Gold nanoparticles confined in lamellar mesophases†
W.
Abidi
a,
B.
Pansu
b,
R.
Krishnaswamy‡
b,
P.
Beaunier
c,
H.
Remita
*a and
M.
Impéror-Clerc
*b
aLaboratoire de Chimie Physique, UMR 8000-CNRS, Université Paris-Sud 11, 91405 Orsay Cedex, France. E-mail: hynd.remita@u-psud.fr
bLaboratoire de Physique des Solides, UMR 8502 CNRS, Bât 510, Université Paris-Sud 11, 91405 Orsay Cedex, France. E-mail: marianne.imperor@u-psud.fr
cLaboratoire de Réactivité de Surface, UPMC Univ., Paris 06, CNRS, UMR 7197, 75252 Paris Cedex 05, France
First published on 17th August 2011
Abstract
A lamellar surfactant mesophase is used as a soft confinement medium for gold nanoparticles that are directly synthesized inside the lamellar mesophase by the radiolytic reduction of a gold salt incorporated into the water medium. By increasing the water layer thickness of the mesophase, spherical gold nanoparticles of increasing size are obtained. The same soft confinement medium is used to synthesize directly in situ by radiolysis gold nanorods (aspect ratio about 10). It is also shown that gold nanorods can be inserted by simple mixing into a sufficiently swollen lamellar phase. In all cases, the structure of the lamellar phase is preserved in the presence of the nanoparticles.
Introduction
Gold nanoparticles have recently attracted a lot of attention because of their exceptional catalytic and optical properties, and their potential applications in different fields like sensing, optical limitation, imaging and cancer therapy.1–3 In particular, many studies report the synthesis of gold nanorods and their optical properties and their potential applications.2 Indeed, these nanorods present two surface plasmon resonances (SPR) corresponding to the electromagnetic-wave-driven oscillation of the quasi-free electrons along (longitudinal SPR) and perpendicular (transverse SPR) to the rods long axis. While the transverse SPR (TSPR) is almost insensitive to the nanorod morphology, the spectral location of the longitudinal one (LSPR) can be easily tuned from green to near infrared by modifying the nanorod aspect ratio: the longer the rod, the smaller the LSPR frequency. Based on these properties, gold nanorods can have different applications:4 hyperthermal therapy against cancer,5 optical data storage,6etc.Including metallic, mineral or biological nano-objects in self-organized systems based on amphiphilic molecules is an important issue, relevant both to the elaboration of novel materials and to transport and vectorisation applications. Numerous studies, in different areas of chemistry, physics and biology rely on doping self-assembled systems and, in particular, lamellar phases. Gold nanoparticles were generated into onion-type multilamellar vesicles (MLVs): monoolein (one of the MLV component) was used as reductant.7 In a more recent study, synthesis of gold nanoparticles by radiolysis and by photoreduction in a sheared lamellar phase has been reported.8 It has been shown by TEM analysis and X-ray diffraction that gold nanoparticles grow within the lamellar phase. The spherical nanoparticles synthesized by radiolysis were aligned along the lamellae.
Radiolysis is a powerful method to synthesize nanoparticles of controlled size and shape3,8–13 in solutions and in heterogeneous media. Solvent radiolysis induces the formation of solvated electrons and radicals, which reduce the metal ions homogeneously in the medium leading to homogeneous nucleation. Small and relatively monodisperse nanoparticles are obtained. Compared to chemical reducing processes that follow a diffusion front, radiolysis presents the advantage of inducing a homogeneous nucleation and growth in the whole volume of the irradiated sample. Metal nanoparticles including 1D, 2D and 3D nanostructures can be directly synthesized in surfactant mesophases. For example, Pd nanowires9 and porous nanoballs of Pt,10 Pd11 and Pd–Au12 have been recently synthesized by radiolysis in hexagonal mesophases. In this paper, we show that a lamellar surfactant mesophase can be used as a soft confinement medium for gold nanoparticles induced by radiolysis. First, we show that the size of spherical gold nanoparticles (NPs) can be tuned by changing the water layer thickness of the lamellar mesophase, revealing a confinement effect between two surfactant bilayers during the NPs growth. Then, we investigate the case of gold nanorods. We have recently reported about the radiolytic one-pot synthesis in solution of gold nanorods.13 Nanorods of controlled aspect ratios were obtained by γ-reduction of AuIII complexes in a micellar solution formed by a mixture of surfactants cetyltrimethylammonium bromide (CTAB) and tetraoctylammonium (TOAB) and in presence of acetone, cyclohexane and silver ions. By increasing the silver ion concentrations, aspect ratios up to 10 are obtained.13 Here we show that these nanorods can be inserted in a sufficiently swollen lamellar mesophases or can be induced directly in these lyotropic lamellar phases by radiolysis.
