One-step synthesis of low defect density carbon nanotube-doped Ni(OH)2 nanosheets with improved electrochemical performances
Sheng
Chen
,
Junwu
Zhu
*,
Hui
Zhou
and
Xin
Wang
*
Key Laboratory for Soft Chemistry and Functional Materials (Nanjing University of Science and Technology), Ministry of Education, Nanjing, 210094, China. E-mail: jwzhu2000@yahoo.com.cn (J. Zhu); wxin@public1.ptt.js.cn (X.Wang)
First published on 11th August 2011
Abstract
A facile soft chemistry route is described to fabricate a composite of α-Ni(OH)2 nanosheets doped with low defect density carbon nanotubes (CNTs) via one step. As a result of the hydrolysis of nickel nitrate in a water/N-methyl-pyrrolidone (NMP) system, the as-produced H+ and NO3− species can react with carbon atoms of CNTs, yielding a small amount of oxygen-containing functional groups which may serve as the anchor sites. It is interesting to find that CNTs simultaneously act as both nucleation centers and templates, eventually leading to the formation of smooth and micro-scale α-Ni(OH)2 nanosheets–CNTs hybrids. Because of the soft oxidation nature of as-proposed synthetic pathway, the conjugation of carbon atoms in the framework is less disrupted and the CNT backbones in the composite have a characteristically low defect density; thereby, the physical properties of CNTs are well preserved in the composite material. As expected, a high specific capacitance of 1302.5 F g−1 of the composite are obtained in comparison with its individual components (372.1 F g−1 for Ni(OH)2 and 101.4 F g−1 for CNTs), highlighting the importance of rational design and synthesis of hybrid nanostructures for high-performance energy storage applications.
Introduction
Since their discovery in 1991 carbon nanotubes (CNTs) have been proposed as novel nanomaterials that can offer significant advantages for applications in many scientific and technological fields.1,2 A combination of extraordinary electrical, thermal and mechanical properties make CNTs not only attractive as robust components in nanoelectronic devices, but also excellent molecular building blocks for assembling new hybrid materials for widespread applications, particularly as electrodes for energy storage devices.2 Generally, the CNTs (single and multi-walled) are produced by three main techniques: arc discharge, laser vaporization and chemical vapor deposition.3 The integration of inorganic nanoparticles and carbon nanotubes is a new way to obtain novel hybrid materials with intriguing properties for various applications such as catalysts, gas sensors as well as electronic and magnetic devices and supercapacitors.3–6 It was found that the combination of CNTs and some inorganic nanoparticles, such as Au,3 Pt–Ru alloy,4MnO2,5Fe3O4,6ZrO2,7InO2,8BaTiO3,9etc. could give the materials some additional properties due to the concerted effect.Currently, the main procedures in fabricating CNT-based hybrids usually involve a cumbersome surface modification of pristine CNTs in advance, producing some oxygen-containing groups such as hydroxyl, epoxy and carboxyl on the surface during oxidation. These functional groups can be used as nucleation centers and consequently make the in situ formed nanoparticles attach on the surface of the CNTs.10,11 The shortcomings of this procedure are that the operation is very laborious, hazardous and usually involves the application of toxic and environmentally harmful agents, such as H2SO4, HNO3etc. A gradual loss in conductivity occurs with increasing the degree of oxidation because of the transformation of carbon atoms from a planar sp2-hybridized to a distorted sp3-hybridized geometry.11,12 It is inevitable to introduce a large amount of framework defects during oxidation, which may severely impair the electronic structure of this intriguing material.13–15
Taking into account of the fact that many well documented novel performances of CNTs are related to its novel electronic structure,1,16 it makes sense to keep the carbon conjugation of CNTs unaltered in composite materials, which may cast light on further exploring distinctive characters of CNT based functional materials for a wide range of practical applications. Unfortunately, recent literatures have revealed that excessive framework defects yield when the CNT surface is modified in advance for composites preparations. Accordingly, we really need a more simple, soft and straightforward approach to functionalize multi-walled carbon nanotubes.
