Switchable Ionic liquids (SILs) based on glycerol and acid gases
Ikenna
Anugwom
*a,
Päivi
Mäki-Arvela
a,
Pasi
Virtanen
a,
Pia
Damlin
d,
Rainer
Sjöholm
c and
Jyri-Pekka
Mikkola
*ab
aLaboratory of Industrial Chemistry and Reaction Engineering, Process Chemistry Centre, Åbo Akademi University, Åbo-Turku, FI-20500, Finland. E-mail: ianugwom@abo.fi; Fax: +35822154479; Tel: +35822154514
bTechnical Chemistry, Department of Chemistry, Chemical-Biological Center, Umea University, SE-901 87, Umea, Sweden. E-mail: Jyri-Pekka.Mikkola@chem.umu.se; Fax: +46 (0)90-13 63 10; Tel: +46 (0)90-786 5284
cLaboratory of Organic Chemistry, Åbo Akademi University, Åbo-Turku, FI-20500, Finland
dLaboratory of Analytical Chemistry, Åbo Akademi University, Process Chemistry Centre, Åbo-Turku, FI-20500, Finland
First published on 10th August 2011
Abstract
New types of switchable ionic liquids (SILs), containing 1,8-diazabicyclo-[5.4.0]-undec-7-ene (DBU), glycerol and an acid gas (CO2, SO2), were synthesized and characterized in this study. [DBU][Carbonate] or [sulfonate] were easily synthesized from a non-ionic mixture of molecular organic polyol and amidine base upon bubbling of an acid gas (CO2, SO2). Moreover, they were switched back to the original molecular solvents by flushing out the acid gas (CO2, SO2) by heating and/or bubbling an inert gas such as N2 through it. The structures of the SILs were confirmed by NMR and FTIR. The change from low polarity (molecular solvent) to high polarity (Switchable Ionic Liquid, SIL) was also indicated by the changes in properties, such as viscosity and miscibility with different organic solvents. The decomposition temperatures of the SILs were determined by means of Thermo Gravimetric Analysis (TGA) and gave values in the range of 50 °C and 120 °C for DBU-glycerol-CO2 (SIL1) and DBU-glycerol-SO2 (SIL2), respectively. Due to the reasonable decomposition temperatures, these novel SILs can be employed in multiple applications.
Introduction
Most of the common chemical processes are often multistep processes which involve reactions, extractions, and separations. Frequently the optimum solvent selection is not trivial. The limitations for a solvent to act optimally in each and every process step, in a complete process, can be related to the inflexibility of its physical properties.1 Thus, a common practice is to add and remove solvent at end of each process step. There are several ways by which physical properties of solvents can be altered, for instance by changing the temperature or pressure. These alterations of solvents properties can, however, only affect the solvent within some limits.1 There are several known examples where changes in pressure can affect the solvents properties. For instance, the properties of supercritical fluids (such as CHF3) and CO2-expanded liquids (i.e., subcritical mixtures of CO2 and an organic liquid) can be continuously and reversibly changed by changing the liquid pressure.1,2 However, typically these kinds of changes occur at rather high pressures, exceeding 40 bar.3A new class of switchable solvents which are capable of reversible compositional change under mild conditions has been studied by Jessop et al.2 Bubbling an acid gas through an equimolar mixture of two molecular compounds, such as an amidine (e.g. 1,8-diazabicyclo-[5.4.0]-undec-7-ene, DBU) or a guanidine (e.g. 2-butyl-1,1,3,3-tetramethylguanidine, BTMG) and an alcohol, causes an exothermic transformation from a mixture of molecular compounds to an ionic liquid. Furthermore, the viscous ionic liquid can be converted back to molecular compounds by bubbling N2 or argon through the liquid at room temperature, or at 80 °C to achieve a more rapid transformation.1–3 This new class of solvents is characterized by a ‘switching’ behavior from molecular liquids to an ionic liquid. Also, owing to the switching, some of the solvent properties such as polarity and viscosity can be changed.2 This behavior introduces new possibilities for ionic liquid recovery and recycling.