Experimental section
Materials
Chloroauric acid (HAuCl4, 3 H2O), silver nitrate AgNO3, cetyltrimethylammonium bromide (CTAB) (98% purity), acetone, hexanol, cyclohexane were obtained from Sigma-Aldrich and used as received. Tetraoctylammonium bromide (TOAB) was obtained from Acros Organics. N2 gas (purity > 99.995%) was purchased from Air Liquide.Irradiation facilities
The solutions were irradiated under N2 atmosphere in glass vessels with a rubber plastic septum. The γ-irradiation source was a 60Co gamma-facility of 7000 Curies with a dose rate of (2.7 kGy h−1) at Orsay.Characterization
For the lamellar mesophases, we used the cetyltrimethylammonium bromide (CTAB)/hexanol/water ternary system, with three different compositions.14 The resulting mesophases obtained after mixing exhibited the characteristic textures of a lamellar liquid crystal phase as checked by optical microscopy between crossed polarizers. Small angle X-ray scattering (SAXS) experiments were performed on the samples using in-house equipment on a copper rotating anode X-ray generator (λ = 0.154 nm). The SAXS intensity was collected on a two dimensional image-plate detector. Samples were sealed in 1.5 mm thick Lindeman glass capillaries. Experiments were also conducted at the D2AM ESRF beamline and at the SWING Soleil synchrotron beamline. The UV-visible spectra of these samples were carried out on HP diode array HP8453 spectrophotometer. The mesophases containing the nanoparticles were dissolved in 2-propanol and a few drops of the solution were deposited on a copper coated carbon grids. Transmission electron microscopy (TEM) observations were performed with a JEOL JEM 100CX II transmission electron microscope operating at 100 kV.Results and discussion
For the lamellar mesophase, we used the cetyltrimethylammonium bromide (CTAB)/hexanol/water ternary system at different compositions, in order to vary the water thickness (dw) to control the confinement.14 The use of different compositions allows us to tune the lamellar spacing d between 4 and 20 nm. SAXS experiments allow us measure the lamellar spacing by the presence of three or more Bragg peaks. The value of the CTAB bilayer thickness (δ) is known from the literature and is equal to 2.7 nm.15 Then the water thickness dw = d−δ is directly deduced from the measured lamellar spacing d.1. Synthesis of gold nanoparticles in lamellar mesophases
Three different compositions in CTAB/hexanol/aqueous solution were used: 40/27/33%, 19/12/69% and 9/6/85% (w/w). The aqueous solution contains the same concentration of the metallic salt HAuCl4 (10−3 M) for the three compositions. The three samples were irradiated (dose 46 kGy) in order to induce the formation of gold nanoparticles by radiolytic reduction (Fig. 1). Radiolysis of polar solvents induces the formation of solvated electrons and radicals, in particular, in the case of water:3| H2O ⇒ eaq−, H3O+, H˙, OH˙, H2, H2O2 | (1) |