Herein, we report an effort to fabricate a low defect density CNT-based composite material from the hydrolysis of nickel nitrate in a water/N-methyl-pyrrolidone (NMP) system. It is noteworthy that the concentration of H+ and NO3− from hydrolysis of nickel nitrate in our procedure is much lower than that in traditional oxidation procedures; our route is considered to be much softer, and leading to lower defected frameworks of CNTs in the composites. To our best knowledge, little research concerning this technology for CNTs has been reported thus far. Since there is insignificant disturbance in electronic structure and conjugation, the functionalization of CNTs is achieved whilst still preserving their excellent physical properties. The as-obtained Ni(OH)2–CNTs composites show elevated electrochemical performances for energy storage devices. Additionally, it is predicated from the experimental results that CNTs have played a role as both nucleation centers and templates for large and smooth Ni(OH)2 nanosheets to grow, which may push the potential utilization of this intriguing material to a broader horizon. We expect the simplicity and effectiveness of fabricating conductive CNT-based nanocomposites with good properties would be relevant for addressing some of the fundamental issues in practical applications.
Results and discussion
Nickel hydroxide is attractive on account of its layered structure with large interlayer spacing, well-defined electrochemical redox activity and the possibility of the enhanced performance by varying the preparative procedure.17 Being an important electrochemical active material, the Faradic reactions give a Ni(OH)2 film a higher specific capacitance than that of RuO2.18 CNTs provide a high specific surface area, mechanically robust electrode support structure with good conductivity in energy storage devices. If the combination of CNTs and Ni(OH)2 can be accomplished, it should be possible to obtain an interesting nanocomposite presumably endowed with exceptional properties.19,20One essential character of our procedure is the utilization of pristine multi-walled carbon nanotubes (MWNTs) as the starting material. The representative method involves using an MWNTs–NMP dispersion in conjunction with a nickel nitrate aqueous solution in a solvothermal system to yield a composite of low defect density MWNTs doped with Ni(OH)2 nanocrystals (denoted as CN). The components of the as-obtained hybrid were characterized with XRD and Raman analyses. Fig. 1a shows the XRD patterns of CN, together with pure MWNTs and Ni(OH)2 for comparison. The XRD profiles of MWNTs exhibited a predominant peak at 26.1° corresponding to the reflections from the (002) planes of hexagonal graphite (JCPDS No. 75-1621). The diffraction peaks of CN are similar to those of a nano hexagonal phase of α-Ni(OH)2 (JCPDS 22-0444, a = b = 5.34 Å, c = 7.5 Å), while the (002) reflection of MWNTs is obviously weakened. However, Raman spectra in Fig. 1b verify the existence of MWNTs in the product. Apart from those contributions around 1360, 1600, 2700 and 3000 cm−1 attributed to the D, G, 2D and 3S bands of MWNTs,21,22 the Raman peak at ∼500 cm−1 of CN is the fingerprints of the Ni–OH translational vibration lattice mode.23 Thus from XRD and Raman data, it is speculated that α-Ni(OH)2 and MWNTs coexist as the components of the as-obtained composite.
