Synthesis and characterization of ultralong La2O3:Eu3+ photoluminescent nanowires†
Tian
Xia
*a,
Jingping
Wang
b,
Nan
Lin
a,
Hui
Zhao
a,
Lihua
Huo
a and
Grigoris
Mountrichas
c
aKey Laboratory of Functional Inorganic Materials Chemistry, Ministry of Education, School of Chemistry, Chemical Engineering and Materials, Heilongjiang University, Harbin, 150080, P. R. China. E-mail: xiatian0621@yahoo.com.cn; Fax: 86 0451 8660 8426; Tel: 86 0451 8660 8426
bCollege of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin, 150001, P. R. China
cTheoretical and Physical Chemistry Institute, National Hellenic Research Centre, Athens, 11635, Greece
First published on 10th August 2011
Abstract
Ultralong La2O3:Eu3+ single-crystalline nanowires were successfully fabricated through a large-scale and facile composite-hydroxide-mediated method followed by a subsequent heat treatment process. The as-synthesized products were characterized by powder X-ray diffraction (PXRD), scanning electron microscopy (SEM) combined with energy dispersive X-ray spectroscopy (EDS), transmission electron microscopy (TEM), and photoluminescence spectra. The product formed via the composite-hydroxide-mediated method, La(OH)3:Eu3+, can transform into La2O3:Eu3+ with the same wire-like morphology after a postannealing process. The possible mechanistic pathways for the formation of the one-dimensional (1D) La2O3:Eu3+ superstructure have been proposed. La(OH)3:Eu3+and La2O3:Eu3+ nanowires are high-surface-area materials. Under the excitation of 283 nm ultraviolet light, La2O3:Eu3+ nanowires show a strong red emission line corresponding to 5D0 → 7F2 transition at 625 nm.
1. Introduction
The preparation of one-dimension (1D) nanomaterials has been the focus of scientific research due to their outstanding electrical, magnetic and optical properties. Many efforts have been devoted to fabricating 1D nanocrystals, such as nanowires, nanotubes, nanorods and nanobelts etc.1–6 However, the synthesis of 1D ultralong nanostructures for practical applications remains a difficult and demanding task. Recently, there are several attempts to synthesize 1D ultralong nanocrystals based on nanobelts, nanowires and nanofibres.7–14 These superstructures can open new pathways in chemistry and technology.As a well-known inorganic functional material, rare earth oxides have been extensively used in high-performance luminescent and magnetoresistance devices, catalysts and solid oxide fuel cells due to their chemical and physical characteristics arising from their unique 4f electronic shells.15–20 These properties depend not only on their chemical composition and crystal structure but also on their dimensionality, morphology and size.21,22 The use of rare earth oxides, in the form of 1D superstructures, could lead to the high quality materials because of both the shape-specific nature and the quantum effect, and can act as the functional host material as well. Although a large number of 1D rare earth oxides have been investigated,23–30 there are relatively few reports on La2O3 for application in the preparation of red-emitting phosphors for field emission displays and luminescent lamps. In addition, it is also a promising candidate for sensor applications.31 Because of these important properties, several kinds of techniques have been developed to fabricate 1D La2O3 nanomaterials, which mainly include the hydrothermal synthesis route.32–35 Although the synthesis of 1D La2O3 has been reported previously, there are only a few reports on the preparation of separated, uniform and well-defined 1D Eu3+-doped La2O3, which is one of the most important red phosphors.
From the viewpoint of application, nanomaterials should not only synthesized in a shape-controllable strategy with the desired composition, reproducible morphology, size and structure but also should be prepared using simple and environmentally friendly methodologies. Environmentally friendly synthetic methodologies that include template synthesis, molten-salt synthesis, and hydrothermal processes have gradually been implemented as effective techniques in the preparation of nanostructures. In particular, the molten-salt technique is an important method for the preparation of low-dimensional nanomaterials with the advantages of high quality, cost-efficiency and good homogeneity. The environmental advantage of this method results from the fact that neither a template nor catalyst is required. Recently, a general method, based on a reaction in a eutectic composite-hydroxide, appears to be a plausible and diverse way for producing the single-crystalline complex oxides as demonstrated by Wang and co-workers.36 This simple process has also been used to prepare lanthanum hydroxide and oxide.31
In this article, we report the preparation of ultralong La2O3:Eu3+ nanowires through a mild composite-hydroxide-mediated (CHM) technique followed by a subsequent heat treatment process. The superstructures of La2O3:Eu3+ consist of 50–100 μm long single-crystalline nanowires with a diameter of tens to more than one hundred nanometres. To the best of our knowledge, this is the first time that 1D single-crystalline rare earth oxide phosphor with superstructure have been reported.
