO powder and the complex formation of the MB-dye with oxygen molecules prior to the UV irradiation must influence the photocatalysis13 as well as the corresponding MFE.
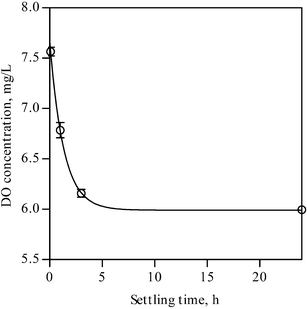 | ||
| Fig. 4 Relationship between the DO levels and the settling time. | ||
As described in previous studies, MFEs were mostly explained in terms of the radical pair mechanism. Kiwi4 explained that when radical species diffuse to the photocatalyst surface, an applied magnetic field interferes with the reactivity of the radical species and the recombination rate of the photo-generated radicals becomes dependent on the strength of the magnetic field. It is also reported that the MFEs may occur through variations on the reencounter of the reactive free radicals14,15 or the recombination of electrons and holes.9 Wakasa et al claimed that observed MFEs over the photocatalysts are not caused by the reencounter of free radicals but by magnetically induced acceleration of recombination of photoexcited electrons and holes.9,10
Our experiments of varying the settling time, however, suggest that the involvement of another factor such as dissolved oxygen (DO) must be considered for an emergence of the MFE. In this respect, the following interesting reports may support our speculation; the diffusion of paramagnetic DO molecules in water16,17,18 (and also the dissolution of the oxygen molecules into water19) are accelerated by a strong gradient static magnetic field. On considering our heterogeneous photocatalytic system with the estimated powder/solution interphase interface area of 190 cm2, the non-negligible amount of local fluctuation on various physical and chemical properties—viscosity, conductivity, electric charge density, concentrations of various species adsorbed or in the buffer layer of the MB solution, and the sort of dangling bonds and their distribution of the ZnO powder—should exist within the narrow surface volume in the vicinity of the powder. It is then natural to assume that the gradient in the electrostatic surface potential and the corresponding magnetic flux density exist in the narrow volume. This may suggest that the product of the magnetic flux density and its gradient be comparable with those reported, causing non-negligible magnetic force on the oxygen molecules around the photocatalyst.
In order to elucidate the role of DO on the MFE, the MB solution with settling time of 24 h is aerated with argon gas (99.9999% purity, Japan Air Gases) for 1 h at a flow rate of 0.1 L min−1 before dispersing photocatalyst powders, which produces the MB solution with less dissolved oxygen (1.06 mg L−1). Fig. 5 demonstrates the photodegradation of MB over ZnO with an argon aerated solution. It is found that the MFE totally disappears for the argon aeration system. Each run is carried out at least three times and the results are reproducible.
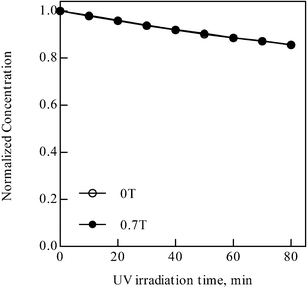 | ||
| Fig. 5 Photodegradation of MB as a function of irradiation time with an argon saturated solution using ZnO. | ||
The indication of the DO involvement with the MFE leads to an idea of the mechanism that the magnetic field promotes the short range diffusion (molecular transport) of DO molecules in the MB solution near the photocatalyst, which may result in the increased rate of supplying active oxygen radicals for the MB dye degradation with magnetic field on. Yet, besides the transport of oxygen molecules, there are several other potential factors for the MFE, such as the radical formation process or the chemical reaction of MB degradation, both of which are involved with the adsorption of oxygen molecules on the powder and the dye-oxygen complex formation as mentioned above. Some discrepancy between the MFE variation in Fig. 3 and the DO level with the settling time in Fig. 4 indicates one of those, i.e. the possible involvement of the MB-dye complex formation with oxygen molecules. Further detailed investigation is thus required to elucidate the mechanism of the novel phenomena.
This work was supported by the Global Center of Excellence (GCOE) Program and Grant-in-Aid for Scientific Research (B) by MEXT, Japan.
References
- S.S. Bhatagnar, R.N. Mathur and R.N. Kapur, Philos. Mag. Lett., 1929, 8, 457 Search PubMed.
- L.R. Faulkner, H. Tachikawa and A.J. Bard, J. Am. Chem. Soc., 1972, 94, 691–699 CrossRef CAS.
- E.S. Ulrich and U. Thomas, Chem. Rev., 1989, 89, 51–147 CrossRef.
- J. Kiwi, J. Phys. Chem., 1983, 87, 2274–2276 CrossRef CAS.
- W. Zhang, X. Wang and X. Fu, Chem. Commun., 2003, 2196–2197 RSC.
- B. Dindar and S. Icli, J. Photochem. Photobiol., A, 2001, 140, 263–268 CrossRef CAS.
- J. Marto, M.P. Sao, T. Trindade and J.A. Labrincha, J. Hazard. Mater., 2009, 163, 36–42 CrossRef CAS.
- M. Wakasa, S. Suda, H. Hayashi, N. Ishii and M. Okano, J. Phys. Chem. B, 2004, 108, 11882–11885 CrossRef CAS.
- M. Wakasa, N. Ishii and M. Okano, C. R. Chim., 2006, 9, 836–840 CrossRef CAS.
- M. Wakasa, Y. Kobayashi and M. Okano, J. Soc. Photogr. Sci. Technol. Jpn., 2006, 69, 271 CAS.
- J. Lea and A. A. Adesina, J. Photochem. Photobiol., A, 1998, 118, 111–112 CrossRef CAS.
- H.C. Liang, X.Z. Li, Y.H. Yang and K.H. Sze, Chemosphere, 2008, 73, 805–812 CrossRef CAS.
- P. R. Ogilby and J. Sanetra, J. Phys. Chem., 1993, 97, 4689–4694 CrossRef CAS.
- U. Till, C.R. Timmel, B. Brocklehurst and P.J. Hore, Chem. Phys. Lett., 1998, 298, 7 CrossRef CAS.
- S. Nagakura, H. Hayashi and T. Azumi, Dynamic Spin Chemistry, Kodansha and Wiley, Tokyo and New York, 1998 Search PubMed.
- S. Ueno and K. Harada, IEEE Trans. Magn., 1982, 18, 1704–1706 CrossRef.
- S. Ueno, M. Iwasaka and G. Furukawa, IEEE Trans. Magn., 1995, 31, 4259 CrossRef CAS.
- S. Aoyagi, A. Yano, Y. Yanagida, E. Tanihira, A. Tagawa and M. Iimoto, Chem. Phys., 2006, 331, 137–141 CrossRef CAS.
- N. Hirota, Y. Ikezoe, H. Uetake, J. Nakagawa and K. Kitazawa, Mater. Trans., 2000, 41, 976–980 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: (1) an example of original UV-Vis spectra of the monitoring MB concentration, (2) temperature calibration. See DOI: 10.1039/c1ra00051a |
| This journal is © The Royal Society of Chemistry 2011 |