 | ||
| Fig. 1 (a) XRD patterns of MWNTs, CN, Ni(OH)2; (b) Raman spectra of CN and MWNTs; (c) XPS spectra of CN; (d) FTIR spectra of CN and MWNTs. | ||
The significance of our work is highlighted by using less defected CNTs as a precursor. One problem is that anchor sites are scarce on the MWNTs surfaces for chemical interaction with Ni(OH)2. As such, the H+ from a hydrolysis of metal nitrate aqueous solution along with NO3− of metal nitrate was employed to react with MWNTs, producing a small amount of oxygenation-containing functional groups as anchors. The evidence that MWNTs backbones in CN have a low defect density is verified using XPS and FTIR analyses. XPS spectra indicate that the product contains C, O and Ni as the main elements. The C1s peak in Fig. 1c can be deconvoluted into four components at binding energies of 284.6 eV(C–C), 285.8 eV (C–OH), 287.8 eV (C–O–C), and 289.3 eV (HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). After integrating the area of each deconvoluted peak, the amount of incorporated functional groups is calculated. Remarkably, the percentage of incorporated overall functional groups (13.9%) and carboxyl groups (1.3%) in MWNTs in composites is smaller than other reports.24,25 Because it is generally accepted in carbon nanotubes that a higher percentage of carboxyl and oxygenation-containing functional groups indicates more carbonaceous fragments and defects introduced in the carbon backbones,24,25 our results suggest relatively low amount of fragments and defects of MWNTs in the composite.
O). After integrating the area of each deconvoluted peak, the amount of incorporated functional groups is calculated. Remarkably, the percentage of incorporated overall functional groups (13.9%) and carboxyl groups (1.3%) in MWNTs in composites is smaller than other reports.24,25 Because it is generally accepted in carbon nanotubes that a higher percentage of carboxyl and oxygenation-containing functional groups indicates more carbonaceous fragments and defects introduced in the carbon backbones,24,25 our results suggest relatively low amount of fragments and defects of MWNTs in the composite.
In addition, the FTIR spectra suggest some functional groups like hydroxy (C–OH) and epoxy (C–O–C) groups are introduced in the MWNTs in CN (Fig. 1d). A significant contribution at around 660 cm−1 is distinguished as the stretching vibration of the Ni–O–H stretching mode of Ni(OH)2.26 Notably not even a weak absorption around 1700 cm−1 for CN was observed, probably owing to the low amount of incorporated carboxyl groups,9 consistent with XPS analyses.
The morphologies of the products were observed using TEM and SEM. Fig. 2a,b reveal that individual nano-Ni(OH)2 nanosheets have a typical size of less than 1 μm with obvious microscopic roughening instead of perfectly flat sheets. Wrinkles or deformations can be extensively observed in these pseudo 2D sheets. High resolution TEM (HRTEM) results suggest that the crystallinity of Ni(OH)2 is not very high (not shown). Fig. 3a reveals the Ni(OH)2 material consists of randomly aggregated, crumpled sheets closely associated with each other and forming a disordered solid. Since the core of the particle is often inactive due to diffusion barriers, the aggregation which occurs in preparation process may result in limited utilization efficiency of the active material. Therefore, individual nano-Ni(OH)2 would demonstrate a moderate electrochemical activity. It is anticipated that if the restacking of the nanostructured nickel electrode can be effectively inhibited, which offers a smaller crystalline size to facilitate fast charging and discharging because the required ionic transport distance is reduced, the overall system may show superior electrochemical performances.
 | ||
| Fig. 2 TEM images of (a–b) nano-Ni(OH)2 and (c–f) CN. | ||
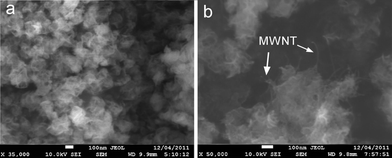 | ||
| Fig. 3 SEM images of (a) nano-Ni(OH)2 and (b) CN. | ||
Fortunately, it is worth noting that when we used this same method to synthesize Ni(OH)2 in the presence of MWNTs, smooth and micro-scale Ni(OH)2 nanosheets were obtained (Fig. 2c–f and Fig. 3b). The Ni(OH)2 sheets is electron transparent (as expected) with some wrinkles visible in the micrographs. Comparing the bright- and dark-field TEM images, the brinks and edges of the sheets can be clearly distinguished from the background. It should be pointed out that in our system without any surfactants, the relative flattening and large Ni(OH)2 sheets were obtained, while they were hardly found in the absence of carbon nanotubes. Analogous to our recent reports regarding the graphene–Cu2O system27 and taking into account the fact that the MWNTs and α-Ni(OH)2 obtained here are both hexagonal phase (as mentioned in the XRD analyses), we assume that in this system the carbon tubes may play a role as template and nucleation centers to make Ni(OH)2 crystals grow along certain directions.