Many “conventional” room-temperature ionic liquids, can stand rather high temperatures often more than 200 °C, and thus can be used in processes where high temperatures are required.4 However, the recovery and reuse of them is challenging, thus limiting their usability in industrial applications.5,6
The switchable solvents composed of primary alcohols together with DBU and CO2 as trigger were originally proposed by Jessop et al.2 and have decomposition temperatures residing between 50–60 °C. Due to the low decomposition temperatures, the industrial applications of these carbonate salts are limited. In this study, the synthesis and characterization of novel switchable ionic liquids (SILs), based on the same concept as in ref. 2, is described. These new switchable solvents are composed of an amidine e.g. 1,8-diazabicyclo-[5.4.0]-undec-7-ene (DBU), 1,2,3- propanetriol (glycerol) and an acid gas, for example SO2 or CO2. Glycerol (glycerin) is a cheap and renewable feedstock obtained e.g. as a by-product of biodiesel production. The production of glycerol is expected to increase, since the production of biodiesel is increasing owing to the new directives, particularly in the EU (the EU directive on the promotion of the use of biofuels or other renewable fuels for transportation, 2003/30/EC).7 At the same time, the availability of glycerol increases and its price is expected to further decrease. Typically, 100 kg of glycerol is produced per ton of biodiesel.8 Most industrial flue gas streams contain SO2, COS and CS2 in addition to CO2 as acid gases, as a result of combustion of (fossil) fuels.9 These gases are easily available for a low price, since efficient flue gas scrubbing methods are used for purification of industrial flue gas streams. Utilization of these gases is of high interest in order to make industrial flue gas streams economically beneficial. A general reaction for the formation of SIL is shown in Scheme 1. The SILs presented in this study might be industrially more attractive than the earlier SILs2 since their decomposition temperature is higher.
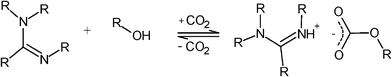 | ||
| Scheme 1 A general mechanism for the formation of SIL. | ||
This paper describes the preparation and characterization of switchable Ionic Liquids (SILs) based on glycerol, 1,8-diazabicyclo-[5.4.0]-undec-7-ene, (DBU), and acid gases (CO2 and SO2).
Results and discussion
SILs were prepared by bubbling either CO2 or SO2 through the mixture containing 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar amounts of DBU and glycerol. The weight increase with the carbonate SIL corresponds to the molar ratio of 3.14
1 molar amounts of DBU and glycerol. The weight increase with the carbonate SIL corresponds to the molar ratio of 3.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for, DBU to CO2, whereas for the glycerol sulfonate SIL, the molar ratios of 3
1 for, DBU to CO2, whereas for the glycerol sulfonate SIL, the molar ratios of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8 and 3
3.8 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for DBU to SO2 and DBU to glycerol were achieved, respectively. These results indicate that all OH-groups of the glycerol in the SO2 version have reacted upon formation of the ionic liquids according to Schemes 2 and 3. This observation was later on supported by NMR and FTIR studies.
1 for DBU to SO2 and DBU to glycerol were achieved, respectively. These results indicate that all OH-groups of the glycerol in the SO2 version have reacted upon formation of the ionic liquids according to Schemes 2 and 3. This observation was later on supported by NMR and FTIR studies.
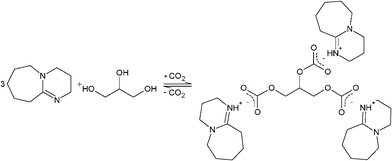 | ||
| Scheme 2 Proposed mechanism for the formation of SIL1, DBU-glycerol-CO2. | ||
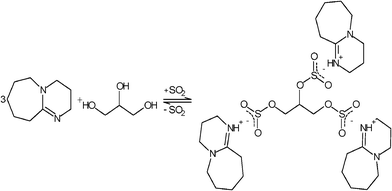 | ||
| Scheme 3 Proposed mechanism for the formation of SIL2, DBU-glycerol-SO2. | ||
Characterization of SILs
The structure of the SIL1 was confirmed by NMR and FTIR. Thereafter, several physico-chemical properties of both switchable ionic liquids were determined, such as viscosity, decomposition and phase transition temperatures, miscibility with various solvents, as well as their water content.Characterization of the DBU/Glycerol/CO2 system by 1H and 13C NMR spectroscopy
NMR spectra were measured on neat samples of DBU and glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), both before and after addition of CO2. Due to the high viscosity of the SILs at room temperature, the NMR spectra were recorded at 60 °C to minimize signal broadening. 1H as well as quantitative 13C NMR spectra were recorded. In order to assign the signals and to confirm the structures, different 2D NMR techniques were also utilized. The spectra of the samples are shown in Fig. 1 and 2.