2. Experimental
2.1 Synthesis
Chemicals: reagent-grade chemicals, La(NO3)3·6H2O (Tianjin Delan Fine Chemical Company, 99.99%), Eu(NO3)3·6H2O (Tianjin Delan Fine Chemical Company, 99.99%), NaOH and KOH (Beijing Fine Chemical Company, 99.9%) were used as received without further purification.The synthesis is performed in the following steps: in a typical procedure, La(NO3)3·6H2O (2.2 g, 5 mmol) and Eu(NO3)3·6H2O (0.117 g, 0.263 mmol) were first added into a 25 mL Teflon beaker as the raw materials for the reaction, resulting in the formation of mixture of La(NO3)3 and Eu(NO3)3 [Eu3+/(La3++Eu3+) = 5 mol%]. 20 g of the mixed hydroxides (NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 51.5
KOH = 51.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.5 in weight) was placed on top of the lanthanide nitrates in the beaker. The beaker was put into a digital oven, which was maintained at 240 °C. After the hydroxides were totally molten, the hydroxide solution was stirred by shaking the beaker to ensure the uniformity of the mixed reactants. After 72 h, the beaker was naturally cooled to room temperature. The white powder was filtered and washed with deionized water several times to remove hydroxides on the surface of the particles. The final products were dried in air at 60 °C. When the as-prepared sample was calcined in air at 800 °C for 2 h, ultralong La2O3:Eu3+ nanowires were obtained.
48.5 in weight) was placed on top of the lanthanide nitrates in the beaker. The beaker was put into a digital oven, which was maintained at 240 °C. After the hydroxides were totally molten, the hydroxide solution was stirred by shaking the beaker to ensure the uniformity of the mixed reactants. After 72 h, the beaker was naturally cooled to room temperature. The white powder was filtered and washed with deionized water several times to remove hydroxides on the surface of the particles. The final products were dried in air at 60 °C. When the as-prepared sample was calcined in air at 800 °C for 2 h, ultralong La2O3:Eu3+ nanowires were obtained.
A possible reaction mechanism for the synthesis of ultralong La(OH)3:Eu3+ and La2O3:Eu3+ nanowires is suggested. The eutectic point at NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 51.5
KOH = 51.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.5 is only about 165 °C. This may be a key factor in synthesizing the products at 240 °C. During the reaction process, hydroxides play a role not only as the solvent but also as the reactant for lowering the reaction temperature. In the molten hydroxides, La(NO3)3 and Eu(NO3)3 react with mixed hydroxides to form La(OH)3:Eu3+. Subsequently, the as-prepared La(OH)3:Eu3+ sample is calcined at 800 °C which is essentially a decomposition process.
48.5 is only about 165 °C. This may be a key factor in synthesizing the products at 240 °C. During the reaction process, hydroxides play a role not only as the solvent but also as the reactant for lowering the reaction temperature. In the molten hydroxides, La(NO3)3 and Eu(NO3)3 react with mixed hydroxides to form La(OH)3:Eu3+. Subsequently, the as-prepared La(OH)3:Eu3+ sample is calcined at 800 °C which is essentially a decomposition process.
2.2 Characterization
Powder X-ray diffraction (XRD) patterns were recorded on a Rigaku D/MAX-3B diffractometer with Cu–Kα radiation (1.5418 Å). The 2θ Bragg angles were scanned over a range of 10–80° at a rate of 2° min−1.Field-emission scanning electron microscopy (FE-SEM, XL-30, Philips) equipped with energy-dispersive X-ray spectroscopy (EDS) analysis was used to characterize the morphology and elemental composition of the samples.
Transmission electron microscopy (TEM), high-resolution TEM (HRTEM) and selected area electron diffraction (SAED) experiments were performed on a JEM-2100 electron microscope (JEOL, Japan) with an acceleration voltage of 200 kV. Samples for TEM measurements were prepared by dropping a diluted suspension of the product powder onto a standard carbon-coated Formvar film on a copper grid (230 mesh).
The Brunauer–Emmett–Teller (BET) surface area was measured on a Quantachrome NOVA 1000 Ver 6.11 system at 77.4 K. All samples were first degassed in a vacuum at 200 °C for 2 h before analysis. The BET-specific area was calculated from the nitrogen adsorption data in the relative pressure range from 0.05 to 0.3. The total volume was estimated from the amount adsorbed at a relative pressure of about 0.99.
The photoluminescence (PL) excitation and emission spectra were taken on a F-4500 spectrophotometer equipped with a 150 W xenon lamp as the excitation source. The measurements were performed at room temperature.