In addition, Fig. 2c,d clearly indicates Ni(OH)2 sheets have been successfully entrusted with tube structural MWNTs. The content of the MWNTs in the composites seems relative low, in good agreement with the calculations (the mass ratios of MWNTs/Ni(OH)2 is ∼6.27%; the mass of Ni(OH)2 is calculated by Ni(NO3)2·6H2O according to the reaction: Ni(NO3)2 + 2H2O → Ni(OH)2 + 2HNO3). Moreover, the optical pictures of the as-obtained powder samples and their dispersions (0.5 mg mL−1) highlighted the stable colloidal nature of the CN dispersion (not shown), which enables the possibility of using this soft oxidation route to create new CNT-based materials and devices. The apparent color changes from yellow of nano-Ni(OH)2 to grey of CN for both their dispersions and powders, suggesting MWNTs have been well distributed into the Ni(OH)2 matrix to form a homogeneous composite material. So it is safe to say that the synthesized Ni(OH)2–MWNTs composite is a nanostructure, with the Ni(OH)2 sheets separated by MWNTs. In such a structure, the “pore” in the composites is actually the gap between two neighboring Ni(OH)2 sheets. Such a structure can obviously prevent the stacking of Ni(OH)2 due to van der Waals interactions, resulting in a large surface area and rich porous structure available for energy storage. On the other hand, the presence of low defect density MWNTs with high electrical conductivity may be favorable to improve the electrical conductivity of the composite electrode.
On the basis of the above experimental results, a possible formation process of CN can be proposed. Initially, bulk MWNTs were subjected to ultrasonication in NMP to give an exfoliated MWNT dispersion, followed by the in situoxidation process. After being transferred into a Teflon-lined stainless steel autoclave and heated at 180 °C, the hydrolysis of Ni(NO3)2 in aqueous solution commences in the presence of weak alkaline NMP, yielding Ni(OH)2, H+ and NO3−. The as-produced species of NO3− and H+ can react with carbon atoms of MWNTs, forming oxygen-containing functional groups, such as C–OH, C–O–C, and HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O etc. on the surfaces of MWNTs. It is worth mentioning that the concentration of H+ from hydrolysis is much lower than that of traditional oxidation procedures, thus our route is considered to be much softer.
O etc. on the surfaces of MWNTs. It is worth mentioning that the concentration of H+ from hydrolysis is much lower than that of traditional oxidation procedures, thus our route is considered to be much softer.
Significantly, the as-produced negatively charged oxygen-containing functional groups can then interact with free Ni2+via electrostatic forces or coordination bonds. The D/G peak intensity ratio of MWNTs Raman spectra in Fig. 1b is relatively high, implying that there are some structural defects created on the surface of pristine MWNTs.21,22 Thus it is possible that both chemisorption and van der Waals interactions between Ni2+, Ni(OH)2 and MWNTs exist, at oxygen-containing defect sites and pristine regions of MWNTs, respectively.
It should be noted that these nucleation centers can serve as the anchor sites. Considering both MWNTs and Ni(OH)2 are hexagonal phase, it possible for CNTs to play a role as templates for the growth of Ni(OH)2 crystals along certain directions. According to XRD results, individual nano-Ni(OH)2 has unobvious growth directions, while Ni(OH)2 sheets in CN show a predominant (001) facet. This fact might suggest that MWNTs could promote Ni(OH)2 to grow along the (110) direction. In addition, controlled experiments suggest that some reaction parameters, including the concentrations of Ni(NO3)2 aqueous solution, the double-solvent system and temperature exert a vital influence on the formation of Ni(OH)2 nanosheets.
