Impregnation of paclitaxel into poly(DL-lactic acid) using high pressure mixture of ethanol and carbon dioxide†
Satoshi
Yoda
*a,
Keisuke
Sato
b and
Hideko T.
Oyama
b
aNanosystem Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. E-mail: s-yoda@aist.go.jp; Fax: +81 29 861 4567; Tel: +81 29 861 9425
bDepartment of Chemistry, Rikkyo University, 3-34-1 Nishiikebukuro, Toshima-ku, Tokyo, 171-8501, Japan. E-mail: hideko-oyama@rikkyo.ac.jp; Fax: +81 3 3985 2363; Tel: +81 3 3985 2363
First published on 22nd July 2011
Abstract
Paclitaxel (PT) is a mitotic inhibitor used in cancer chemotherapy. Impregnation of PT into amorphous poly(DL-lactic acid) (PDLLA) in mixtures of high-pressure ethanol (EtOH) and carbon dioxide (CO2) at various compositions was investigated at 313 K and 20 MPa. It was demonstrated that the high pressure EtOH–CO2 mixture is a promising solvent for fabrication of polymer-based drug delivery systems (DDS) materials, which enables the avoidance of drug deterioration due to processing at elevated temperatures. A mixture with 25 mol% EtOH allowed impregnation of the largest amount of PT in the PDLLA matrix. The amount of impregnated PT in the EtOH–CO2 mixtures was 10 times and 28 times larger than those in supercritical CO2 and liquid EtOH, respectively. The composition of the EtOH–CO2 mixture affected the amount of PT that could be impregnated. The increase in the amount of impregnated PT in the mixture is probably attributed to plasticization of PDLLA and increased solubility of PT into the EtOH–CO2 mixture. The degree of swelling observed in the PDLLA caused by plasticization depended on the composition of the EtOH–CO2 mixture, with the volume increasing to 1.7 times the initial size for a mixture containing 40 mol% EtOH at 313 K and 20 MPa. Physical aging was induced after the swollen PDLLA in supercritical CO2 was vitrified by pressure drop from 20 MPa to atmospheric pressure at 313 K, whereas vitrification hardly occurred in the EtOH–CO2 mixture under the same conditions.
Introduction
Poly(lactic acid) (PLA) is a useful polymer with biodegradability, biocompatibility, and bioresorbability. Hence it is employed in a variety of medical applications including controlled release drug delivery system (DDS).1 There are two major routes for impregnating drugs into a PLA matrix: one is immersion using an organic solvent and the other is mechanical mixing. However, residue of harmful solvents in the PLA matrix and deterioration of drugs processed at elevated temperature on mixing have been serious problems for DDS material fabrication.An alternative route using supercritical carbon dioxide (scCO2) as a medium in fabrication of materials for drug delivery has been developed.2scCO2 is an environmentally friendly, non-flammable, and non-toxic solvent and is easily removed from the polymer matrix after fabrication. Since scCO2 plasticizes a variety of polymers including PLA and enables processing at lower temperature than that in the atmosphere, it can prevent thermal deterioration of drugs. Thus the scCO2 route is quite beneficial for preparation of DDS materials. Polymer microspheres containing drugs and scaffolds (polymer sponges) incorporating drugs are two major applications. Preparation of microspheres has been attempted using a variety of precipitation techniques such as rapid expansion of scCO2 (RESS), and anti-solvent techniques for precipitation.2,3 In recent studies, polymer particles and drug systems such as poly(L-lactic acid (PLLA) and retinyl palmitate,4PLLA and azacytidine,5poly(methyl methacrylate) (PMMA) and 2-acetyloxy-4-(trifluoromethyl) benzoic acid (trifulsal),6 were prepared and characterized. Biodegradable polymer scaffolds prepared by polymer saturation with CO2 (with decreasing glass transition temperature) followed by depressurization induce the formation of the polymer. The scCO2 process is free from residues of organic solvent, which may damage cells or tissues.6 Recently polymer scaffolds incorporating drugs or bioactive agents like PMMA–PLA and ibuprofen systems have been reported.7
In the preparation of these polymeric medical materials, the solubility of drugs into scCO2, the swelling behavior of the polymers in scCO2, the affinity of polymer and drug molecules, are important factors for a successful impregnation process. Solubility of drugs into scCO2 is a first hurdle in many cases. Solubility of drugs into scCO2, which generally have a large molecular weight and/or molecular polarity, is unfortunately quite low.8 For this reason a variety of precipitation techniques using CO2 as an anti-solvent have been employed for the preparation of drug-containing particles.
