Transparent carbon nanotube patterns templated by inkjet-printed graphene oxide nanosheets†
Joong Tark
Han
*a,
Jun Suk
Kim
a,
Donghoon
Kwak
b,
Bo Gyeong
Kim
a,
Bo Hwa
Jeong
a,
Seung Yol
Jeong
a,
Hee Jin
Jeong
a,
Kilwon
Cho
b and
Geon-Woong
Lee
a
aNano Carbon Materials Research Group, Korea Electrotechnology Research Institute, 70 Boolmosangil, Changwon, Korea 641-120. Fax: 82-55-280-1590; Tel: 82-22-280-1677E-mail: jthan@keri.re.kr; gwleephd@keri.re.kr
bDepartment of Chemical Engineering, POSTECH, San 31, Hyojadong, Pohang, Korea 790-784
First published on 18th July 2011
Abstract
Partially reduced graphene oxide (GO) nanosheets served as interfacial adhesive layers for the fabrication of single-walled carbon nanotube (SWCNT) patterns on hydrophilic substrates.
Chemically modified graphene, which is produced through the oxidation of highly oriented pyrolytic graphite, permits fabrication of graphene-templated hybrid materials.1–13Graphene oxide (GO) nanosheets are composed of planar graphene-like aromatic domains, the basal planes and edges of which are decorated with hydroxyl, epoxy, ether, carbonyl or carboxylic groups.14–17 Thermal deoxygenation of GO nanosheets is typically achieved above 200 °C under inert or reducing environments, and the reaction efficiency increases with temperature. It should be noted that oxygen groups gradually detach from GO surfaces even at temperatures below 200 °C under vacuum.18–21 This means that the surface energy of a GO film can be exquisitely controlled by treatment at relatively low temperatures. Surface energy-modified surfaces afford precise control over the deposition of nanomaterials.
Single-walled carbon nanotubes (SWCNTs) provide electrode materials with excellent conductivity and mechanical properties.22–25 Researchers have tried to produce SWCNT patterns by contact printing, plasma etching, photolithographic methods, and inkjet printing.28–35 Inkjet printing processes usually use functionalized SWCNTs and SWCNTs hybridized with conducting polymers. Pristine SWCNTs produce highly conductive patterns. Until recently, highly transparent conductive films could only be prepared from SWCNTs in surfactant solutions.25 However, the SWCNT network films can be easily detached even on alkylsilane-treated SiO2 surfaces during a washing process in water as shown in Fig. 1(a). Even the SWCNT dispersion solution in organic solvents did not form uniform networks on the submicrometer scale on hydrophilic surfaces due to the high interfacial tension between SWCNTs and substrates.33 Moreover, vigorous sonication of pristine SWCNTs to form a dispersion in organic solvents damaged the nanotube surfaces, which decreased the conductivity of the nanotubes.36
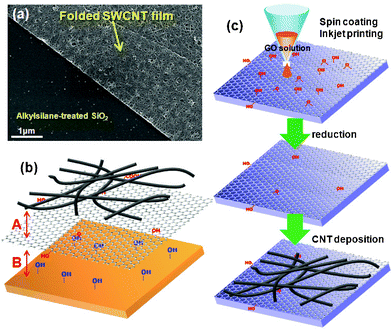 | ||
| Fig. 1 (a) Detached and folded SWCNT film on alkylsilane-treated SiO2 surface. (b) The role of GO at the interface between hydrophilic surfaces and SWCNTs. (c) Schematic representation of the graphene-templated deposition of SWCNTs on hydrophilic surfaces. | ||
Here, we introduce a straightforward method for forming uniform SWCNT network films and patterns on hydrophilic substrates by using partially reduced GO patterns as an interfacial adhesive layer, as shown in Fig. 1(b). This approach relies on the thermal reduction of inkjet-printed GO patterns under vacuum at relatively low temperatures to deposit unfunctionalized SWCNTs.
This strategy has several advantages: (i) the functional groups of the partially reduced GO rely on the interfacial adhesive properties between the substrate and the materials onto which we want to deposit; (ii) the processing techniques are scalable and straightforward; (iii) uniform SWCNT networks may be formed, even by spraying; (iv) plastic substrates are compatible with the low-temperature process; (v) the work function may be easily matched to the conducting and semiconducting materials used in optoelectronic devices.
Graphite oxide was prepared by the modified Hummer's method with pure graphite (average size is 70 μm from natural graphite powder, Alfar Aesar, 99.999% purity, −200 mesh). Graphite oxide was then exfoliated into GO nanosheets in deionized water by bath sonication for 30 min. Carboxylated graphene oxide nanosheets are easily removed in water, and it is difficult to detach carboxyl groups from the edges, even by chemical reduction, at low temperatures.1 Thus, small GO nanosheets with carboxylic acid groups were first removed by centrifuging an aqueous GO solution to efficiently deposit SWCNTs. The aqueous GO solution was centrifuged twice at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The small carboxylated GO sheets were successfully separated in the first supernatant, as determined by X-ray photoelectron spectroscopy (XPS), shown in Fig. 2(a). It was also confirmed that GO sheets in the second supernatant were mainly functionalized with epoxy and hydroxyl groups, as determined by the Fourier transform infrared (FTIR) and XPS spectra, shown in Fig. 2(c) and (d), respectively.
000 rpm for 30 min. The small carboxylated GO sheets were successfully separated in the first supernatant, as determined by X-ray photoelectron spectroscopy (XPS), shown in Fig. 2(a). It was also confirmed that GO sheets in the second supernatant were mainly functionalized with epoxy and hydroxyl groups, as determined by the Fourier transform infrared (FTIR) and XPS spectra, shown in Fig. 2(c) and (d), respectively.
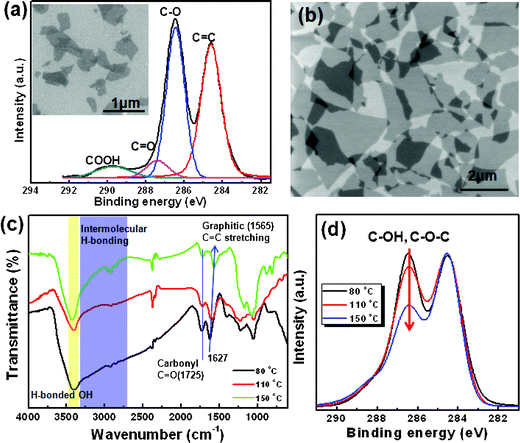 | ||
| Fig. 2 (a) XPS spectrum and SEM image (inset) of small-sized GO nanosheets separated by centrifugation. (b) SEM image of spin-coated large GO sheets on SiO2. (c),(d) FTIR and XPS spectra (C1s) of GO films after thermal treatment at 80, 110, 150 °C under vacuum, respectively. | ||
The second supernatant GO solution was subsequently used for spin-coating and inkjet printing. Prior to inkjet printing, we prepared a uniform GO film on an SiO2 substrate to characterize the surface properties and functionalities. The process of depositing CNTs onto RGO-modified surfaces is schematically described in Fig. 1(c). The SiO2 surfaces were cleaned by UV irradiation prior to deposition of GO. The large GO nanosheets were successfully deposited onto the SiO2 surfaces by spin-casting under a nitrogen gas stream, as shown in Fig. 2(b) and Fig. S1, ESI.†Spin-casting enabled the preparation of highly uniform graphene films over a large area. After deposition, GO thin films were thermally reduced under vacuum by varying the temperature from 50 °C to 150 °C for 3 h. The epoxy and hydroxyl groups on the basal plane of GO sheets, which can interact with the SiO2 surface, should remain after the thermal reduction process under vacuum. The C–O bonds in the GO nanosheets were gradually reduced after thermal treatment under vacuum, as observed in the XPS spectra (Fig. 2(d)). The gradual shift in the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond stretch (1621 cm−1) toward a graphitic C
C bond stretch (1621 cm−1) toward a graphitic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch (1565 cm−1), along with the decrease in the intermolecular OH-hydrogen bonding stretch (from 3400 to 2400 cm−1) in the FTIR spectra (Fig. 2(c) also indicated the reduction of GO at elevated temperatures under vacuum. Fig. 3(a) shows the water contact angle (WCA) on the reduced graphene oxide film as a function of the reduction temperature under vacuum. Below 100 °C, the WCA slowly increased with increasing temperature, then dramatically increased until saturation. The high WCA of slightly reduced GO films containing many oxidative groups indicated that deoxygenation occurred from the outer surface layer at low temperatures under vacuum.