3. Results and discussion
The ultralong Eu3+-doped La2O3 nanowires were synthesized at 240 °C through the CHM method followed by subsequent heat treatment. Fig. 1A shows the X-ray diffraction (XRD) pattern of the products obtained by the CHM method, which indicates the presence of a pure hexagonal La(OH)3 phase (JCPDS card No. 36-1481). No additional peaks of other phases have been found, suggesting that Eu3+ has been effectively introduced into the La(OH)3 host lattice. Fig. 2 shows the SEM, TEM, HRTEM images and the SAED pattern of La(OH)3:Eu3+ nanowires. As shown in the SEM (Fig. 2A and B) and TEM images (Fig. 2C), the as-prepared La(OH)3:Eu3+ products are entirely composed of relatively uniform ultralong nanowires with a length of 50–100 μm, and a diameter of 200 nm. The HRTEM image of an individual nanowire (Fig. 2D) provides that the resolved lattice planes indicate a high degree of crystallinity. The interplanar distance is about 0.287 nm, close to the (200) lattice spacing of the hexagonal phase. The corresponding SAED pattern (inset of Fig. 2C) recorded perpendicular to the growth axis of single nanowire can be attributed to the [100] zone axis diffraction of hexagonal La(OH)3:Eu3+, suggesting that the nanowire is a single-crystalline and grows along the [110] direction. EDS (Fig. 2E) recorded from nanowires indicates that the products are composed of La, Eu, and O, while H can not be detected by EDS.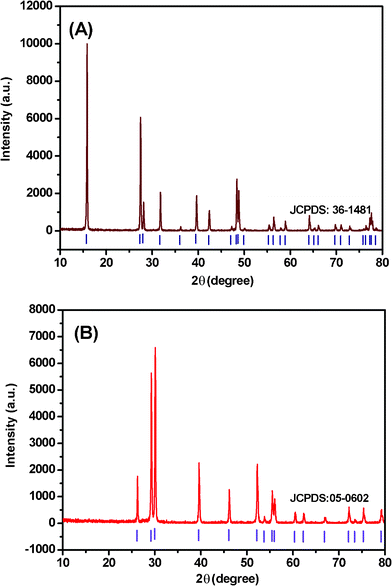 | ||
| Fig. 1 XRD patterns of (A) La(OH)3:Eu3+ and (B) La2O3:Eu3+ nanowires. The standard data (vertical markers) of hexagonal La(OH)3 (JCPDS card no. 36-1481) and La2O3 (JCPDS card No. 05-0602) is also presented in the figure for comparison. | ||
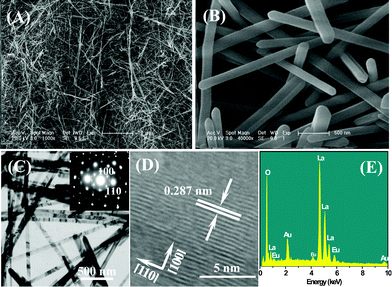 | ||
| Fig. 2 (A) Low-magnification and (B) high-magnification FE-SEM images of the as-prepared ultralong La(OH)3:Eu3+ nanowires; (C) TEM image of La(OH)3:Eu3+ nanowires (inset shows the SAED pattern of an individual nanowire); (D) HRTEM image of a part of an individual nanowire, and (E) EDS of La(OH)3:Eu3+ nanowires. | ||
Fig. 1B shows the XRD pattern of La2O3:Eu3+ nanowires. The diffraction peaks could be indexed to pure hexagonal La2O3 phase (JCPDS card No. 05-0602). Fig. 3A–C show SEM images of La2O3:Eu3+ nanowires. A large number of ultralong La2O3:Eu3+ nannowires are formed as shown in Fig. 3A. Here the superstructures are up to more than one hundred micrometres in length. More detailed morphologies of the nanowires are depicted in Fig. 3B and C. Fig. 3B shows that the products consist of nanowires of about 100 nm in diameter, while a more detailed investigation (Fig. 3C) reveals that the ends of nanowires have a conical shape. To confirm the chemical stoichiometry of the synthesized products, we perform the EDS analysis on the nanowires.