In addition, the density of the as-produced functional groups on MWNTs can be effectively controlled by varying the initial concentration of Ni(NO3)2, thereby opening up the possibility for tailoring the oxidation degree of CNTs just in one step. Given that these oxygen-containing groups are promising anchor sites for different functional nanoparticles, this work makes sense for achieving the targets of manipulating CNTs-based functional material at the nanoscope. Further study on the growth mechanism is still in progress.
Increasing concerns over environmental issues such as climate change and fossil fuel depletion, have put pressure on the development of high-performance electrical energy storage devices to become more environmentally responsible.28 A supercapacitor is a novel energy storage device that can attain greater energy density than those of conventional capacitors and greater power density than batteries; this has kindled considerable interests of both scientific and technological researchers in the field of energy storage.29 Extensive research has been conducted to obtain fundamentally new materials, technologies for the design and manufacture of electrochemical supercapacitors.29,30
To explore the potential applications, the as-obtained samples were fabricated into electrodes and subjected to cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge–discharge measurements. Typical CV responses of CN, MWNTs and Ni(OH)2 at 5 mV s−1 are shown in Fig. 4a,b. A pair of voltammetric peaks in the CV plots of Ni(OH)2 correspond to the redox reactions of Ni(OH)2 + OH− ↔ NiO(OH) + H2O + e−.19 The two redox peaks in the CVs of MWNTs probably ascribe to the redox reaction of electrolyte ions with the residual functional groups and structural defects in the MWNTs matrix.31 With the introduction of MWNTs into Ni(OH)2 nanosheets, the peaks of MWNTs and Ni(OH)2 are blended with each other; only two well-resolved redox peaks are observable for CN. The strong chemical bonds between MWNTs and Ni(OH)2 in the composite is supposed to be responsible for this phenomenon. Moreover, because the specific capacitance (Cs) is proportional to the average areas of CVs,32 integration over the full range of CV curves was conducted to determine the average value. The specific capacitance (Cs) from the CVs was calculated based on the following equation,
 | ||
| Fig. 4 (a–b) CVs and calculated specific capacitance of CN, MWNTs and Ni(OH)2; (c–d) discharge curves and calculated specific capacitance of CN at different current densities; (e) cycle life of Ni(OH)2 and CN at 5 A g−1; (f) EIS spectrum of CN. | ||
The galvanostatic charge–discharge curves of CN are shown in Fig. 4c. The Cs is calculated according to Cs = I/[m(dV/dt)] from the discharge curves, where I is the constant discharge current, m is the mass of these samples, and dV/dt can be obtained from the slope of the discharge curve given by the instrument. The composite of the Ni(OH)2 nanosheets doped with MWNTs shows a specific capacitance as high as ∼1244.2 F g−1 (based on total sample mass) at a charge and discharge current density of 5 A g−1. The specific capacitance is still as high as 771.3 F g−1 even at a high charge and discharge current density of 20 A g−1. Consistently excellent retention of specific capacitance of CN over a wide range of current densities suggests this material is promising as a supercapacitor electrode. Additionally, it is worthwhile mentioning that the Cs value of CN (1244.2 F g−1) is significantly higher than the traditional supercapacitor electrode materials (like RuO2, 720 F g−1),34 and β-Ni(OH)2/graphene hybrids (935 F g−1)35etc., implying good electrochemical capacitance behaviors of the as-prepared nanocomposite.
It is known that the quasi-reversible electronic-transfer reaction offers Ni(OH)2 a high theoretical Cs value. However, aggregation usually occurs via van der Waals interactions in the preparation process which leads to a lower utilization of Ni(OH)2 active material. Herein, the introduction of MWNTs into Ni(OH)2 is highlighted as an effective pathway for inhibiting the stacking of Ni(OH)2 nanosheets. The integration of Ni(OH)2 and MWNTs on one hand gives a larger surface area and porous structures, convenient for the insertion/deinsertion of ions from electrolyte to electrode; on the other hand, the excellent conductivity of low defect density MWNTs is beneficial to improve the electrical conductivity of hybrid electrodes, thereby resulting in an dramatic increment of the specific capacitance.
