Target-induced biomolecular release for sensitive aptamer-based electrochemical detection of small molecules from magnetic graphene†
Dianping
Tang
*,
Juan
Tang
,
Qunfang
Li
,
Bingqian
Liu
,
Huanghao
Yang
and
Guonan
Chen
Laboratory of Analysis and Detection of Food Safety (Ministry of Education and Fujian Province), Department of Chemistry, Fuzhou University, Fuzhou, 350108, China. E-mail: dianping.tang@fzu.edu.cn; Fax: +86 591 22866135; Tel: +86 591 22866125
First published on 26th July 2011
Abstract
A novel magneto-controlled electrochemical sensing strategy for simple, sensitive and rapid determination of small molecules was designed by using target-induced release of ferrocene-labeled aptamers from a magnetic graphene platform.
The development of assays capable of determining disease-related biomolecules in a sensitive, rapid and simple fashion has become a major analytical tool in clinical diagnosis, drug delivery and gene expression profiling.1 Despite many advances in this field, it is still a challenge to explore new approaches and strategies for the improvement of the sensitivity, simplification and feasibility of assays. Magneto-controlled molecular electronics and bioelectronics have been new topics on monitoring the biomolecules with the aid of external magnetic field based on the electronics signal of functional magnetic beads associated with probes.2 Attraction of the functional magnetic beads to the probe with an external magnet activates the electrical contact between the immobilized proteins and the base electrode, and the sensor’s circuit is switched on. Positioning the magnet above the cell retracts the magnetic beads from the probe, and the electronics behaviors of the functional magnetic particles is switched off.3 Magnetic sorting protein assay systems with various throughputs have been built and employed for the determination of biomolecules in flowing fluids by using an external magnet.4
Carbon nanostructures provide a broad survey of numerous carbon-based nanomaterials in the context of commercially available nanoscales as well as emerging technologies and future applications in the fields of molecular electronics, nano- and micro electromechanic devices.5Graphene, a one-atom thick and two-dimensional sheet of carbon atoms arranged in a honeycomb-like structure, has captured worldwide interest because of its attractive electronic properties, such as high electrical conductivity, high surface-to-volume ratio, high electron transfer rate, and exceptional thermal stability.5 Recently, our research works showed that graphene nanosheets could strongly bind single stranded DNA, such as aptamers, as a result of hydrophobic and π-stacking interactions between the nucleobases and graphene.6 The interaction could protect the release of aptamers from the surface of graphene nanosheets. Upon the addition of the target, however, the fluorophore-labeled aptamers formed a stable and rigid structure, and released from the graphene surface, thus resulting in the signal change.6 We reasoned that if such assays could be extended to detect species other than fluorescence assay, it might be generally useful for other types of highly sensitive and selective assays. Electrochemistry holds great potential as the next-generation detection strategy because of its high sensitivity, simple instrumentation, and excellent compatibility with miniaturization technologies.7 Up to date, however, there is still no report focusing on the electrochemical detection of biomolecules based on the direct interaction between the low-cost graphene and biomolecules. Herein we synthesized magnetic graphene nanosheets (MGP), which were used for sensitive electrochemical detection of biomolecules with a flow-through system based on the target-induced release of the ferrocene-labeled aptamers (as signal tags) from the MGP (see ESI† for experimental details).
Prior to experiments, MGP was synthesized according to the literature with a small modification.8 Firstly, two solutions were prepared as follows: solution (A) including 200 mg NaOH and 20 mL diethylene glycol (DEG) was initially heated for 1 h at 120 °C under N2, and then cooled down to 70 °C; solution (B) including 15 mg graphene oxide, 60 mg FeCl3, and 10 mL DEG was heated for 30 min at 220 °C under N2. Following that, 5 mL solution (A) was rapidly added into the solution (B), and further heated for another 1 h. Finally, magnetic graphene nanosheets (MGP, ∼40 mg) were obtained by centrifugation and washed with ethanol several times. The synthesized MGP was characterized by using TEM and XPS techniques (see ESI† in Fig. S1 and S2).
To realize our design, we fabricated a MGP-based sensing platform for the detection of adenosine triphosphate (ATP, as an example). Fig. 1 shows a schematic representation of the magneto-controlled graphene sensing platform. Due to the strong noncovalent binding of MGP with nucleobases and aromatic compounds, ferrocene-labeled aptamers (Fc-P0, 5'-Fc-(CH2)3-ACCTG GGGGA GTATT GCGGA GGAAG GT-3') are initially bound onto the surface of MGP. With an external magnet, the Fc-P0-MGP is attached onto the gold substrate, which exhibited a strong electrochemical signal due to the presence of ferrocene (Fig. 1a). In the presence of a target, the binding between the Fc-P0 and the target can alter the conformation of Fc-P0, and disturb the interaction between the Fc-P0 and MGP. Such interactions enable the release of the Fc-P0 from the MGP (Fig. 1b), resulting in the decrease of the electrochemical signal. The decrease in the current depends on the concentration of ATP in the sample.