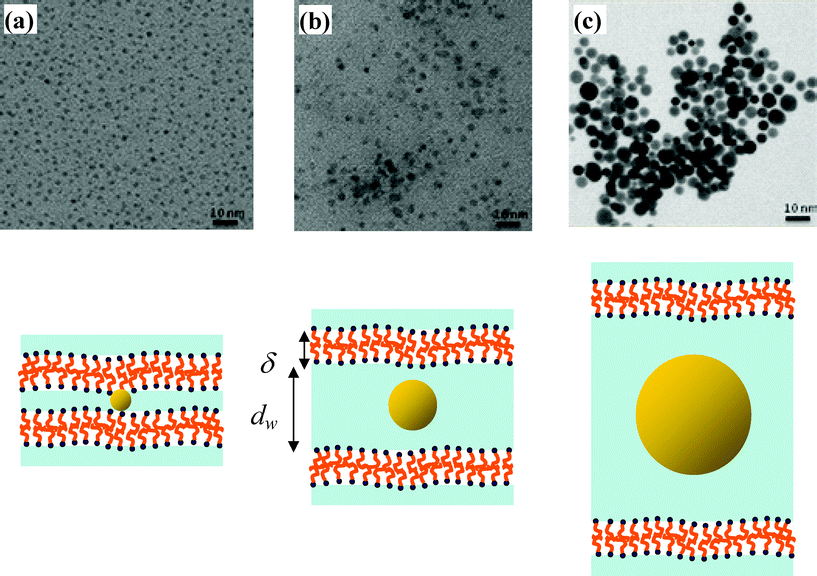 | ||
| Fig. 1 TEM pictures of the gold nanoparticles obtained for the three lamellar phases with increasing water layer thicknesses (a) dw = 1.3 nm, (b) dw = 5.9 nm and (c) dw = 14.3 nm and a schematic representation of the different lamellar phases containing gold NPs. Nanoparticles are spherical and have a mean diameter measured by TEM of 1.5 ± 0.5 nm, 3.5 ± 0.5 nm and 8.5 ± 1.5 nm, respectively. For simplicity, only the gold cores of the NPs are drawn without the CTAB ligands. | ||
H˙ and OH˙ are scavenged by primary or secondary alcohols (in our case by hexanol) to yield alcohols radicals:
| RCHOH + OH˙ (or H˙) → RC˙OH + H2O (or H2) | (2) |
Solvated electrons e−aq (E0(H2O/e−aq) = −2.87 VNHE)16,17 and alcohol radicals are strong reducing agents, which are able to reduce metal ions to lower valences and finally to metal atoms.
The colour of the irradiated AuIII-doped lamellar mesophases change from light yellow to light pink, indicating the formation of gold nanoparticles (see Fig. S1, ESI†).
SAXS spectra (see Fig. S2, ESI†) performed a few days after irradiation show that the geometry of the irradiated lamellar phase is conserved, with the same lamellar spacing as the pre-irradiated form. For the three different compositions, the lamellar spacing are d = 4 nm, d = 8.6 nm and d = 17 nm, respectively. The water thicknesses dw = d−δ are so deduced and correspond to 1.3 nm, 5.9 nm and 14.3 nm, respectively (Fig. 1). Consequently, the radiolysis method does not disrupt the structure of the mesophase. The nanoparticles do not give a detectable signal on these SAXS spectra, because of their small concentration and small size. Then, TEM characterization has been used for the spherical gold NPs. The nanoparticles were extracted to be observed by TEM.
Transmission electron microscopy (TEM) pictures show that the nanoparticles obtained in the three different lamellar phases are spherical and display different sizes (Fig. 1). The size of the nanoparticles increases with the water layer thickness inside the lamellar phase. For a water thickness dw = 1.3 nm, the nanoparticles are the smallest and have a mean diameter of 1.5 ± 0.5 nm, while the NPs obtained with dw = 5.9 nm and dw = 14.3 nm have a mean size of 3.5 ± 0.5 nm and 8.5 ± 1.5 nm, respectively. The polydispersity in size is around 15–20% as derived from the TEM pictures.
These results show that the gold nanoparticles most probably grow between the surfactant bilayers. Indeed, their sizes correspond quite accurately to the water layer thickness, showing that a confinement effect is obtained. It is known that CTAB is present on the NPs surface as a bilayer in a water medium. When the NPs are inserted in the lamellar phase, this bilayer could be a part of the stacking layers and the NPs partially attached to the lamellae. For simplicity, only the gold cores of the NPs are drawn in Fig. 1 and 3 without the CTAB ligands.