Furthermore, the electrochemical stability of CN and nano Ni(OH)2 is investigated at 5 A g−1 (Fig. 4e). It is found that the CN electrode retained about 51.8% of initial capacitance after 1000 cycles, while that of the nano-Ni(OH)2 retained only about 38.8%. The relative poor electrochemical stability is attributable to phase transformation after cycling in alkali solution.18 Moreover, the discrepant electrochemical stability between CN and nano-Ni(OH)2 may be attributable to the different double-layer and pseudo-capacitive contributions. As well known, the double-layer process only involves a charge rearrangement, while pseudo-capacitive is related to a chemical reaction, and the double-layer capacitors have a better electrochemical stability but lower Cs as compared with those of pseudocapacitators.36 Accordingly, the as-synthesized CN, making more double-layer contribution compared with that of nano-Ni(OH)2 owning to the effect of MWNTs, have a higher electrochemical stability. It should be noted that the stability of Ni(OH)2 in alkali solution can be improved by doping of some elements like Co, Pb, etc.,37 therefore, our synthesized composites are still valuable for practical use.
To understand the electrochemical conductivity behavior of the as-prepared sample, the EIS data of CN was analyzed using Nyquist plots. Nyquist plots show the frequency response of the electrode/electrolyte system and are a plot of the imaginary component (Z′′) of the impedance against the real component (Z′).38 Among all the evaluation parameters, CN shows a comparative value than that of pure Ni(OH)2 material (the EIS data of pure Ni(OH)2 is not shown here). Notably the low equivalent series resistance (ESR) value of 0.92 Ω, as determined by the intersection of the curve at real part Z′ in the high frequency range, makes CN promising as a supercapacitor electrode.
It is worth mentioning that this methodology is readily adaptable for fabricating a series of low defected density CNT based functional materials. Moreover, given that graphene can be considered as an unrolled CNT, we replace CNTs with graphene; accordingly some low defect density graphene-based nanocomposites were dramatically accomplished. In view of the unique structure and exceptional physicochemical properties of conductive CNTs and graphene, there may be some distinctive characters of the as-prepared products, which will give those materials more intriguing applications.
Experimental
Synthesis of carbon nanotubes–Ni(OH)2 nanocomposites
The multi-walled carbon nanotubes (MWNTs, purity: >95%; outer diameter: 30–50 nm; length: 5–15 μm) used in this study is commercially purchased from Jiangsu fullerene and new carbon materials Co. Ltd., China. The MWNTs were prepared by a catalytic chemical vapor deposition technique using ethylene and Ni particle as the carbon precursor and catalyst, respectively. MWNTs were dispersed in NMP at a starting concentration of 0.04 mg mL−1 with ultrasonication for 0.5 h.39 The as-obtained MWNTs dispersion (100 mL) was vigorously stirred, where the Ni(NO3)2 aqueous solution (200 mg of Ni(NO3)2·6H2O dissolved in deionized water (5 mL) was added. The as-produced mixture was loaded into a Teflon-lined stainless steel autoclave and heated at 180 °C for 12 h. After being cooled to room temperature, the product was collected, washed, and finally dried at 60 °C. For reliable comparison, pure Ni(OH)2 was prepared in the absence of MWNTs via a similar procedure.Characterization