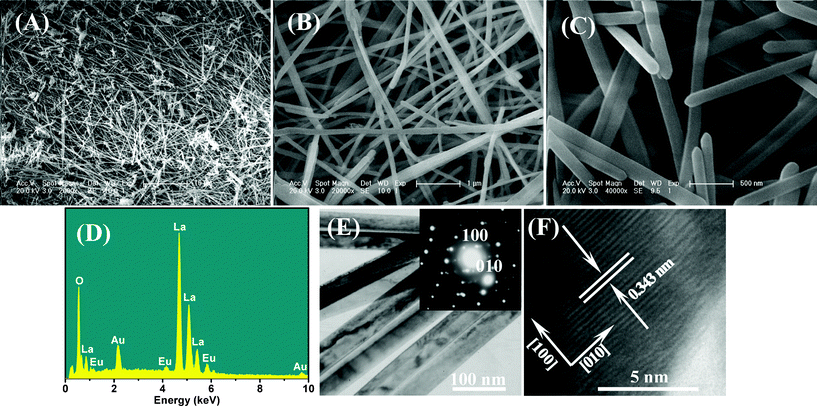 | ||
| Fig. 3 FE-SEM images of La(OH)3:Eu3+ samples obtained by CHM reaction for (A) Low-magnification and (B) high-magnification FE-SEM images of the as-prepared ultralong La2O3:Eu3+ nanowires; (C) top-view of nanowires; (D) EDS of La2O3:Eu3+ nanowires; (E) TEM image of La2O3:Eu3+ nanowires (inset shows the SAED pattern of La2O3:Eu3+ nanowires); (F) HRTEM image of a part of an individual nanowire. | ||
A representative EDS pattern (Fig. 3D) recorded from the superstructures suggests the presence of La, Eu and O, and the ratio of lanthanum to europium atoms is 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.97 (19.6), which is close to the theoretical 19
0.97 (19.6), which is close to the theoretical 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (19.0) ratio expected for 5 mol% Eu3+ doped-La2O3. Further structural characterizations of the La2O3:Eu3+ nanostructures were performed using TEM and HRTEM. Fig. 3E shows a typical TEM image and SAED pattern of La2O3:Eu3+ nanowires, while a HRTEM image of an individual nanowire is shown in Fig. 3F. The lattice spacing of 0.343 nm corresponds to the (100) planar spacing of hexagonal La2O3. Moreover, it is noted that a highly single crystalline nature is observed for the La2O3:Eu3+ nanowires with a [010] growth direction.
1 (19.0) ratio expected for 5 mol% Eu3+ doped-La2O3. Further structural characterizations of the La2O3:Eu3+ nanostructures were performed using TEM and HRTEM. Fig. 3E shows a typical TEM image and SAED pattern of La2O3:Eu3+ nanowires, while a HRTEM image of an individual nanowire is shown in Fig. 3F. The lattice spacing of 0.343 nm corresponds to the (100) planar spacing of hexagonal La2O3. Moreover, it is noted that a highly single crystalline nature is observed for the La2O3:Eu3+ nanowires with a [010] growth direction.
In order to elucidate the growth process of this La2O3:Eu3+ superstructure, a clear time-dependent experiment was carried out under similar reaction conditions. We studied the samples (obtained at different stages of the reaction under 240 °C CHM treatment) using SEM and XRD techniques. The corresponding SEM images and XRD patterns of the intermediates obtained at different reaction time intervals are shown in Fig. 4 and Fig. 5, respectively. Initially, after 1 h, a white powder was obtained which consists of nanoparticles and large-scale nanorod bundles of around 200 nm in length, and the nanorod bundles are composed of individual parallel nanorods with diameters of about 30 nm (Fig. 4A). The EDS pattern (inset of Fig. 4A) and XRD pattern (Fig. 5A) indicate that the nanorod bundles are La(OH)3:Eu3+. In the samples obtained after reaction for 6 h and 12 h, some nanowires together with nanorod bundles are observed in the products as shown in Fig. 4B and C. The corresponding XRD patterns show a preferential growth of the (100) plane of the hexagonal La(OH)3 crystals (Fig. 5B and C), which implies anisotropic growth along identical orientations ([110] direction). This is in agreement with the HRTEM observations. The above mentioned results suggest that La(OH)3:Eu3+ nanorods begin to grow gradually into nanowires during this time frame. After 24h, the nanorod bundles almost disappear and large-scale nanowires exist (Fig. 4D). In the corresponding XRD patterns, the diffraction peak of the (100) plane is strengthened greatly compared with the other peaks (Fig. 5D). The complete conversion to La(OH)3:Eu3+ superstructures can be achieved only after 72 h of CHM treatment, as shown by the disappearance of nanorod bundles (Fig. 4E and F).