Addition of polar solvents to scCO2 such as ethanol (EtOH) is known to be effective to increase the solubility of many polar substances. Additionally, polar solvents are also effective for plasticization, swelling and possibly dissolution of polymers. Recently Kiran applied a high pressure acetone–CO2 mixture to the formation process of PLLA.9 He reported that the PLLA was solubilized in the high pressure mixtures at optimized conditions, and that the higher incorporation with the mixture affected the properties. High pressure mixtures of CO2 and organic solvent for drug incorporation into polymers should be used efficiently. However, mainly due to difficulty in measurements, there are limited data on drug solubility and polymer plasticization in mixtures of scCO2 and organic solvents. Lack of solubility and plasicization data is a technical barrier for effective use of the mixture.
In the present paper we report on a novel fabrication of PLA-based DDS material using a high-pressure EtOH and CO2 mixture. EtOH is believed to be the only organic solvent acceptable for the preparation of medical materials. Conveniently, high-pressure EtOH and CO2 mixtures form the homogeneous phase over a wide range of compositions.10 The homogeneous mixture is expected to increase the solubility of drugs and plasticization of PLA. Therefore, the homogeneous mixture is expected to be very useful for preparation of DDS materials.
Here we demonstrated impregnation of an anticancer drug, paclitaxel (PT) (Fig. 1) into amorphous poly(D,L-lactic acid) (PDLLA) in a high pressure EtOH–CO2 mixture at 313 K and 20 MPa. PT is a bioactive natural product of alkaloid and is widely employed in chemotherapy for lung, ovarian, and breast cancers. Solubility of PT into scCO2 has been already reported,11–14 which ranges from 10−7 to 10−6 in molar fraction. The solubility of PT in the EtOH–CO2 (10 mol% EtOH) mixture was also mentioned previously11 and described as about 10 times larger than that in scCO2. Detailed solubility measurements of PT in an EtOH–CO2 mixture are under investigation in our group and similar results were obtained. Therefore the EtOH–CO2 system is expected to be more beneficial for impregnation of PT compared to scCO2. The effects of scCO2 on PLA swelling caused by plasticization have been studied,15–17 but there is little literature available on high pressure EtOH–CO2 mixtures. We conducted an evaluation of PDLLA swelling in EtOH–CO2 mixtures using a high pressure view cell, and measured the PT amount impregnated into the PDLLA under different compositions of high pressure EtOH–CO2 mixtures. The relationship between swelling and PT impregnation, and the efficacy of the EtOH addition to CO2, are discussed in detail.
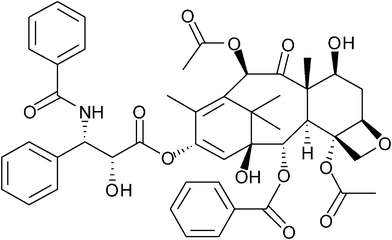 | ||
| Fig. 1 Chemical structure of paclitaxel (PT). | ||
Experimental
Materials
Paclitaxel (PT) (purity 99.74%, Tokyo Supply, Co. Ltd) was used without further purification. Amorphous PDLLA (Lacea (H280), Mitsui Chemicals, Inc., Japan) was used for this work, which is reported to contain about 12% of D-lactyl units in its structure. Exposure of PLA to scCO2 at high pressures results in dissolution of the fluid in the polymer which leads to plasticization and swelling and morphological modifications such as “recrystallization”.9 This “recrystallization” is particular in semi-crystalline PLA. The recrystallization progresses after implanting in living organism and can cause infections there.18 Because of this, amorphous PDLLA was employed to avoid any such effect of crystallization. Measurements by gel permeation chromatography (GPC) and differential scanning calorimetry (DSC) in our laboratory estimated that the number-average molecular weight (Mn) and the weight-average molecular weight (Mw) are 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 174
000 g mol−1 and 174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, respectively, and the glass transition temperature (Tg) is 330.0 K. The PDLLA dried at 353 K in a vacuum oven overnight was hot-pressed at 463 K and 10 MPa for 5 min and then quickly cold-pressed at 273 K to prepare films with ca. 500 μm or 1 mm thickness. CO2 gas (99.99%, Showa Tansan Co. Ltd.) was dehydrated using a zeolite column and used for all experiments. Conventional EtOH (99.9%) was used without further purification.