Powder X-ray diffraction (XRD) analyses were performed on a Bruker D8 Advance diffractometer with Cu Kα radiation (λ ≈ 1.54 Å). Raman spectra were run on a Renishaw Raman microscope. FT-IR spectra of KBr powder pressed pellets were recorded on a Bruker VECTOR 22 spectrometer. X-ray photoelectron spectra (XPS) were recorded on a Perkin-Elmer PHI5000C X-ray photoelectron spectrometer, using Mg Kα (hυ = 1253.6 eV) X-ray as the excitation source. Morphologies of the as-obtained products were observed on a transmission electron microscope (TEM, JEOL JEM-2100). SEM images were obtained using a JEOL JSM 7001F scanning electron microscope.Electrochemical Measurement
The electrochemical properties of the as-obtained products were investigated under a three-electrode cell configuration at room temperature. The working electrodes were fabricated by mixing the prepared powders with 15 wt% acetylene black and 5 wt% polytetrafluorene-ethylene (PTFE) binder. A small amount of DI-water was added to the mixture to produce a homogeneous paste. The mixture was pressed onto nickel foam current-collectors (1.0 cm × 1.0 cm) to make electrodes. The mass of the active material was in a range of 2.3–5.4 mg. Before electrochemical testing, the prepared electrode was soaked overnight in a 6 M KOH solution. Electrochemical characterization was carried out in a conventional three-electrode cell with 6 M KOH aqueous solution as the electrolyte. Platinum foil and a saturated calomel electrode (SCE) were used as the counter and reference electrodes, respectively. The scan rate of CV was 5 mV s−1. EIS was recorded under the following conditions: AC voltage amplitude 5 mV, frequency ranges 105 to 0.1 Hz, and open circuit. Galvanostatic charge–discharge testing was done from −0.15 to 0.35 V. All measurements were conducted on a CHI 660C electrochemical workstation (Shanghai CH Instrument Company, China).Conclusions
Collectively, a facile in situoxidation methodology was proposed to fabricate a composite of low defect density MWNT doped Ni(OH)2 nanosheets. The formation of the nanocomposites does not require cumbersome and costly surface modification of CNTs in advance. The hydrolysis of nickel nitrate precursor in the double-solvent system plays a vital role in the whole process. The electrochemical performance of Ni(OH)2 is substantially improved upon after introduction of MWNTs. In view of the easy synthesis and facile processability of this procedure, this approach is readily available in a wide range of CNT-based functional materials.Acknowledgements
This investigation was supported by the Natural Science Foundation of China (50902070), the Natural Science Foundation of Jiangsu province (No. BK2009391), the Research Fund for the Doctoral Program of Higher Education of China (No. 20093219120011), and NUST Research Funding, NO. ZDJH07.References
- M. A. Correa-Duarte and L. M. Liz-Marzán, J. Mater. Chem., 2006, 16, 22–25 RSC.
- E. S. Snow, Science, 2005, 307, 1942–1945 CrossRef CAS.
- R. Singh, T. Premkumar, J. Y. Shin and K. Geckeler, Chem.–Eur. J., 2010, 16, 1728–1743 CrossRef CAS.
- Y. Lin, X. Cui, C. H. Yen and C. M. Wai, Langmuir, 2005, 21, 11474–11479 CrossRef CAS.
- K. Gong, P. Yu, L. Su, S. Xiong and L. Mao, J. Phys. Chem. C, 2007, 111, 1882–1887 CAS.
- B. Jia and L. Gao, J. Phys. Chem. B, 2007, 111, 5337–5343 CrossRef CAS.
- Z. Sun, X. Zhang, N. Na, Z. Liu, B. Han and G. An, J. Phys. Chem. B, 2006, 110, 13410–13414 CrossRef CAS.
- Q. Zhang, M. Zhu, Q. Zhang, Y. Li and H. Wang, J. Phys. Chem. C, 2009, 113, 15538–15543 CAS.
- V. Bedekar, M. Murayama, R. L. Mahajan and S. Priya, J. Am. Ceram. Soc., 2010, 93, 3618–3623 CrossRef CAS.
- S.-M. Yoon, S. J. Kim, H.-J. Shin, A. Benayad, S. J. Choi, K. K. Kim, S. M. Kim, Y. J. Park, G. Kim, J.-Y. Choi and Y. H. Lee, J. Am. Chem. Soc., 2008, 130, 2610–2616 CrossRef CAS.