![SAXS spectra of (a) the gold nanorods aqueous suspension (TEM observation in the inset) (b) the reference lamellar phase in pure water [9% CTAB/6% hexanol/85% water (w/w)] and (c) the lamellar phase containing the gold nanorods obtained by simple mixing. The two insets (2D SAXS patterns) reveal a flow orientation of the lamellar phase inside the samples.](/image/article/2011/RA/c1ra00259g/c1ra00259g-f2.gif) | ||
| Fig. 2 SAXS spectra of (a) the gold nanorods aqueous suspension (TEM observation in the inset) (b) the reference lamellar phase in pure water [9% CTAB/6% hexanol/85% water (w/w)] and (c) the lamellar phase containing the gold nanorods obtained by simple mixing. The two insets (2D SAXS patterns) reveal a flow orientation of the lamellar phase inside the samples. | ||
In order to evidence that the lamellar phase is playing such a role in the confinement, blank experiments were conducted on dilute micellar CTAB solutions (0.082 M) using the same concentration of HAuCl4 (10−3 M) and the same proportion of hexanol (0.19 M) with respect to CTAB (ratio CTAB/hexanol = 3/2 (w/w)). After irradiation at the same dose rate, the obtained NPs are still spherical, but much larger and polydisperse in size (mean size of 17 ± 11 nm) as the ones synthesized in lamellar mesophases (see TEM images in Fig. S4, ESI†). This blank experiment gives another argument to prove the confinement effect of the lamellar phase.
2. Insertion of gold nanorods into the lamellar mesophases by mixing
Various synthesis approaches of gold nanorods based on seed mediated growth have been reported.18–21 Gold nanorods of well-controlled aspect ratios were also synthesized by electrochemical reduction,22,23 photochemical reduction,24 ultrasonic irradiation25 and more recently by radiolysis.13 In all these studies, the presence of CTAB and silver ions were found necessary to obtain anisotropic growth.Gold nanorods were first synthesized in solutions by radiolysis using a recently reported method.13 The initial solution contains 0.082 M CTAB, 7.5 × 10−4 M TOAB, 1.9 × 10−3 M HAuCl4, 0.139 M cyclohexane, 0.266 M acetone and 2.4 × 10−4 AgNO3. The TEM pictures of the gold nanorods obtained after radiolysis (dose = 32.2 kGy) show that these nanorods (NRs) have a mean diameter of 7.5 nm and an aspect ratio around 10 (Fig. 2 inset). As expected for a rodlike morphology, they display two plasmon bands located at 510 nm (TSPR) and 950 nm (LSPR). The average dimensions of the NRs were more accurately determined by SAXS (Fig. 2a). In Fig. 2a, the red curve corresponds to the fit of the experimental data by the form factor of cylindrical particles with a mean diameter D = 7.5 nm (polydispersity of 20%) and an average length L = 90 nm, leading to an average aspect ratio of 12.
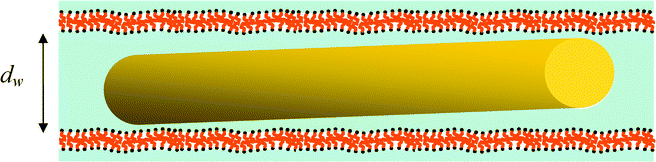 | ||
| Fig. 3 Drawing of a gold nanorod confined within the lamellar mesophase. Because of the NP dimensions (D < dW < L), its long axis is expected to be oriented along the lamellar planes. For simplicity, only the gold cores of the NRs are drawn without the CTAB ligands. | ||
These nanorods were collected by centrifugation and washing before insertion in a lamellar phase by simple mixing. A sufficiently swollen lamellar phase was used to incorporate these nanorods by mixing, with a water thickness (dw = 15.7 nm) greater than the diameter of the nanorods (7.5 nm). In Fig. 2, the SAXS spectra from the NRs solution, the host lamellar phase without particles (pure water) and the mixture are compared.
The SAXS spectrum of a reference mesophase in pure water without nanorods (CTAB/hexanol/water: 9/6/85% (w/w)) exhibits three well defined Bragg peaks, characteristic of the lamellar mesophase (Fig. 2b) and corresponding to a lamellar spacing d = 18.4 nm and a water thickness dw = 15.7 nm. In the insert, the 2D pattern appears strongly anisotropic, due to a partial shear alignment of the lamellar phase when it is introduced inside the capillary. The SAXS spectrum of the mesophase doped with nanorods by mixing is shown in Fig. 2c. It is clear from this spectrum that the signal of the lamellar phase is superimposed to the nanorods signal (red curve). The observation of the Bragg peaks demonstrates that a lamellar phase is conserved in the presence of the inserted gold nanorods. In the inset of Fig. 2c, again, a partial alignment of the lamellar phase is detected. Moreover, a very weak anisotropy of the nanorods signal around the beam-stop indicates that the nanorods may have their long axis confined inside the water sheets. As shown in Fig. 3, because of the NP dimensions (D < dW < L), their long axis is indeed expected to be oriented along the lamellar planes.
The positions of the lamellar Bragg peaks (Fig. 2c) are slightly shifted to the right compared to the spectra of the reference mesophase (Fig. 2b), corresponding to a slightly smaller lamellar spacing d = 15.7 nm and a water thickness dw = 13 nm. This is because the nanorod solution used for the mixture contains some amount of CTAB, and the resulting CTAB concentration is slightly greater than that for the reference composition in pure water.
3. One-pot synthesis of gold nanorods in lamellar phases
Here, we report about the direct radiolytic synthesis of gold nanorods in situ in the lamellar phase.Gold nanorods were induced from the same initial solution used previously in solution.13 This initial solution (containing 0.082 M CTAB, 7.5 × 10−4 M TOAB, 1.9 × 10−3 M HAuCl4, 0.139 M cyclohexane, 0.266 M acetone, and 2.4 × 10−4 AgNO3) was considered as the aqueous phase of the ternary system and was mixed with CTAB and hexanol in the composition 9% CTAB/6% hexanol/85% aqueous solution. Again, because the synthesis solution contains CTAB, the composition of the lamellar phase prior to radiolysis was slightly shifted to: 11.2% CTAB/5.8% hexanol/82.9% aqueous solution (w/w). In Fig. S5 (ESI†), the SAXS spectra of this lamellar phase is compared to the one in pure water of composition 9% CTAB/6% hexanol/85% water.
When viewed between cross polarizers the doped mesophase after irradiation shows a lamellar texture (Fig. 4a). The SAXS pattern recorded before irradiation shows that the use of this aqueous phase induces a lamellar phase with characteristic dimensions d = 15 nm for a water thickness dw = 12.3 nm. These values are very close to the ones observed for the sample prepared by simple mixing. We have chosen this largest spacing to allow nanorods having diameter below this value to be synthesized inside this spacing. The mesophases doped with AuIII precursors were submitted to irradiation. The obtained mesophases turn from light yellow to brown. The same final colour was also obtained by irradiation of the micellar solution leading to the formation of nanorods.13 The TEM images (Fig. 4b) show that nanorods with an aspect ratio of about 7 were synthesized in the lamellar phase. Their UV-visible absorption spectrum is shown in Fig. 4c: two surface plasmon resonance bands located at 560 and 1000 nm correspond to the longitudinal (LSPR) and transverse (TSPR), which characterize the presence of nanorods. SAXS experiments after irradiation (not shown) allow us to deduce the dimensions of the nanorods, with an average aspect ratio of 7, an average diameter D = 9 ± 1 nm (polydispersity of 10%), which are all in agreement with the TEM results. The lamellar spacing is the same before and after irradiation, showing that the lamellar arrangement is not affected by radiolysis or by the growth of the nanorods inside. Therefore, it is possible to transpose the synthesis of nanorods in solution to a confined medium situation and the nanorods aspect ratios are similar in both cases.