C stretch (1565 cm−1), along with the decrease in the intermolecular OH-hydrogen bonding stretch (from 3400 to 2400 cm−1) in the FTIR spectra (Fig. 2(c) also indicated the reduction of GO at elevated temperatures under vacuum. Fig. 3(a) shows the water contact angle (WCA) on the reduced graphene oxide film as a function of the reduction temperature under vacuum. Below 100 °C, the WCA slowly increased with increasing temperature, then dramatically increased until saturation. The high WCA of slightly reduced GO films containing many oxidative groups indicated that deoxygenation occurred from the outer surface layer at low temperatures under vacuum.
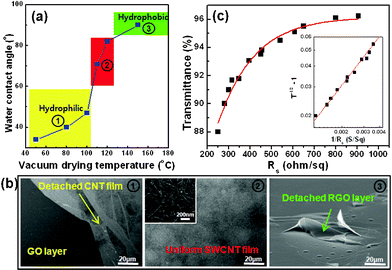 | ||
| Fig. 3 (a) Water contact angles of GO films as a function of vacuum drying temperature. (b) SEM images of SWCNT films on underlying GO layers. The numbers shown in (b) correspond to those in (a). (c) Transmittance vs. sheet resistance plot for SWCNT network films on moderately reduced GO surfaces. Inset plot: Transmittance versus sheet resistance data plotted to emphasize the goodness of fit to Eq. 1, ESI.† | ||
We next spray-coated an aqueous dispersion of the arc-SWCNTs (purchased from Nano Solutions, Korea) in a 1% sodium dodecylbenzenesulfonate solution onto GO-treated substrates, and washed with water to remove the surfactant. As shown in Fig. 3(b), moderately hydrophobic GO surfaces with 70–80° WCAs favored the formation of a uniform SWCNT film, which may be allowed by interfacial π–π interactions. However, GO surfaces treated below the critical temperature (∼110 °C) displayed detached SWCNT films during washing in water because the underlying GO film was insufficiently reduced, and a significant fraction of the carbonyl, epoxy, and hydroxy groups remained at the basal planes and edges. Furthermore, beyond the critical temperature, the underlying GO films detached from the hydrophilic surface in an aqueous solution because the hydrophobic characteristics of the rGO layer after thermal treatment at 150 °C destabilized the SWCNT layer. These results indicated that the moderately hydrophobic GO layer acted as an interfacial adhesive layer to facilitate deposition of a uniform SWCNT film on the hydrophilic substrates. The sheet resistance and transmittance of the films were found to be 250 Ω sq−1 and 88%, respectively (Fig. 3(c)). These properties corresponded to a ratio between the DC (σDC) and optical (σOP) conductivities (σDC/σOP) of 12.5, which was relatively large compared with that reported previously.22–27
We next fabricated GO patterns on UV–ozone-treated SiO2 surfaces by inkjet printing. Evaporation during the drying process played a vital role in controlling the film morphology and distribution of the GO nanosheets within the inkjet-printed films. To prepare a uniform GO layer, substrates were treated with UV–ozone to produce a hydrophilic surface, and the temperature was tuned from room temperature to 80 °C. We successfully fabricated uniform GO patterns at 70 °C using an aqueous GO solution on the SiO2 surfaces (Fig. S2, ESI†). Onto the pattern was deposited a SWCNT/surfactant solution by spraying, followed by washing with water. Fig. 4(a) shows the dot and line patterns of SWCNTs on underlying GO patterns treated at 110 °C under vacuum. Fig. 4(b) shows the macroscopic patterns on the SiO2 surfaces, and the aqueous inks on the patterned surface clearly reveal the deposition of hydrophobic SWCNTs (high WCAs on the dark area) on hydrophilic SiO2 surfaces (low WCAs on the bright area). The magnified SEM images (Fig. 4(c) and (d)) showed that the SWCNTs were selectively deposited onto the GO patterns.