1), both before and after addition of CO2. Due to the high viscosity of the SILs at room temperature, the NMR spectra were recorded at 60 °C to minimize signal broadening. 1H as well as quantitative 13C NMR spectra were recorded. In order to assign the signals and to confirm the structures, different 2D NMR techniques were also utilized. The spectra of the samples are shown in Fig. 1 and 2.
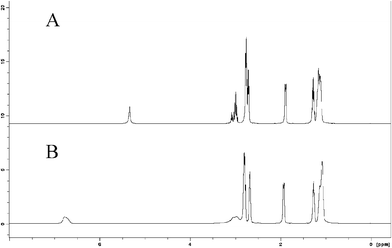 | ||
Fig. 1 (A) The 1H NMR spectra of a neat sample of DBU/glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (B) DBU/glycerol (3 1), (B) DBU/glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/CO2 recorded at 60 °C using external DMSO-d6 as a reference. 1)/CO2 recorded at 60 °C using external DMSO-d6 as a reference. | ||
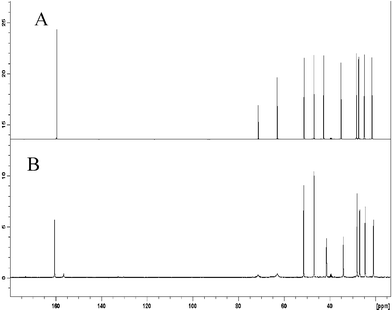 | ||
Fig. 2 (A) The 13C NMR spectra of a neat sample of DBU/glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (B) DBU/glycerol (3 1), (B) DBU/glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/CO2 recorded at 60 °C using external DMSO-d6 as a chemical shift reference. 1)/CO2 recorded at 60 °C using external DMSO-d6 as a chemical shift reference. | ||
The 1H NMR spectral data are shown in Table 1 and the 13C NMR spectral data are shown in Table 2. The atoms of DBU are numbered according to Fig. 3.
 | ||
| Fig. 3 Numbering of the carbon atoms in DBU. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and DBU:Glycerol (3
1) and DBU:Glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1):CO2. Shifts are referred to an external standard, DMSO (δ = 2.50 ppm)
1):CO2. Shifts are referred to an external standard, DMSO (δ = 2.50 ppm)
DBU:Glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DBU:Glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1):CO2 1):CO2 |
||||
|---|---|---|---|---|---|
| a Atom numbering shown in Fig. 3. | |||||
| Ha | nH | δ, ppm | H | nH | δ, ppm |
| DBU 3, 4, 5 | 18 | 1.06–1.23 | DBU 3, 4, 5 | 18 | 1.00–1.21 |
| DBU 10 | 6 | 1.28 | DBU 10 | 6 | 1.27 |
| DBU 6 | 6 | 1.89 | DBU 6 | 6 | 1.93 |
| DBU 9 | 6 | 2.71 | DBU 9 | 6 | 2.68 |
| DBU 2,11 | 12 | 2.73–2.82 | DBU 2,11 | 12 | 2.74–2.87 |
| Gly 1,3 | 4 | 2.93–3.04 | Gly 1, 2, 3 | 5 | 2.89–3.14 |
| Gly 2 | 1 | 3.08 | |||
| NH, OH | 3 | 5.34 | NH, OH | 3 | 6.73 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and DBU:Glycerol (3
1) and DBU:Glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1):CO2. Shifts are referred to an external standard, DMSO (δ = 39.50 ppm)
1):CO2. Shifts are referred to an external standard, DMSO (δ = 39.50 ppm)
DBU:Glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DBU:Glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1):CO2 1):CO2 |
||||
|---|---|---|---|---|---|
| a Atom numbering shown in Fig. 3. b Approximate values measured by quant. 13C NMR. c Strongly broadened signals. | |||||
| Ca | Rel. int.b | δ, ppm | C | Rel. int.b | δ, ppm |
| DBU 10 | 3 | 21.558 | DBU 10 | 3 | 20.882 |
| DBU 5 | 3 | 24.905 | DBU 5 | 3 | 24.489 |
| DBU 3 | 3 | 27.384 | DBU 3 | 3 | 26.926 |
| DBU 4 | 3 | 28.349 | DBU 4 | 3 | 28.086 |