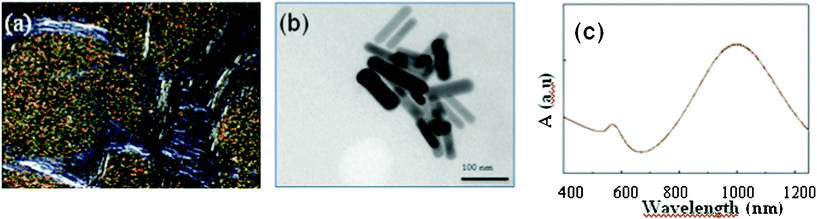 | ||
| Fig. 4 Gold nanorods induced by radiolysis directly in a lamellar mesophase with dw = 12.3 nm, (a) optical texture of the lamellar phase after irradiation between cross polarizers, (b) TEM of the nanorods, and (c) UV-visible absorption spectrum of the nanorods. | ||
Mechanism of reduction and formation of gold spherical nanoparticles and nanorods
In the presence of a sufficient concentration of CTAB, the main AuIII precursor is the complex AuBr4−–CTAB. Solvated electrons and the reducing alcohol radicals formed in situ by radiolysis are able to reduce the gold complexes. The reduction proceeds in two successive steps: first, the reduction of AuIII into the unstable bivalent state AuII (reactions 3 and 4), which dimerizes to form (AuII)2,26 and disproportionates into AuIII and AuI (reaction 5), and then the reduction of AuI into atoms and clusters (reactions 6 and 7).3,13,26 The intermediate AuI and Au0, which are complexed by CTA+, can diffuse along the bilayer surface and subsequently associate to form metal clusters confined in the water domain.The different reduction reactions of AuIII lead finally to Au0 are then the following:
| AuIIIBr4− + eaq− → AuIIBr42− | (3) |
| AuIIIBr4− + RC˙OH → AuIIBr42− + RCO + H+ | (4) |
| AuII + AuII → (AuII)2 → AuIII + AuI | (5) |
| AuI + eaq− → Au0 | (6) |
| AuI + RC˙OH → Au0 + RCO + H+ | (7) |
| AuIII + Au0 → AuII + AuI,26 | (8) |
| eaq− + (CH3)2CO → (CH3)2C˙OH + OH− | (9) |
In this case, the only reducing species of the gold complexes are the alcohol radicals. The effect of acetone is to slow down the reduction kinetics leading to, in presence of CTAB and silver, the formation of gold nanorods.13 The dose necessary to reduce the gold complexes is high because the radiolytic reduction yield is low. This is due to back oxidation of gold (AuI or gold atoms) by the oxidative radicals Br2˙− and Br3−/Br2 (E0(Br2˙−/2Br) = 1.7 VNHE ; E0(Br2/Br2˙−) = 0.43 VNHE)27 formed under our radiolytic conditions.13 (Br−,CTA+) and (AgBr2−,CTA+) complexes play the role of growth directing agents because of their efficient adsorption on preferential facets.28,29 These aspects were discussed in the previous paper on radiolytic synthesis of gold nanorods in solutions.13
Conclusion
Radiolysis is a powerful method to induce the formation of nanoparticles in situ within mesophases. One-pot radiolytic synthesis of both spherical and rod-like gold NPs was achieved in lamellar phases. The structure of the lamellar phase is preserved during the synthesis of the NPs. For spherical NPs, the confinement limits their size: by increasing the water layer thickness of the mesophase, spherical gold NPs of increasing size are obtained. This strong correlation between the water layer thickness and the NPs size is evidenced for the first time and proves that the lamellar phase acts as a soft confinement medium. More precisely, the mechanism of this confinement effect is probably not only a simple geometrical effect, but rather involves modifications of both the nucleation and growth rates of the NPs. In addition, for the first time, gold nanorods have been successfully synthesized in a soft confined medium. The incorporation of gold nanorods with high aspect ratios into lamellar phases is of high interest for original applications.In future works, we will investigate if this confinement effect can be extended to the case of the gold nanorods, performing their synthesis in less swollen lamellar phases. The confinement should then influence the value of the diameter of the nanorods, and thus their final aspect ratio. The present study has been conducted at the same gold salt concentration of 10−3 M for all samples, corresponding to a final volume fraction occupied by the NPs of only about φ = 10−5. From the volume fraction φ, one can estimate the average distance L between the nanoparticles of average volume v confined in the water sheets of thickness dw using the relation: φ = v/(L2dw). The average distance L between the NPs is quite large, of about 50–100 times the size of the NPs (taking the diameter for the spherical NPs and the length for the rod-like ones). In such a dilute regime, no interactions are present between the NPs. Further experiments will be conducted at higher salt concentrations. Since the diffusion of the gold ions inside the confined medium plays a role in the growth step of the NPs, the concentration of the gold salt should also influence the final NPs dimensions. Finally, in the very high salt concentration regime, interactions between NPs and coupling effects are expected to take place.
Acknowledgements
Cyrille Rochas (ESRF) and Florian Meneau (Soleil) are gratefully acknowledged for their assistance during the synchrotron experiments.References
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS
.