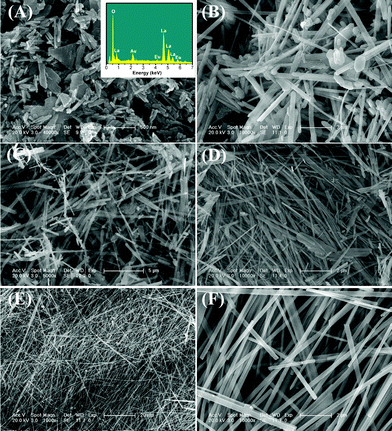 | ||
| Fig. 4 (A) Low-magnification and (B) high-magnification FE-SEM images of La(OH)3:Eu3+ samples obtained by CHM reaction for (A) 1 h (insert shows the EDS pattern), (B) 6 h, (C) 12 h and (D) 24 h. (E) Low-magnification and (F) high-magnification FE-SEM images of as-formed La(OH)3:Eu3+ nanowires by CHM reaction for 72 h. | ||
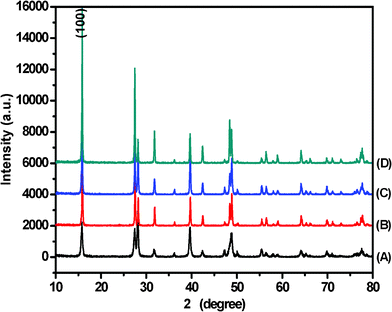 | ||
| Fig. 5 XRD patterns of La(OH)3:Eu3+ prepared by CHM method for (A) 1 h, (B) 6h, (C) 12 h, and (D) 24 h. | ||
SEM investigations on the early stages of the superstructure’s development, as shown in Fig. 4A, indicate that the nascent nanocrystals are in the form of nanorod bundles which are developed by 1D growth, starting from end faces and growing along the direction perpendicular to the surfaces. This growth mechanism is different from that in the previously reported hydrothermal method,37,38 in which 1D lanthanide hydroxides are formed through a disolution–recrystallization–growth process. Although detailed investigation is necessary to further elucidate the growth mechanism of 1D superstructure, we believe that strong interactions between nanoparticles and the anisotropic growth nature of lanthanide hydroxides are responsible for the formation of ultralong nanowires. During the reaction process, the mixed hydroxides (NaOH and KOH) are used not only as the solvent but also as reactants for decreasing the reaction temperature. In the molten hydroxides, lanthanide nitrates are dissolved and subsequently a large numbers of La(OH)3:Eu3+ seeds are formed. Then in situ anisotropic growth of agglomerated La(OH)3:Eu3+ nanoparticles is governed by a liquid–solid (LS) process, which leads to the formation of nanorod bundles. By increasing the reaction time, the nanorods grow along the [110] direction and are transformed into uniformly ultralong nanowires. Therefore, we can conclude that the promotion of the anisotropic growth of nanowires in this system may be mainly explained by a general coorperation effect, including the intrinsic structural features and the local reaction detail, and so on. The mechanism resembles to the vapor–solid (VS) process presented for the growth of semiconductor nanobelts by Wang’s group.39 In our case, we propose a related LS mechanism in which the precursor is delivered in the liquid phase rather than in the vapor phase, in analogy to the VS mechanism. A possible schematic illustration of formation mechanism for this wire-like structure is shown in Scheme 1. Ultralong La2O3:Eu3+ nanowires can be obtained through the following heat treatment process.
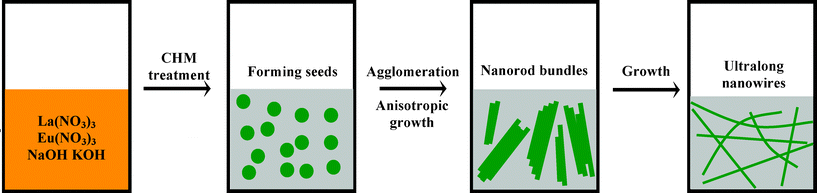 | ||
| Scheme 1 Illustration of formation mechanism for the ultralong La(OH)3:Eu3+ nanowires. | ||
To investigate the difference in the surface areas between ultralong La(OH)3:Eu3+ and La2O3:Eu3+ nanowires, nitrogen adsorption and desorption experiments were carried out. From the adsorption and desorption isotherms in Fig. 6A, it is concluded that it could be categorized as a type IV system with an inconspicuous hysteresis loop observed in the range of 0.1–1.0 P/P0 for La(OH)3:Eu3+ nanowires and 0.5–0.9 P/P0 for La2O3:Eu3+ nanowires. The BET model surface areas of these nanomaterials were calculated to be 217.7 and 131.1 m2 g−1. Fig. 6B shows the pore size distribution plots of La(OH)3:Eu3+ and La2O3:Eu3+ nanowires. The 0.20 cm3 g−1pore volume of the La(OH)3:Eu3+ nanowires is distributed over a broad range of pore sizes (up to 80 nm) with a volume-weighted peak at ca. 23 nm, which is significantly higher than the pore volume of the La2O3:Eu3+ nanowires (0.14 cm3 g−1). The as-prepared La2O3:Eu3+ nanowires also exhibit high specific surface area and a broad range of pore sizes (up to 80 nm), but the volume-weighted peak decreases to 21 nm. Calcining the as-prepared nanoarchitectures decreases the surface area and pore volume, and rearranges the pore-size distribution. The high surface areas of the rare earth compounds might make them show a strong binding force with biomolecules. In addition, the existence of nanopores on the surfaces makes them a kind of special material which may be used as a catalyst or sorbent for drug delivery.