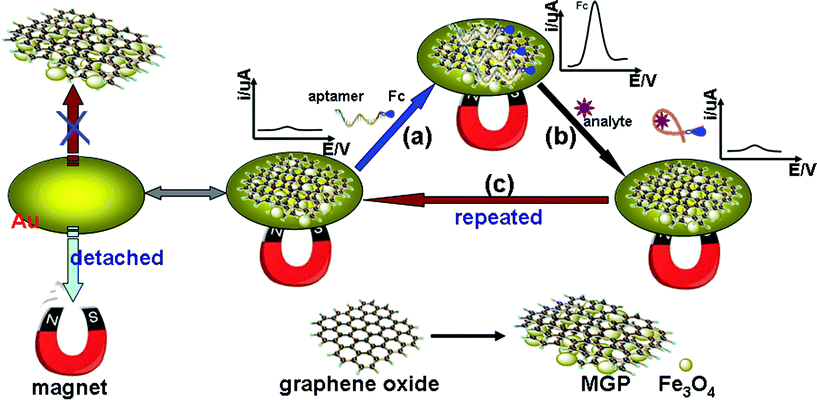 | ||
| Fig. 1 Schematic illustration of magneto-controlled graphene sensing platform for aptamer-based electronic detection of small molecules. | ||
First, we used quartz crystal microbalance (QCM) to investigate the validity of the method. Measurements were carried out at the flow. Fig. 2a shows the QCM frequency response of the gold substrate toward the immobilized process of MGP with an external magnet. Upon addition of Fc-P0 (50 μL, C[MGP] = 10 mg/mL) into the detection cell, the QCM frequency increased, and then leveled off (Fig. 2b). Significantly, the shifts in frequency increased with the increase of Fc-P0 concentrations. The results indicated that Fc-P0 could be bound onto the MGP. Importantly, we also observed that the QCM frequency decreased upon the injection of excess ATP target (200 μM) (Fig. 2c). The decrease in frequency was mainly attributed to the release of Fc-P0 from the MGP. Moreover, when the excess ATP was flowed through the cell, the frequency was almost restored to the value of MGP. These results indicated that magneto-controlled graphene platform could be preliminarily utilized for the detection of ATP by using target-induced release of the aptamers from the MGP.
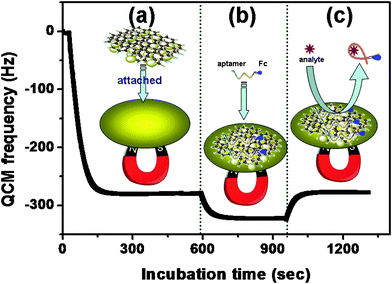 | ||
| Fig. 2 QCM responses of the magneto-controlled sensing platform on the gold substrate: (a) MGP attachment, (b) Fc-P0 binding and (c) target-induced release of aptamer. | ||
Next, the dynamic behaviors of the Fc-P0 and MGP, as well as of the Fc-P0-MGP complex with the target, were on-line monitored in 0.1 M pH 7.4 phosphate buffered saline (PBS) solution by using a typical chronoamperometric method with a conventional three-electrode system in the sequential injection mode. Fig. 3a shows the amperometric response of the MGP-modified gold electrode in the presence of Fc-P0 as a function of the incubation time. The binding of Fc-P0 on the MGP was very fast at RT, and reached equilibrium within 5 min. The increase in the current mainly derived from the Fc-labeled aptamers. The labeled ferrocene could act as an electron mediator, and improved the electrochemical behaviors of the modified electrode. When the ATP target was injected into the cell, however, the amperometric response decreased with the increment of incubation time (Fig. 3b). The reason might be attributed to the target-induced release of the Fc-P0 aptamers from the MGP (i.e. the reaction of the aptamers with the targets). The release was relatively slow. To further elucidate the advantage of the labeled ferrocene, the aptamers without the ferrocene were also studied as control test. The control test consisted of the binding and release of the aptamers from the MGP all alike. As shown in Fig. 3c, no significant shifts in the current were observed. The result indicated that ferrocene could be used as signal tags for the detection of the target in the system. In addition, we also investigated the non-specific adsorption of the gold substrate without the MGP modification toward Fc-P0 and the targets. No significant non-specific adsorption was acquired.