000 g mol−1, respectively, and the glass transition temperature (Tg) is 330.0 K. The PDLLA dried at 353 K in a vacuum oven overnight was hot-pressed at 463 K and 10 MPa for 5 min and then quickly cold-pressed at 273 K to prepare films with ca. 500 μm or 1 mm thickness. CO2 gas (99.99%, Showa Tansan Co. Ltd.) was dehydrated using a zeolite column and used for all experiments. Conventional EtOH (99.9%) was used without further purification.
Observation of swelling of PDLLA in high pressure EtOH–CO2 mixture
PDLLA swelling in EtOH–CO2 mixtures was observed at 313 K and 6–20 MPa by direct visualization using a high pressure view cell.19Fig. 2(a) shows a schematic diagram of the apparatus for this experiment. The details of the view cell (9) are shown in Fig. 2(b). PDLLA disk (20) (6 mm in diameter and 0.5 mm in the thickness) was compressed between sapphire windows (18) with c-shaped spacer (19) (stainless steel, 0.5 mm in thickness). Since the view cell (9) volume was small (0.4 cm3), a buffer tank (5) (25 cm3) was connected to the system to stabilize the pressure. The temperature of the system was controlled within ±0.1 K by a heater and a PID thermostatic controller. The experimental temperature was monitored at the view cell (9) by a thermocouple and an indicator (13). The pressure of the system was controlled by a back pressure regulator (14) within ±0.5 MPa. The system was equipped with a screw pump (6) for fine adjustment of pressure.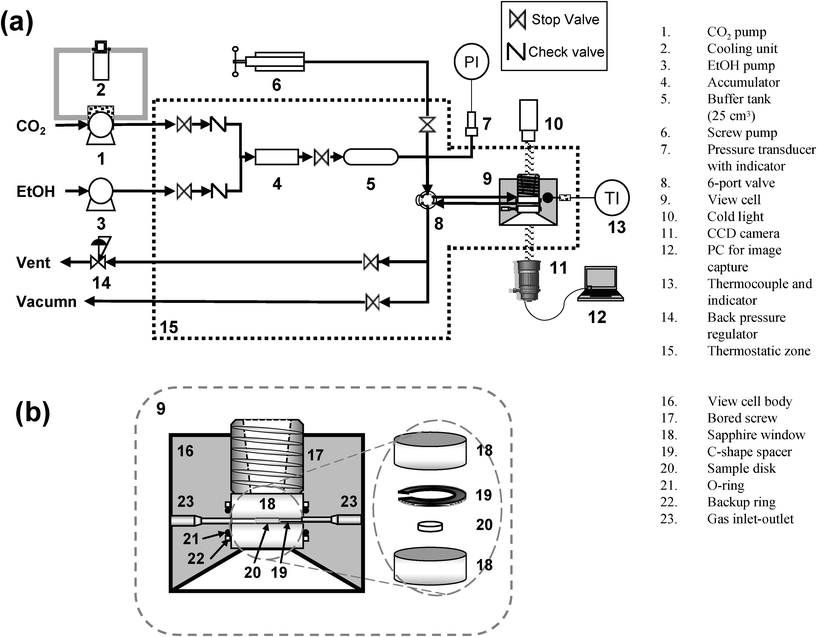 | ||
| Fig. 2 (a) Schematic diagram of apparatus for swelling observation of PDLLA in EtOH–CO2 mixtures. (b) Close-up of the view cell. | ||
The PDLLA disk (20) was evacuated in the cell (9) at 313 K, at least 24 h before starting measurement. CO2 was liquefied through the cooling unit (2) at 273 K. The liquefied CO2 and EtOH were sent by high pressure pumps (1) and (3) (Shodex DS-4) and then mixed with each other at the accumulator (4) (10 cm3 high pressure column filled with 1 mm glass beads). The molar ratio of EtOH/CO2 was controlled by changing the flow rates of the pumps. The mixture then passed through the buffer tank (5) in a thermostatic zone (15) at an experimental temperature which was controlled to ±0.1 K. After reaching equilibrium, the mixture was introduced into the cell (9) by switching the 6-port valve (8), and left for 24 h under these conditions. The images of PDLLA in the view cell were recorded every 5 min at the same magnitude by a CCD camera (11) and PC (12). Since the PDLLA is fixed between the windows with a constant gap (0.5 mm), vertical swelling could be neglected. Under these conditions, the degree of swelling (ΔS) is defined as follows:
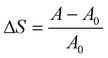 | (1) |
Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) was carried out for samples treated in scCO2 and EtOH–CO2 with equipment similar to that used in the swelling experiments. After treatment, the pressure was released rapidly (within 1 min) for all cases. Samples were dried in vacuum at room temperature for at least one week to a constant weight. No weight gain was observed after the high pressure treatment. The PDLLA pellets as received were analyzed for comparison. DSC measurements were performed under a nitrogen atmosphere with a differential scanning calorimeter (TA Instruments, DSC-Q200, manufactured in USA). Firstly, a 3–5 mg sample was heated at 10 K min−1 from 273 to 483 K and kept for 3 min (Cycle 1). After cooling rapidly to 273 K, the same procedure was re-applied to the same sample (Cycle 2). Cycle 1 was performed to remove thermal hysteresis and any remaining EtOH trapped in the polymer. The data were analyzed by TA-Q series software.Impregnation of PT in PDLLA
The apparatus for the impregnation experiments is shown in Fig. 3. The PDLLA disk (12) (5 mm in diameter and 1 mm in thickness) and 10 mg of PT were placed separately in a high pressure vessel (50 cm3) (7). High pressure EtOH–CO2 mixtures (0–100 mol% EtOH) at 313 K and 6–20 MPa were produced in similar way to that in the swelling observation. The EtOH–CO2 mixture was then introduced into the cell by a switching 6-port valve (6). After the temperature and pressure become stable, the PDLLA sample disk and PT were left in the EtOH–CO2 mixture for 24 h without flowing. After cooling the high pressure vessel (7) to room temperature, the EtOH–CO2 mixture was released gently to prevent PT precipitation on the surface of the PDLLA and formation of the polymer. The pressure was controlled by the back pressure regulator (9) and programmable back pressure controller (10). The rate of decreasing pressure was 2 MPa per hour. After removing from the vessel, the surface of the PDLLA disk was carefully blown off and it was confirmed that no visible PT precipitate had formed on the surface. The PT impregnated PDLLA was evacuated to remove any remaining EtOH.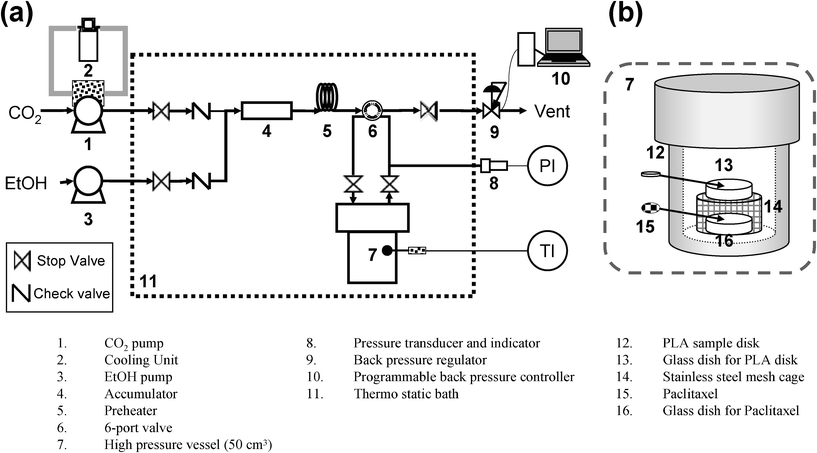 | ||
| Fig. 3 (a) Schematic diagram of apparatus for impregnation of PT in PDLLA. (b) Close-up of the high pressure vessel. | ||
The amount of impregnated PT in the PDLLA disk was determined using gel permeation chromatography (GPC). The sample was first dissolved in chloroform. The GPC analysis was conducted using TSK-GEL (300 mm (L) × 7.8 mm (ID), Tosoh Corp.) and chloroform as eluent at (313 K). Details of GPC measurements are described elsewhere.20 The amount of PT was estimated by both UV-VIS and fluorescence detectors. The UV-VIS absorbance of PT was measured at 250 nm by the former detector and the fluorescence intensity of PT was monitored at 310 nm with the excitation wavelength of 250 nm by the latter detector.