| DBU 6 | 3 | 35.117 | DBU 6 | 3 | 34.087 |
| DBU 9 | 3 | 42.681 | DBU 9 | 3 | 41.437 |
| DBU11 | 3 | 46.984 | DBU11 | 3 | 46.952 |
| DBU 2 | 3 | 51.253 | DBU 2 | 3 | 51.422 |
| DBU 7 | 3 | 159.540c | DBU 7 | 3 | 160.545c |
| -O-COO− | 0.5 | 156.513 | |||
| Gly 1, 3 | 2 | 63.076c | Gly 1, 3 | 2 | 63.021c |
| Gly 2 | 1 | 71.375 | Gly 2 | 1 | 71.430 |
Most of the signals in the 1H NMR spectra were broad and complex and a detailed spectral analysis was not performed. However, the assignments of 1H and 13C NMR signals were performed using standard 2D correlation NMR spectroscopy.
The addition of CO2 resulted in quite small chemical shift changes (Δδ ≈ 0.01–0.05 ppm) in the 1H NMR spectrum of the mixture of DBU and glycerol (Fig. 1). However, the addition resulted in broadening of the signals. This was most marked in the signals of the C–H protons of glycerol (δ ≈ 3.89–3.14 ppm). The signal at δ = 6.73 ppm, representing a mixed signal of the exchangeable protons (O–H of glycerol and N–H of protonated DBU) was also broadened and shifted downfield from δ = 5.34, Fig. 1A to 6.73 ppm, Fig. 1B (Δδ ≈ 1.4 ppm), indicating an increase of acidity.
Upon addition of CO2, the signals in the 13C NMR spectrum shifted between 0.0 and 1.2 ppm (Fig. 2). The largest shifts (Δδ ≈ 1 ppm) were shown by the signals of the DBU carbons closest to the protonation site (C-6, C-9, upfield shifts, C-7, downfield shift), as expected. The signals of the glycerol carbons (centered at δ = 63.021 and 71.430 ppm, respectively), were very broad and contained signals of both glycerol and carbonated glycerol. Furthermore, a new signal appeared after CO2 addition at δ = 156.513 ppm, Fig. 2B. This broad signal was assigned to the carbonyl carbon of the formed carbonate.
In order to find definitive evidence of the formation of a carbonate, another experiment with a glycerol-DBU molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was performed. The 1H NMR and 13C NMR spectra now gave signals which could be assigned to underivatised glycerol as well as signals probably derived from the glyceryl carbonate. The intensity of the 13C NMR signal previously assigned to the carbonate carbon at δ = 156.513 ppm also increased, Fig. 4.
1 was performed. The 1H NMR and 13C NMR spectra now gave signals which could be assigned to underivatised glycerol as well as signals probably derived from the glyceryl carbonate. The intensity of the 13C NMR signal previously assigned to the carbonate carbon at δ = 156.513 ppm also increased, Fig. 4.
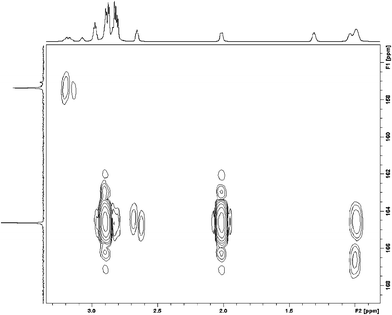 | ||
Fig. 4 The partial HMBC 2D NMR spectrum of DBU/Glycerol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/CO2. 1)/CO2. | ||
The presence of glycerol carbonate was shown by long-range 1H/13C correlation spectroscopy (HMBC). In this spectrum (Fig. 4), connectivity was observed between the H-1 protons in glycerol and the carbonate carbon as shown by the presence of a correlation signal situated at 3.18/157.34 (H/C) ppm. The other cross peaks seen in Fig. 4 are connectivities between the ring hydrogens and the bridgehead carbon in DBU.