- B. C. Satishkumar, J. Phys. D: Appl. Phys., 1996, 29, 3173 CrossRef CAS.
- I. Dumitrescu, N. R. Wilson and J. V. Macpherson, J. Phys. Chem. C, 2007, 111, 12944–12953 CAS.
- L. B. Casabianca, M. A. Shaibat, W. W. Cai, S. Park, R. Piner, R. S. Ruoff and Y. Ishii, J. Am. Chem. Soc., 2010, 132, 5672–5676 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217–224 CrossRef CAS.
- Y. Miyata, Y. Maniwa and H. Kataura, J. Phys. Chem. B, 2006, 110, 25–29 CrossRef CAS.
- S. Ida, D. Shiga, M. Koinuma and Y. Matsumoto, J. Am. Chem. Soc., 2008, 130, 14038–14039 CrossRef CAS.
- G. Hu, C. Li and H. Gong, J. Power Sources, 2010, 195, 6977–6981 CrossRef CAS.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS.
- S. Nohara, T. Asahina, H. Wada, N. Furukawa, H. Inoue, N. Sugoh, H. Iwasaki and C. Iwakura, J. Power Sources, 2006, 157, 605–609 CrossRef CAS.
- S. Alwarappan, A. Erdem, C. Liu and C.-Z. Li, J. Phys. Chem. C, 2009, 113, 8853–8857 CAS.
- H. Wang, J. T. Robinson, X. Li and H. Dai, J. Am. Chem. Soc., 2009, 131, 9910–9911 CrossRef CAS.
- T. Bordjiba, M. Mohamedi and L. H. Dao, J. Power Sources, 2007, 172, 991–998 CrossRef CAS.
- C. G. Salzmann, S. A. Llewellyn, G. Tobias, M. A. H. Ward, Y. Huh and M. L. H. Green, Adv. Mater., 2007, 19, 883–887 CrossRef CAS.
- X. Zhang, T. V. Sreekumar, T. Liu and S. Kumar, J. Phys. Chem. B, 2004, 108, 16435–16440 CrossRef CAS.
- Y. L. Zhaoa, J. M. Wanga, H. Chena, T. Pana, J. Q. Zhanga and C. N. Caoa, Int. J. Hydrogen Energy, 2004, 29, 889–896 CrossRef.
- C. Xu, X. Wang, L. Yang and Y. Wu, J. Solid State Chem., 2009, 182, 2486–2490 CrossRef CAS.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294–1301 CAS.
- F.-L. Zheng, G.-R. Li, Y.-N. Ou, Z.-L. Wang, C.-Y. Su and Y.-X. Tong, Chem. Commun., 2010, 46, 5021 RSC.
- S. Chen, J. Zhu and X. Wang, J. Phys. Chem. C, 2010, 114, 11829–11834 CAS.
- W. Choi, S. Choi, B. Hong, D. Lim, K. Yang and J. Lee, Mater. Sci. Eng., C, 2006, 26, 1211–1214 CrossRef CAS.
- V. Srinivasan and J. W. Weidner, J. Power Sources, 2002, 108, 15–20 CrossRef CAS.
- Z.-S. Wu, D.-W. Wang, W. Ren, J. Zhao, G. Zhou, F. Li and H.-M. Cheng, Adv. Funct. Mater., 2010, 20, 3595–3602 CrossRef CAS.
- J. P. Zheng, P. J. Cygan and T. R. Jow, J. Electrochem. Soc., 1995, 142, 2699–2703 CrossRef CAS.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS.
- C.-C. Hu and C.-Y. Cheng, Electrochem. Solid-State Lett., 2002, 5, A43–A46 CrossRef CAS.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS.
- J. N. Coleman, Adv. Funct. Mater., 2009, 19, 3680–3695 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |