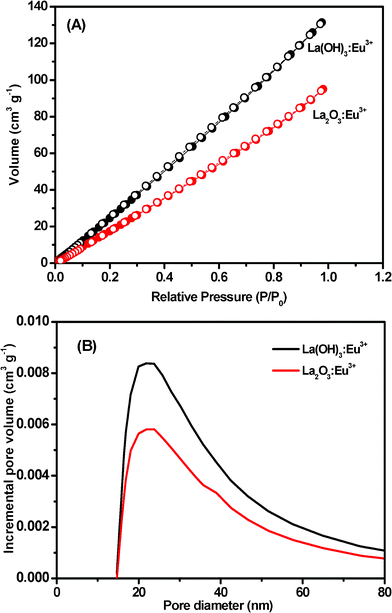 | ||
| Fig. 6 (A) N2 adsorption (•) and desorption (○) isotherms and (B) the BJH pore size distributions for La(OH)3:Eu3+ and La2O3:Eu3+ nanowires. | ||
Photoluminescent (PL) studies are also carried out to investigate the optical properties of the La2O3:Eu3+ nanowires. The as-prepared nanomaterial exhibits a nice luminescence compared with the La2O3:Eu3+ bulk materials (ESI†, here it should be mentioned that all the experimental conditions were kept identical to avoid experimental errors). It shows that the luminescence intensity of our La2O3: Eu3+ nanowires is much higher than that of the bulk. This could be attributed to the different local environments surrounding the Eu3+ luminescence centers; similar results are also obtained by Song et al.33 Detailed experimental work is still in progress. The excitation spectrum was obtained by monitoring the emission of the Eu3+ 5D0 → 7F2 transition (625 nm). It can be clearly seen that the excitation spectrum (ESI†) consists of two broad bands with maxima at 230 nm and 283 nm, which are attributed to the La2O3 host excitation band and the charge-transfer band (CTB) between O2− and Eu3+, respectively. The presence of the La2O3 host excitation band in the excitation spectrum of Eu3+ indicates that there exists an energy transfer from the La2O3 host and La3+ to the doped Eu3+.40 In the longer wavelength region, the f–f transition lines of Eu3+ (assigned in the ESI†) can be observed with very weak intensity compared with the CTB of the O2−–Eu3+ transition.
The room temperature PL emission spectrum of La2O3:Eu3+ nanowires is shown in Fig. 7A. Under excitation at 283 nm, a strong emission at 625 nm is observed. This strong red luminescence at 625 nm can be attributed to the emission of Eu3+ 5D0 → 7F2 transition. Commonly, the centrosymmetric degree for the local environment of the Eu3+ ions depends on the relative intensity ratio (R) of 5D0 → 7F2 to 5D0 → 7F1.41 Our results indicate that the Eu3+ ions prefer to hold a low symmetry site in the La2O3 host material. This emission process can be seen from digital image (Fig. 7B) of La2O3:Eu3+ nanowires dispersed in water under 310 nm excitation with a UV lamp. For comparison, La(OH)3:Eu3+ does not show a photoluminescent emission under the same excitation conditions. All the other emission peaks attributed to 5D0,1,2 → 7FJ (J = 0, 1, 2, 3, 4) transition lines of Eu3+ have also been assigned in Fig. 7A. The presence of emission lines from higher excited states of Eu3+ (5D1, 5D2) is attributed to the low vibration energy of the La–O band (500 cm−1). The multiphoton relaxation by La–O vibration is not able to bridge the gaps between the higher energy levels (5D1, 5D2) and the 5D0 level of Eu3+ completely, leading to emission from these levels. The surface defects in nanocrystals should be greatly increased compared with those in bulk materials. One can consider that in La2O3:Eu3+ nanowires, most of the exciton energies are transferred to the surface defects instead of the Eu3+ ions, causing the intensity of the Eu3+ exciton band to decrease. However, the La2O3:Eu3+ nanowires show a strong red emission under UV light excitation, indicating that there are little surface defects in La2O3:Eu3+ nanowires prepared in this work.42 In addition, the luminescence intensity is also related to the Eu–O bond distance.43 Namely, the luminescence intensity is proportional to CTB. This result is in agreement with our experiments.