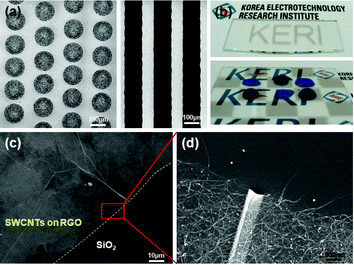 | ||
| Fig. 4 (a) Dot and line SWCNT patterns on GO patterns prepared by inkjet printing with GO dispersion solution in water. (b) Digital photo images of the macroscopic SWCNT patterns on the SiO2 surfaces: the bottom image is the angled view of aqueous ink droplets on the SWCNT patterned surface. (c) Selective deposition of the SWCNTs on moderately reduced GO layer. (d) Magnified image of the rectangular area in (c). | ||
We have demonstrated a promising route for preparing partially reduced GO films as interfacial adhesive layers for the fabrication of SWCNT patterns on hydrophilic surfaces. To this end, highly oxidized and small GO sheets were successfully removed from the initial GO solution. Uniform GO films and patterns were then fabricated by spin-coating and inkjet printing, respectively, and the surface energy of the films was modulated by thermal treatment under vacuum. We found that the SWCNTs were selectively deposited onto partially reduced GO films with moderately hydrophobic properties.
Acknowledgements
This work was supported by a grant from the Fundamental R&D Program for Core Technology of Materials funded by the Ministry of Knowledge Economy, and from KERI (grant# 10-50-H0102-04 and 11-12-N0101-45), Republic of Korea.References
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217 CrossRef CAS.
- G. Eda and M. Chhowalla, Adv. Mater., 2010, 22, 1 CrossRef.
- R. Muszynski, B. Seger and P. V. Kamat, J. Phys. Chem. C, 2008, 112, 5263 CAS.
- G. Williams, B. Seger and P. V. Kamat, ACS Nano, 2008, 2, 1487 CrossRef CAS.
- C. Xu, X. Wang and J. W. Zhu, J. Phys. Chem. C, 2008, 112, 19841 CAS.
- K. Jasuja and V. Berry, ACS Nano, 2009, 3, 2358 CrossRef CAS.
- J. M. Lee, Y. B. Pyun, J. Yi, J. W. Choung and W. I. Park, J. Phys. Chem. C, 2009, 113, 19134 CAS.
- G. Lu, S. Mao, S. Park, R. S. Ruoff and J. Chen, Nano Res., 2009, 2, 192 CrossRef CAS.
- G. Goncalves, P. A. A. P. Marques, C. M. Granadeiro, H. I. S. Nogueira, M. K. Singh and J. Gracio, Chem. Mater., 2009, 21, 4796 CrossRef CAS.
- S. Wang, B. M. Goh, K. K. Manga, Q. Bao, P. Yang and K. P. Loh, ACS Nano, 2010, 4, 6180 CrossRef CAS.
- J. Xu, K. Wang, S.-Z. Zu, B.-H. Han and Z. Wei, ACS Nano, 2010, 4, 5019 CrossRef CAS.
- B. H. Kim, J. Y. Kim, S.-J. Jeong, J. O. Hwang, D. H. Lee, D. O. Shin, S.-Y. Choi and S. O. Kim, ACS Nano, 2010, 4, 5464 CrossRef CAS.
- X.-Z. Tang, Z. Cao, H.-B. Zhang, J. Liu and Z.-Z. Yu, Chem. Commun., 2011, 47, 3084 RSC.
- A. Lerf, H. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477 CrossRef CAS.