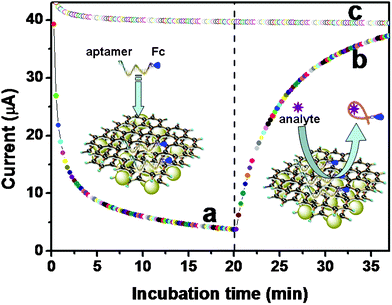 | ||
| Fig. 3 Chronoamperometric responses of (a) the binding of Fc-P0 with the MGP, (b) target-induced release of Fc-P0 from the MGP, and (c) the binding and release of the aptamer without the labeled Fc from the MGP in pH 7.4 PBS. | ||
Following that, a square wave voltammetric (SWV) measurement was employed for the determination of ATP based on target-induced release of Fc-P0 from the MGP with the labeled ferrocene as tracer. As seen from Fig. 4, the SWV peak currents decreased with the increase of ATP concentrations in the sample in pH 7.4 PBS. The calibration plots exhibited a good linear relationship between the peak currents and ATP concentrations in the range from 1.0 nM to 300 μM with a correlation coefficient of 0.9931 (inset of Fig. 4, n = 27). The detection limit (LOD) was 0.1 nM estimated at 3sB, which was greatly lower than that of our previous resport.6 Although the system has not yet been optimized for maximum efficiency, the assay sensitivity was 2-5 order-of-magnitude higher than those of target-induced displacement or conformational changes of aptamers.9 The precision of the assay was evaluated by calculating the intra- and inter-batch variation coefficients (CVs). Experimental results indicated that the CVs of the assays with the same batch MGP were 8.4%, 9.1%, and 6.3% at the 5 nM, 1.0 μM and 200 μM ATP, respectively, while the CVs of the assays using the MGP with different batches were 5.4%, 7.9%, and 6.5% at the above-mentioned analyte concentrations. The MGP exhibited satisfactory stability. In fact, as much as 90% of the initial amperometric response was preserved after storage of the MGP in PBS for 41 days.
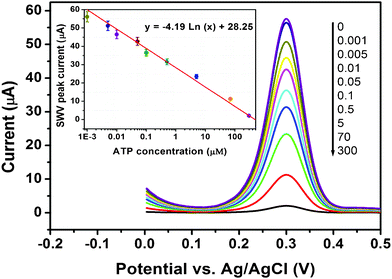 | ||
| Fig. 4 SWV responses of magneto-controlled sensing platform toward ATP standards in pH 7.4 PBS (Inset: calibration curve). | ||
To evaluate the specificity of the strategy, we challenged the system with several ATP analogues: cytosine triphosphate (CTP), guanidine triphosphate (GTP) and uridine triphosphate (UTP). Significantly, higher current changes were observed with the target ATP than those of its analogues (Fig. 5). These results clearly verified the high specificity of the magneto-controlled graphene sensing platform. Importantly, the MGP could be used for the repeated detection of ATP through the re-binding of Fc-P0 aptamer after it was released.
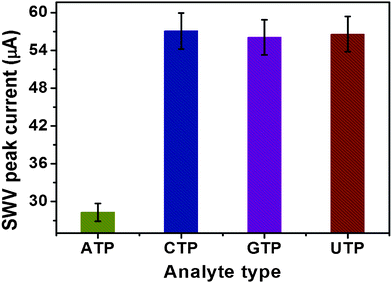 | ||
| Fig. 5 SWV peak current changes of the magneto-controlled sensing platform against ATP and its analogues (1.0 μM as examples). | ||
To further illustrate the universality of the method, the synthesized MGP were applied for the detection of small molecules (cocaine as an example). Ferrocene molecules were linked to the cocaine aptamer (5'-Fc-(CH2)3-GACAA GGAAA ATCCT TCAAT GAAGT GGGTC-3'). As seen from Fig. S3 (see ESI†), the method could likewise display a low LOD of 0.1 nM toward cocaine in comparison with other strategies.9b,10
In summary, we for the first time demonstrate the ability of magnetic graphene nanosheets as a sensing platform for the direct electronic detection of small molecules in this work. Compared with other strategies, the method is sensitive, rapid, simple and reusable. Significantly, the assay approach does not require sophisticated fabrication, and is well suited for high-throughput biomedical sensing and application in both clinical and biodefense areas.
Support by the NSFC of China (21075019, 20877019), the National “973” Basic Research Program (2010CB732403) and the Research Fund for the Doctoral Program of Higher Education of China (20103514120003) is gratefully acknowledged.
References
- (a) N. Maillard, P. Babiak, S. Syed, R. Biswas, L. Mandrich, G. Manco and J. Reymond, Anal. Chem., 2011, 83, 1437 CrossRef CAS; (b) V. Fluxa, N. Maillard, M. Page and J. Reymond, Chem. Commun., 2011, 47, 1434 RSC; (c) M. Swiderska and J. Reymond, Nat. Chem., 2009, 1, 527 CrossRef CAS.