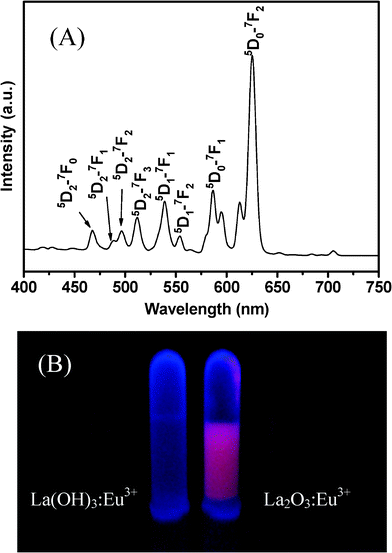 | ||
| Fig. 7 (A) Room temperature PL emission spectrum of La2O3:Eu3+ nanowires under excitation at 283 nm; (B) digital image of La(OH)3:Eu3+ and La2O3:Eu3+ nanowires under a 310 nm UV lamp irradiation. | ||
4. Conclusions
In conclusion, novel ultralong La2O3:Eu3+ single-crystalline nanowires have been synthesized in high quality and yield via a facile CHM method followed by a heat treatment process, without using any catalyst or template. The as-prepared La2O3:Eu3+ nanowires with effective doping of rare earth ions (Eu3+) in the interior and excellent PL properties make them ideal candidates for the optical device applications. To the best of our knowledge, this is the first example of the preparation of a single-cystalline superstructure of La2O3:Eu3+, which is expected to show particular physical properties. Our study may provide a new method for the direct synthesis of doping rare earth oxides in 1D superstructures and the related functional materials.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20701013, 20910102029 and 20801016), the Natural Science Foundation of Heilongjiang Province (No. LC201030) and the Youth Scientific Foundation of Heilongjiang University (QL200625). Dr Tian Xia gratefully acknowledges the special starting research fund of Heilongjiang University.References
- I. Boukai, Y. Bunimovich, J. T. Kheli, J. K. Yu, W. A. Goddard and J. R. Healh, Nature, 2008, 451, 168–171 CrossRef
.
- C. Bae, S. Kim, B. Ahn, J. Kim, M. M. Sung and H. Shin, J. Mater. Chem., 2008, 18, 1362–1367 RSC
.
-
C. N. R. Rao and A. Govindaraj, Nanotubes and Nanowires, The Royal Society of Chemistry, Cambridge, 2005 Search PubMed
.
- C. O’Sullivan, R. D. Gunning, C. A. Barrett, A. Singh and K. M. Ryan, J. Mater. Chem., 2010, 20, 7875–7880 RSC
.
- S. Xu, Y. Ding, Y. G. Wei, H. Fang, Y. Shen, A. K. Sood, D. L. Polla and Z. L. Wang, J. Am. Chem. Soc., 2009, 131, 6670–6671 CrossRef CAS
.
- Z. L. Wang, R. P. Gao, Z. W. Pan and Z. R. Dai, Adv. Eng. Mater., 2001, 3, 657–661 CrossRef CAS
.
- J. W. Liu, J. H. Zhu, C. L. Zhang, H. W. Liang and S. H. Yu, J. Am. Chem. Soc., 2010, 132, 8945–8952 CrossRef CAS
.
- H. Y. Shi, B. Hu, X. C. Yu, R. L. Zhao, X. F. Ren, S. L. Liu, J. W. Liu, M. Feng, A. W. Xu and S. H. Yu, Adv. Funct. Mater., 2010, 20, 958–964 CrossRef CAS
.
- C. H. Lu, J. H. Yang, L. Tang, D. Y. Zhang and L. M. Ma, Chem. Commun., 2006, 3551–3553 RSC
.
- W. Z. Wang, B. Q. Zeng, J. Yang, B. Poudel, J. Y. Huang, M. J. Naughton and Z. F. Ren, Adv. Mater., 2006, 18, 3275–3278 CrossRef CAS
.
- L. H. Zhang, H. Q. Yang, L. Li, R. G. Zhang, R. N. Liu, J. H. Ma, X. L. Xie and F. Gao, Inorg. Chem., 2008, 47, 11950–11957 CrossRef CAS
.
- X. P. Sun and M. Hagner, Chem. Mater., 2008, 20, 2869–2871 CrossRef CAS
.
- J. T. McCann, M. Marquez and Y. N. Xia, Nano Lett., 2006, 6, 2868–2872 CrossRef CAS
.
- F. Ye, Y. Peng, G. Y. Chen, B. Deng and A. W. Xu, J. Phys. Chem. C, 2009, 113, 10407–10415 CAS
.
- M. S. Palmer, M. Neurock and M. M. Olken, J. Am. Chem. Soc., 2002, 124, 8452–8461 CrossRef CAS
.
- P. P. Wang, B. Bai, S. Hu, J. Zhuang and X. Wang, J. Am. Chem. Soc., 2009, 131, 16953–16960 CrossRef CAS
.
- J. A. Capobianco, F. Vetrone, J. C. Boyer, A. Speghini and M. Bettinelli, Opt. Mater., 2002, 19, 259–268 CrossRef CAS
.
- F. Frayret, A. Willesuzanne, M. Pouchard, F. Mauvy, J. M. Bassat and J. C. Grenier, J. Phys. Chem. C, 2010, 114, 19062–19076 Search PubMed
.