- L. T. Szabó and O. Berkesi, Carbon, 2005, 43, 3186 CrossRef.
- E. Fuente, J. A. Menendez, M. A. Diez, D. Suarez and M. A. Montes-Moran, J. Phys. Chem. B, 2003, 107, 6350 CrossRef CAS.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270 CrossRef CAS.
- H. Y. He, J. Klinowski, M. Forster and A. Lerf, Chem. Phys. Lett., 1998, 287, 53 CrossRef CAS.
- A. Lerf, H. Y. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477 CrossRef CAS.
- Y. Matsuo and Y. Sugie, Carbon, 1998, 36, 301 CrossRef CAS.
- Y. Matsuo, K. Tahara and Y. Sugie, Carbon, 1997, 35, 113 CrossRef CAS.
- Z. C. Wu, Z. Chen, X. Du, J. M. Logan, J. Sippel, M. Nikolou, K. Kamaras, J. R. Reynolds, D. B. Tanner, A. F. Hebard and A. G. Rinzler, Science, 2004, 305, 1273 CrossRef CAS.
- L. Hu, D. S. Hecht and G. Grüner, Nano Lett., 2004, 4, 2513 CrossRef CAS.
- D.S. Hecht, D. Thomas, L. Hu, C. Ladous, T. Lam, Y. Park, G. Irvin and P. Drzaic, J. Soc. Inf. Disp., 2009, 17, 941 CrossRef CAS.
- S. De, P. E. Lyons, S. Sorel, E. M. Doherty, P. J. King, W. J. Blau, P. N. Nirmalraj, J. J. Boland, V. Scardaci, J. Joimel and J. N. Coleman, ACS Nano, 2009, 3, 714 CrossRef CAS.
- J. T. Han, J. S. Kim, H. D. Jeong, H. J. Jeong, S. Y. Jeong and G.-W. Lee, ACS Nano, 2010, 4, 4551–4558 CrossRef CAS.
- P. J. King, U. Khan, M. Lotya, S. De and J. N. Coleman, ACS Nano, 2010, 4, 4238 CrossRef CAS.
- S. R. Lustig, E. D. Boyes, R. H. French, T. D. Gierke, M. A. Harmer, P. B. Hietpas, A. Jagota, R. S. McLean, G. P. Mitchell, G. B. Onoa and K. D. Sams, Nano Lett., 2003, 3, 1007 CrossRef CAS.
- J. Zhu, M. Yudasaka, M. Zhang, D. Kasuya and S. Iijima, Nano Lett., 2003, 3, 1239 CrossRef CAS.
- M. A. Meitl, Y. Zhou, A. Gaur, S. Jeon, M. L. Usrey, M. S. Strano and J. A. Rogers, Nano Lett., 2004, 4, 1643 CrossRef CAS.
- K. Kordás, T. Mustonen, G. Tóth, H. Jantunen, M. Lajunen, C. Soldano, S. Talapatra, S. Kar, R. Vajtai and P. M. Ajayan, Small, 2006, 2, 1021 CrossRef.
- W. R. Small and M. Panhuis, Small, 2007, 3, 1500 CrossRef CAS.
- J.-W. Song, J. Kim, Y.-H. Yoon, B.-S. Choi, J.-H. Kim and C.-S. Han, Nanotechnology, 2008, 19, 095702 CrossRef.
- T. Fujigaya, S. Haraguchi, T. Fukumaru and N. Nakashima, Adv. Mater., 2008, 20, 2151 CrossRef CAS.
- K. N. Han, C. A. Li, M.-P. N. Bui and G. H. Seong, Langmuir, 2010, 26, 598 CrossRef CAS.
- S.-H. Jeong, O.-J. Lee, K.-H. Lee, S. H. Oh and C.-G. Pakr, Chem. Mater., 2002, 14, 1859 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: experimental details, AFM image of the GO film and SEM images of GO dot patterns fabricated by inkjet printing. See DOI: 10.1039/c1ra00213a |
| This journal is © The Royal Society of Chemistry 2011 |