Results and discussion
Swelling of PDLLA in high pressure EtOH–CO2 mixture
Fig. 4(a) shows profiles of PDLLA swelling degree (ΔS) in the EtOH–CO2 mixtures with different compositions at 313 K and 6–20 MPa. Typical changes in the appearance of PDLLA by treatment in the EtOH–CO2 mixture (with 50 mol% of EtOH at 12 MPa) is shown in Fig. 4(b). ΔS in scCO2 (at 0 mol% of “the EtOH content in the mixture”) increased monotonically with pressure, like similar experiments reported in the literature.11, 12 Interestingly, a larger ΔS was observed for all runs in the EtOH–CO2 mixtures, than those in scCO2. The ΔS value increased with pressure except for the mixture with 75 mol% EtOH, and increments were large at 25–50 mol% of EtOH. This figure clearly indicates that the mixture with 25–50 mol% EtOH induced substantial swelling of PDLLA, in which an increase of the free volume would be a cause. The maximum swelling was observed in the mixture containing 40 mol% EtOH at 20 MPa. On the other hand, no swelling was observed in pure EtOH at high pressures (6 and 20 MPa), in which similar results are reported for crystalline poly(L-lactic acid) (PLLA) in the literature.21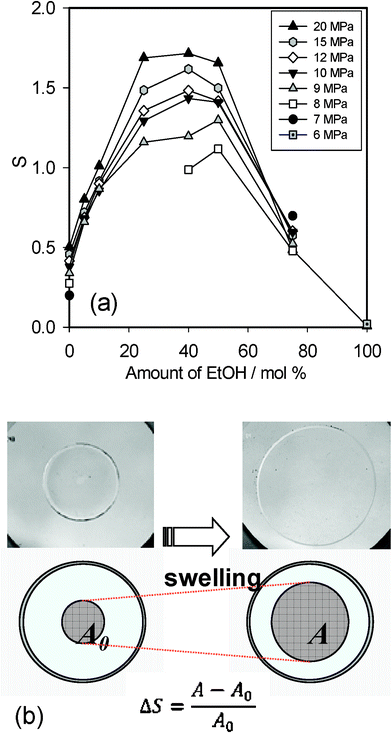 | ||
| Fig. 4 (a) Profiles of PDLLA swelling degree (ΔS) in EtOH–CO2 mixtures at 313 K, 6–20 MPa. (b) Typical appearance of PDLLA before and after swelling with 50 mol% of EtOH at 12 MPa. | ||
This type of EtOH–CO2 mixture effect on polymer swelling has not been previously reported. Matsuyama et al. reported a higher solubility of poly(ethylene glycol) (PEG, molecular weight average = 4000) than pure scCO2 and than pure EtOH (insoluble) was observed.22 They found the maximum solubility of PEG in a 50 wt% EtOH mixture. In the report they performed similar measurements with other polymers including PLA (molecular weight = 5000), but no particular effect was found for PLA in their report. This might be due to the number of measurement points and to the polymer type. As mentioned their report, the polarity and the van der Waals force of the mixed solvent were highly important factors. The solubility and swelling of polymers in EtOH–CO2 mixtures were fundamentally related to the interactions between polymer, EtOH, and CO2. For polymers having some polarity (such as PLA and PEG), interactions between the polymer and pure CO2 were too small, whereas pure EtOH was too polar and their molecules tend to be self-associated.
Pöhler et al. investigated the density of high pressure EtOH–CO2 mixtures at 2–23 MPa and 0–50 mol% EtOH and reported that the maximum density of the mixture was at 30 mol% EtOH, 15–23 MPa.23 This indicates that self-association of both CO2 and EtOH molecules was diluted and the association of these molecules was optimized in this composition. For the polymer–EtOH–CO2 system, there would be good conditions where interactions among those molecules are balanced, so that solvent molecules are more available to the polymer network. It was presumed that swelling of PDLLA in our work is related the amount of solvent included in the polymer network.
Differential scanning calorimetry (DSC) analyses
Fig. 5 shows typical DSC curves of PDLLA treated in the EtOH–CO2 mixtures. Fig. 5(a) is for the first heating (Cycle1) and Fig. 5(b) for the second heating (Cycle2). As expected, there were no crystallization and melting peaks of PDLLA observed in the DSC thermograms, since the exothermic peak for crystallization (of PLA) and the endothermic peak for melting usually appear at about 373 K and 443 K, respectively.20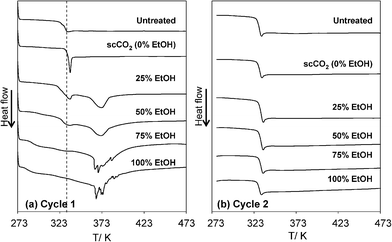 | ||
| Fig. 5 Typical DSC curves (around Tg) of PDLLA treated in EtOH–CO2 mixtures at 313 K, 20MPa. (a) The first heating (Cycle 1) and (b) the second heating (Cycle 2). The dotted line shown in (a) indicates the Tg obtained from Cycle 2. | ||
The glass transition temperature (Tg) of untreated PDLLA film was determined at 329.5 K for the first heating (Cycle 1). In contrast, a sample treated in scCO2 showed a sharp endothermic peak at 334.0 K increasing the Tg by 4.5 K. The result indicates that the treatment in scCO2 induces physical aging of PDLLA, in which Tg shifted to higher temperatures and more heat was required for the rearrangement of polymer chains for glass transition. Physical aging is a phenomenon that occurs when a polymer is cooled from above to below its Tg, a reduction in the specific volume or enthalpy, and an apparent shift to the creep response to longer times occurs.24 Here, the materials with non-equilibrium glassy states tend to slowly rearrange their molecules to reach the equilibrium state, since a rapid cooling through Tg slows down the molecular mobility and creates thermodynamically unstable glass. Here in the present study, a treatment at high pressure gas increases the free volume of PDLLA, thereby causing plasticization and swelling. Furthermore, the depressurization of CO2 from 20 MPa to atmospheric pressure at 313 K transforms PDLLA from the rubbery state to the glassy state. Along with this change, physical aging is induced in PDLLA to rearrange the polymer chains to reach a thermodynamically more stable state. Such stabilized polymeric materials need more energy for the glass transition, thereby increasing Tg and the area of the endothermic peak as shown in Fig. 5. Since the first heating eliminates the structure formed by physical aging and thermal history, in the second heating (Cycle 2) of DSC measurements the Tg of all specimens showed almost the same value of 330.1 K. In Fig. 5(a) the Tg obtained by the second heating is shown by the dotted line. A similar physical aging phenomenon of PLLA and PDLLA is also observed during annealing at 313 K at atmospheric pressure, in which the temperature at the endothermic peak and enthalpy loss during annealing increased linearly with the logarithm of aging time.25 However, there are only few reports on physical aging induced by a high gas pressure treatment involving supercritical CO2.
In samples treated in EtOH–CO2 mixtures and EtOH, an endothermic peak around 334.0 K became broader and weaker in intensity, and shifted to a lower temperature with increased EtOH content. Physical aging was obviously prevented in EtOH–CO2 mixtures. In these samples, another large endothermic peak was detected around 373 K. The peaks on the sample treated in 75% EtOH and 100% EtOH are multimodal and noisy. After investigating some possible reasons, we confirmed that a similar peak was observed for the PDLLA immersed in liquid EtOH at atmospheric pressure and incompletely dried. Even with thorough evacuation (without heating), a small amount of EtOH might be tightly trapped in these high-pressure treated samples. Probably due to a variety of EtOH molecular states in inhomogeneously swelled PDLLA, split peaks were observed in samples treated in 75% and 100% EtOH. The EtOH molecules probably play a role in plasticization and thereby prevent physical aging.
Impregnation of PT in PDLLAviaEtOH–CO2 mixture
Fig. 6 shows the relationship between the EtOH molar ratio of EtOH–CO2 mixtures and the amount of impregnated PT at 20 MPa, 313 K. The amount of impregnated PT in pure scCO2 (at 0% EtOH content as in Fig. 5) was 0.11 μg mg−1 (impregnated PT/PDLLA), whereas those in EtOH–CO2 mixtures were 4–10 times larger than that in pure scCO2 (0.4–1.1 μg mg−1). A small amount of PT was impregnated into PDLLA in the EtOH solution of PT. The degree of swelling, ΔS, under the same conditions is also shown in Fig. 6 for comparison. The amount of PT was approximately correlated with the degree of swelling, in which the larger swelling induced the higher amount of impregnated PT. It clearly indicates that an increase in free volume facilitates diffusion of PT in the PDLLA, thereby increasing the amount of impregnated PT. After the process, a small decrease in the molecular weight of the PDLLA (6% for Mn at the maximum) was found, but there was only a poor correlation with EtOH content (these data are shown in ESI†).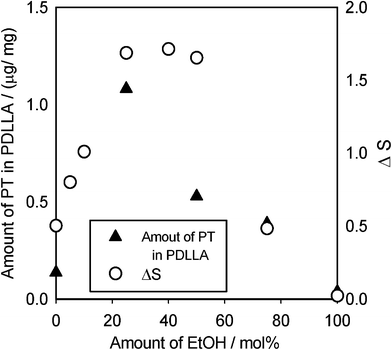 | ||
| Fig. 6 The relationship between the EtOH content of EtOH–CO2 mixtures and the amount of impregnated PT in PDLLA at 20 MPa, 313 K. ΔS values under the same conditions are shown for comparison. | ||
The result verified that a larger solubility is not necessary for a larger amount of impregnation. As mentioned above, the solubility of PT increases significantly and monotonically with enhancement of the EtOH content. Solubility of PT in EtOH (molar fraction) was much higher than that in EtOH–CO2 mixtures (at least larger than 10−3).18In EtOH-rich solvents, a distribution equilibrium of PT should be considered. It is possible that the affinity of PT for PDLLA becomes lower than that for the EtOH–CO2 mixture. As a result of these competitive influences, i.e. solubility of PT, swelling of PDLLA and distribution equilibrium of PT, the amount of impregnated PT peaks at 25% of EtOH mixture and then decreases with further increasing EtOH content.
Conclusions
Impregnation of PT into PDLLA in various compositions of EtOH–CO2 mixtures was investigated at 313 K and 20 MPa. It was shown to be more advantageous to use the EtOH–CO2 mixture for impregnation of PT than the pure scCO2. The amount of impregnated PT in EtOH–scCO2 mixtures (0.4–1.1 μg mg−1) was 4–10 times larger than that in pure scCO2 (0.11 μg mg−1), and was 10–28 times larger than that in EtOH (0.04 μg mg−1). The EtOH–CO2 mixture with 25 mol% EtOH showed the maximum amount of impregnated PT in PDLLA. The increase in the amount of impregnated PT was probably attributed to an enhancement of solubility of PT and the swelling of PDLLA in the EtOH–CO2 mixture. The maximum swelling of PDLLA was observed as 1.7 times larger than the initial size in the mixture with 40 mol% EtOH at 313 K and 20 MPa. The high pressure EtOH–CO2 system is promising for fabrication of a variety of polymer-based DDS materials, since the swelling effect of polymer and high solubility of drugs will be compatible.Acknowledgements
Financial support was kindly provided by the Frontier Project supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan and Rikkyo University Special Fund for Research.References
- R. Langer and N. A. Peppas, AIChE J., 2003, 49, 2990–3006 CrossRef CAS.
- O. R. Davies, A. L. Lewis, M. J. Whitaker, H. Tai, K. M. Shakesheff and S. M. Howdle, Adv. Drug Delivery Rev., 2008, 60, 373–387 CrossRef CAS.
- K. Mishima, Adv. Drug Delivery Rev., 2008, 60, 411–432 CrossRef CAS.
- A. Sane and J. Limtrakul, J. Supercrit. Fluids, 2009, 51, 230–237 CrossRef CAS.
- A. Argemí, A. Vega, P. Subra-Paternault and J. Saurina, J. Pharm. Biomed. Anal., 2009, 50, 847–852 CrossRef.
- A. López-Periago, A. Argemí, J. M. Andanson, V. Fernández, C. A. García-González, S. G. Kazarian, J. Saurina and C. Domingo, J. Supercrit. Fluids, 2009, 48, 56–63 CrossRef.
- D. Velasco, L. Benito, M. Fernández-Gutiérez, J. San Román and C. Elvira, J. Supercrit. Fluids, 2010, 54, 335–341 CrossRef CAS.
- R. B. Gupta and J.-J. Shim, Solubility in supercritical carbon dioxide, CRC press, 2007 Search PubMed.
- E. Kiran, J. Supercrit. Fluids, 2010, 54, 296–307 CrossRef CAS.
- K. Suzuki, H. Sue, M. Itou, R. L. Smith, H. Inomata, K. Arai and S. Saito, J. Chem. Eng. Data, 1990, 35, 63–66 CrossRef CAS.
- V. Vandana and S. Teja Amyn, ACS Symp. Ser., 1995, 429–443 CrossRef CAS.
- V. Vandana and S. Teja Amyn, Fluid Phase Equilib., 1997, 135, 83–87 CrossRef CAS.
- C. A. Nalesnik, B. N. Hansen and J. T. Hsu, Fluid Phase Equilib., 1998, 146, 315–323 CrossRef CAS.
- D. Suleiman, L. A. Estévez, J. C. Pulido, J. E. García and C. Mojica, J. Chem. Eng. Data, 2005, 50, 1234–1241 CrossRef CAS.
- T. Fujiwara, T. Yamaoka, Y. Kimura and K. J. Wynne, Biomacromolecules, 2005, 6, 2370–2373 CrossRef CAS.
- R. Pini, G. Storti, M. Mazzotti, H. Tai, K. M. Shakesheff and S. M. Howdle, Macromol. Symp., 2007, 259, 197–202 CrossRef CAS.
- R. Pini, G. Storti, M. Mazzotti, H. Tai, K. M. Shakesheff and S. M. Howdle, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 483–496 CrossRef CAS.
- G. Perego, T. Vercellio and G. Balbontin, Makromol. Chem., 1993, 194, 2463–2467 CrossRef CAS.
- Y. Ozaki, K. Otake, S. Yoda, Y. Takebayashi, T. Sugeta, N. Nakazawa, H. Sakai and M. Abe, in The 6th International Symposium on Supercritical Fluids, Versailles, France, 2004, PMp14 Search PubMed.
- H. T. Oyama, Y. Tanaka and A. Kadosaka, Polym. Degrad. Stab., 2009, 94, 1419–1426 CrossRef CAS.
- H. Tsuji and K. Sumida, J. Appl. Polym. Sci., 2001, 79, 1582–1589 CrossRef CAS.
- K. Matsuyama, K. Mishima, K.-I. Hayashi, H. Ishikawa, H. Matsuyama and T. Harada, J. Appl. Polym. Sci., 2003, 89, 742–752 CrossRef CAS.
- H. Pöhler and E. Kiran, J. Chem. Eng. Data, 1997, 42, 384–388 CrossRef.
- J. M. Hutchinson, Prog. Polym. Sci., 1995, 20, 703–760 CrossRef CAS.
- P. Pan, B. Zhu and Y. Inoue, Macromolecules, 2007, 40, 9664–9671 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Supplement table 1. See DOI:10.1039/c1ra00070e/ |
| This journal is © The Royal Society of Chemistry 2011 |