- Y. Qiao, H. F. Chen, Y. Y. Lin, Z. Y. Yang, X. H. Cheng and J. B. Huang, J. Phys. Chem. C, 2011, 115, 7323–7330 CAS
.
- T. Domínguez, M. E. Pérez-Bernal, R. J. Ruano-Casero, C. Barriga, V. Rives, R. A. S. Ferreira, L. D. Carlos and J. Rocha, Chem. Mater., 2011, 23, 1993–2004 CrossRef
.
- A. P. Alivisatas, Science, 1996, 271, 933–937 Search PubMed
.
- C. Burda, X. B. Chen, R. Narayanan and M. A. Ei-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS
.
- G. Jia, Y. H. Zheng, K. Liu, Y. H. Song, H. P. You and H. J. Zhang, J. Phys. Chem. C, 2009, 113, 153–158 CAS
.
- A. W. Xu, Y. P. Fang, L. P. You and H. Q. Liu, J. Am. Chem. Soc., 2003, 125, 1494–1495 CrossRef CAS
.
- G. S. Wu, L. D. Zhang, B. C. Cheng, T. Xie and X. Y. Yuan, J. Am. Chem. Soc., 2004, 126, 5976–5977 CrossRef CAS
.
- J. Yang, C. X. Li, Z. W. Quan, C. M. Zhang, P. P. Yang, Y. Y. Li, C. C. Yu and J. Lin, J. Phys. Chem. C, 2008, 112, 12777–12785 CAS
.
- Y. B. Mao, J. Y. Huang, R. Ostroumov, K. L. Wang and J. P. Chang, J. Phys. Chem. C, 2008, 112, 2278–2285 CAS
.
- Z. L. Wang, G. R. Li, Y. N. Ou, Z. P. Feng, D. L. Qun and Y. X. Tong, J. Phys. Chem. C, 2011, 115, 351–356 CAS
.
- S. F. Wang, F. Gu, C. Z. Li and M. K. Lü, Cryst. Growth Des., 2007, 7, 2670–2674 CAS
.
- X. Li, Q. Li, Z. G. Xia, L. Wang, W. X. Yan, J. Y. Wang and R. I. Boughton, Cryst. Growth Des., 2006, 6, 2193–2196 CAS
.
- C. G. Hu, H. Liu, W. T. Dong, Y. Y. Zhang, G. Bao, C. S. Lao and Z. L. Wang, Adv. Mater., 2007, 19, 470–474 CrossRef CAS
.
- X. Wang and Y. D. Li, Chem.–Eur. J., 2003, 9, 5627–5635 CrossRef CAS
.
- L. X. Yu, H. W. Song, Z. X. Liu, L. M. Yang and S. Z. Lu, Phys. Chem. Chem. Phys., 2006, 8, 303–308 RSC
.
- G. G. Li, C. Peng, C. M. Zhang, Z. H. Xu, M. M. Shang, D. M. Yang, X. J. Kang, W. X. Wang, C. X. Li, Z. Y. Cheng and J. Lin, Inorg. Chem., 2010, 49, 10522–10535 CrossRef CAS
.
- J. S. Xie, Q. S. Wu, D. Zhang and Y. P. Ding, Cryst. Growth Des., 2009, 9, 3889–3897 CAS
.
- H. Liu, C. G. Hu and Z. L. Wang, Nano Lett., 2006, 6, 1535–1540 CrossRef CAS
.
- X. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2002, 41, 4790–4793 CrossRef CAS
.
- T. Xia, N. Lin, J. P. Wang, L. H. Huo, H. Zhao and G. Mountrichas, J. Alloys Compd., 2010, 507, 245–252 CrossRef CAS
.
- Z. W. Pan, Z. R. Dai and Z. L. Wang, Science, 2001, 291, 1947–1949 CrossRef CAS
.
- H. Q. Liu, L. L. Wang, W. Q. Huang and Z. W. Peng, Mater. Lett., 2007, 61, 1968–1970 CrossRef CAS
.
-
G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, Berlin, 1994 Search PubMed
.
- Z. G. Wei, L. D. Sun, C. S. Liao, C. H. Yan and S. H. Huang, Appl. Phys. Lett., 2002, 80, 1447–1449 CrossRef CAS
.
- Y. W. Jun, J. H. Lee, J. S. Choi and J. Cheon, J. Phys. Chem. B, 2005, 109, 14795–14806 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: PL emission spectra of La2O3:Eu3+ nanowires and La2O3:Eu3+ bulk materials, and PL excitation spectrum of La2O3:Eu3+ nanowires. See DOI: 10.1039/c1ra00136a |
| This journal is © The Royal Society of Chemistry 2011 |