- (a) I. Willner and E. Katz, Angew. Chem., Int. Ed., 2003, 42, 4576 CrossRef CAS; (b) Y. Ling, T. Pong, C. Vassiliou, P. Huang and M. Cima, Nat. Biotechnol., 2011, 29, 273 CrossRef CAS; (c) A. Barb and J. Prestegard, Nat. Chem. Biol., 2011, 7, 147 CrossRef CAS.
- (a) A. Rodriguez-Villarreal, M. Tarn, L. Madden, J. Lutz, J. Greenman, J. Samitier and N. Pamme, Lab Chip, 2011, 11, 1240 RSC; (b) B. Xu, X. Lin, Z. He, Z. Lin and R. Cao, Chem. Commun., 2011, 47, 3766 RSC.
- (a) H. Lee, J. Jung, S. Han and K. Han, Lab Chip, 2010, 10, 2764 RSC; (b) P. Car, M. Perfetti, M. Mannini, A. Favre, A. Caneschi and R. Sessoli, Chem. Commun., 2011, 47, 3751 RSC.
- (a) C. Shen, A. Brozena and Y. Wang, Nanoscale, 2011, 3, 503 RSC; (b) R. Leary and A. Westwood, Carbon, 2011, 49, 741 CrossRef CAS; (c) M. Cracium, S. Russo, M. Yamamoto and S. Tarucha, Nano Today, 2011, 6, 42 CrossRef; (d) M. Pumera, Chem. Soc. Rev., 2010, 39, 4146 RSC; (e) M. Pumera, Chem. Rev., 2009, 9, 211 CAS; (f) M. Pumera, A. Ambrosi, A. Bonanni, E. Chng and H. Poh, TrAC, Trends Anal. Chem., 2010, 29, 954 CrossRef CAS.
- (a) C. Lu, J. Li, M. Lin, Y. Wang, H. Yang, X. Chen and G. Chen, Angew. Chem., Int. Ed., 2010, 49, 8454 CrossRef CAS; (b) C. Lu, H. Yang, C. Zhu, X. Chen and G. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785 CrossRef CAS; (c) C. Zhu, C. Lu, X. Song, H. Yang and X. Wang, J. Am. Chem. Soc., 2011, 133, 1278 CrossRef CAS.
- (a) D. Tang, B. Su, J. Tang, J. Ren and G. Chen, Anal. Chem., 2010, 82, 1572; (b) D. Tang, J. Tang, B. Su and G. Chen, J. Agric. Food Chem., 2010, 58, 10824 CrossRef CAS; (c) D. Tang and J. Ren, Anal. Chem., 2008, 80, 8064 CrossRef CAS; (d) D. Tang, R. Yuan and Y. Chai, Anal. Chem., 2008, 80, 1582 CrossRef CAS.
- H. He and C. Gao, ACS Appl. Mater. Interfaces, 2010, 2, 3201 CrossRef CAS.
- (a) B. Das, C. Tlili, S. Badhulika, L. Cella, W. Chen and A. Mulchandani, Chem. Commun., 2011, 47, 3793 RSC; (b) Y. Du, C. Chen, M. Zhou, S. Dong and E. Wang, Anal. Chem., 2011, 83, 1532; (c) Y. Ying, H. Wang, T. Sutherland and Y. Long, Small, 2011, 7, 87 CrossRef CAS; (d) X. Zhang, Y. Zhao, S. Li and S. Zhang, Chem. Commun., 2010, 46, 9173 RSC; (e) S. Zhen, L. Chen, S. Xiao, Y. Li, P. Hu, L. Zhan, L. Peng, E. Song and C. Huang, Anal. Chem., 2010, 82, 8432 CrossRef CAS.
- (a) C. Ma, W. Wang, Q. Yang, C. Shi and L. Cao, Biosens. Bioelectron., 2011, 26, 3309 CrossRef; (b) Y. Du, B. Li, S. Guo, Z. Zhou, M. Zhou, E. Wang and S. Dong, Analyst, 2011, 136, 493 RSC; (c) B. Gulbakan, E. Yasun, M. Shukoor, M. You, X. Tan, H. Sanchez, D. Powell, H. Dai and W. Tan, J. Am. Chem. Soc., 2010, 132, 17408 CrossRef CAS; (d) B. Sun, H. Qi, F. Ma, Q. Gao, C. Zhang and W. Miao, Anal. Chem., 2010, 82, 5046 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, the characterization of the synthesized MGP, the universality of the method. See DOI: 10.1039/c1ra00114k |
| This journal is © The Royal Society of Chemistry 2011 |