The yield of DBUH+ [Gly Carbonate]− in the experiment with a DBU/glycerol ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is quite low, based on the NMR spectral data. Furthermore, the molar ratio of glycerol to glyceryl carbonate is ca. 2, according to the 13C NMR spectrum. The results also indicate that most of the carbonate formed is a monocarbonate at C-1 of glycerol.
1 is quite low, based on the NMR spectral data. Furthermore, the molar ratio of glycerol to glyceryl carbonate is ca. 2, according to the 13C NMR spectrum. The results also indicate that most of the carbonate formed is a monocarbonate at C-1 of glycerol.
On the addition of CO2, a quite complex mixture of equilibria is formed, in which the main reactions are the protonation of DBU and the formation of glyceryl monocarbonate. The result is thus roughly a mixture of two salts, [DBUH][Gly-O] and [DBUH][Gly-OCO2] (Scheme 4). Moreover, the mixture probably contains small amounts of carbonate formed by reaction at C-2 of glycerol in addition to bi- and tricarbonates.
![Proposed reactions for the formation of DBUH+ [Gly-Carbonate].](/image/article/2011/RA/c1ra00154j/c1ra00154j-s4.gif) | ||
| Scheme 4 Proposed reactions for the formation of DBUH+ [Gly-Carbonate]. | ||
FT-IR spectrum of the DBU/glycerol/CO2 system
The FT-IR spectra show a comparison of the starting materials (a) glycerol, (b) DBU to the product formed after bubbling CO2 through the DBU/glycerol mixture (c) (Fig. 5). Protonation of DBU, reduction of the OH vibrations from glycerol, new vibrations from the C–O–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and shifts in the C–O vibration should be observed according to Scheme 2. In the OH stretching region glycerol shows an IR band at 3286 cm−1. For the SIL, on the other hand, a broad band with maximum at 3255 cm−1 can be seen. The shift indicates the following changes; due to protonation of DBU, a N–H stretching vibration together with the unreacted OH-group gives the broad spectral response for SIL at 3000–3600 cm−1(Fig. 5c). The bands at 2935 and 2879 cm−1 were from the C–H stretching vibration in the ring and from the alcohol and should therefore also be seen even after bubbling CO2. DBU shows in the range of 1600–1300 cm−1 bands from the C
O group and shifts in the C–O vibration should be observed according to Scheme 2. In the OH stretching region glycerol shows an IR band at 3286 cm−1. For the SIL, on the other hand, a broad band with maximum at 3255 cm−1 can be seen. The shift indicates the following changes; due to protonation of DBU, a N–H stretching vibration together with the unreacted OH-group gives the broad spectral response for SIL at 3000–3600 cm−1(Fig. 5c). The bands at 2935 and 2879 cm−1 were from the C–H stretching vibration in the ring and from the alcohol and should therefore also be seen even after bubbling CO2. DBU shows in the range of 1600–1300 cm−1 bands from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N ring stretching vibrations. In SILs the band at 1610 cm−1 is still strong (from C
N ring stretching vibrations. In SILs the band at 1610 cm−1 is still strong (from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) but the protonation of DBU gives rise to the new band at 1643 cm−1(Fig. 5c).12 At 1274 and 1050 cm−1, in SIL spectrum new bands can be observed owing to the asymmetric and symmetric C–O–C stretching vibrations, respectively. C–O stretching vibrations can be seen in Fig. 5 for glycerol at 1030 cm−1. Ketones, aldehydes, carboxylic acids, lactones, amides and alike show a strong C
N) but the protonation of DBU gives rise to the new band at 1643 cm−1(Fig. 5c).12 At 1274 and 1050 cm−1, in SIL spectrum new bands can be observed owing to the asymmetric and symmetric C–O–C stretching vibrations, respectively. C–O stretching vibrations can be seen in Fig. 5 for glycerol at 1030 cm−1. Ketones, aldehydes, carboxylic acids, lactones, amides and alike show a strong C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching absorption band in the region of 1870–1540 cm−1.12–15 Furthermore, a new band at 1786 cm−1 was observed in the spectrum of the SIL but it is weak.