- E Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759 RSC
.
- W. Abidi and H. Remita, Recent Patent on Engineering, Special Issue: Light-driven reactions and materials in the environmental technology, 2010, 4, 170 CAS
.
- X. Huang, S. Neretina and M. A. El-Sayed, Adv. Mater., 2009, 21, 4880 CrossRef CAS
.
- P.K. Jain, I. H. El-Sayed and M. A. El-Sayed, Nanotoday, 2007, 2, 18–9 CrossRef
.
- P. Zijlstra, J. W. M. Chon and M. Gu, Nature, 2009, 459, 410–3 CrossRef CAS
.
- O. Regev, R. Backov and C. Faure, Chem. Mater., 2004, 16, 5280 CrossRef CAS
.
- M.-E. Meyre, M. Tréguer-Delapierre and C. Faure, Langmuir, 2008, 24, 4421 CrossRef CAS
.
- F. Ksar, G. Surendran, L. Ramos, B. Keita, L. Nadjo, E. Prouzet, P. Beaunier, A. Hagège, F. Audonnet and H. Remita, Chem. Mater., 2009, 21, 1612 CrossRef CAS
.
- G. Surendran, L. Ramos, B. Pansu, E. Prouzet, F. Audonnet, P. Beaunier and H. Remita, Chem. Mater., 2007, 19, 5045 CrossRef CAS
.
- G. Surendran, F. Ksar, L. Ramos, B. Keita, L. Nadjo, E. Prouzet, P. Beaunier, F. Audonnet and H. Remita, J. Phys. Chem. C, 2008, 112, 10740 CAS
.
- F. Ksar, G. Ramos, B. Keita, L. Nadjo, P. Beaunier and H. Remita, Chem. Mater., 2009, 21, 3677 CrossRef CAS
.
- W. Abidi, P. R. Selvakanan, Y. Guillet, I. Lampre, P. Beaunier, B. Pansu, B. Palpant and H. Remita, J. Phys. Chem. C, 2010, 114, 14794 CAS
.
- P. Ekwall, L. Mandell and K. Fontell, J. Colloid Interface Sci., 1969, 29, 639 CrossRef CAS
.
- K. Fontell, A. Khan, B. Linstrôm, D. Maciejewka and S. Puang-Ngern, Colloid Polym. Sci., 1991, 269, 727 CAS
.
-
A. Swallow, J. Radiation Chemistry: an Introduction, Wiley, New York, 1973 Search PubMed
.
- A. H. Schwarz, J. Chem. Educ., 1981, 58, 101 CrossRef
.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389 CrossRef CAS
.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065 CrossRef CAS
.
- M. A. El-Sayed and B. Nikkobakht, Chem. Mater., 2003, 15, 746 CrossRef CAS
.
- B. Nikkobakht and M. A. El-Sayed, Langmuir, 2001, 17, 6368 CrossRef
.
- Y. Y. Yu, S.-S. Chang, C.-L. Lee and C. R. C. Wang, J. Phys. Chem. B, 1997, 101, 6661 CrossRef CAS
.
- S.-S. Chang, C.-W. Shih, C.-D. Chen, W.-C. Lai and C. R. C. Wang, Langmuir, 1999, 15, 701 CrossRef CAS
.
- F. Kim, J. H. Song and P. Yang, J. Am. Chem. Soc., 2002, 124, 14316 CrossRef CAS
.
- K. Okitsu, K. Sharyo and R. Nishimura, Langmuir, 2009, 25, 7786 CrossRef CAS
.
- G. R. Dey, A. K. El Omar, J. A. Jacob, M. Mostafavi and J. Belloni, J. Phys. Chem. A, 2011, 115, 383 CrossRef CAS
.
- A. Henglein, Radiat. Phys. Chem., 1980, 15, 151 CrossRef CAS
.
- F. Hubert, F. Testard and O. Spalla, Langmuir, 2008, 24, 9219 CrossRef CAS
.
- F. Hubert, F. Testard, G. Rizza and O. Spalla, Langmuir, 2010, 26, 6887 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1ra00259g |
| ‡ present address: Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, 560 064, India |
| This journal is © The Royal Society of Chemistry 2011 |