O stretching absorption band in the region of 1870–1540 cm−1.12–15 Furthermore, a new band at 1786 cm−1 was observed in the spectrum of the SIL but it is weak.
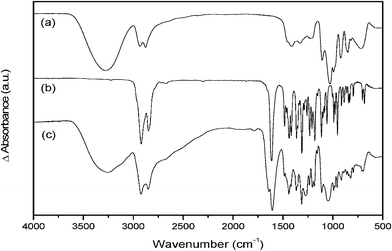 | ||
| Fig. 5 FT-IR spectra of (a) glycerol, (b) DBU and (c) DBU/glycerol after bubbling CO2. | ||
Physical properties of the SILs
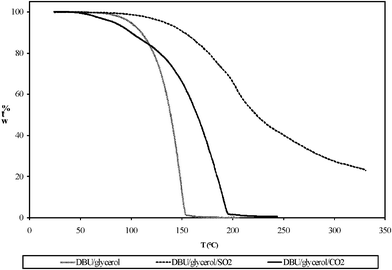
The physical properties of the switchable ionic liquids synthesized from either bubbling CO2 or SO2 through a mixture of DBU and glycerol are presented in Table 3. The viscosities of the SILs were much higher than the corresponding ones in their non-ionic form (Table 4). The onset of the decomposition temperature of these switchable ionic liquids were, according to TGA, at ∼60 °C for SIL1 and ∼120 °C for SIL2, respectively, under nitrogen atmosphere (Fig. 6). However, a shoulder in the weight-loss curves is observed at ∼85 °C, for the SIL1, and at more than 200 °C, for the SIL2, possibly indicating a change (‘collapse’) in the nature of the compound. The reason might be that the acid gas liberated to the solution from a deteriorating carbonate/sulfonate structure is until that temperature able to loosely maintain the equilibrium in between the released, soluted gas and the carbonate/sulfonate structure. The SO2 SIL exhibited higher decomposition temperature compared to the CO2 SIL due to the strength of the acid gas binding explained by Heldebrant et al.13 The strength of the acid gas binding was linearly correlated to the Lewis acidity of the acid gas, thus the decomposition temperature of SO2 SIL is higher compared to the CO2 SIL.13 These ionic liquids do not have clear melting/freezing points. However, the switchable ionic liquid containing SO2 exhibited slightly higher glass transition temperature compared to the one containing CO2 (Fig. 7). According to the Karl-Fischer analysis, both of the SILs contained less than 0.1 wt% of moisture.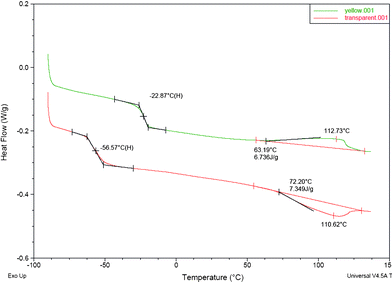 | ||
| Fig. 7 The DSC graphs of (a) SIL2 and (b) SIL1. | ||
| SIL | Water content (%) | Glass transition temperature (°C) | Decomposition temperature (onset) (°C) | Viscosity (PaS) |
|---|---|---|---|---|
| a The viscosity was measured from solution when no more weight increase was observed at 25 °C, viscosity of DBU-glycerol was 0.96 PaS. The decomposition temperatures were measured in nitrogen atmosphere. | ||||
| DBU/glycerol/CO2 | 0.08 | −57 | 60 | 160a |
| DBU/glycerol/SO2 | 0.04 | −23 | 120 | n.d |
| Solvent | Dielectric constant εr | Non-ionic form | Ionic form | |||
|---|---|---|---|---|---|---|
| (25 °C)16 | DBU/glycerol mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molar ratio) 1, molar ratio) |
DBU | Glycerol | DBU/glycerol/SO2 | DBU/glycerol/CO2 | |
| a y: miscible, n: immiscible, *: formation of white crystals. | ||||||
| Hexane | 1.88 | n | y | n | n.d. | n |
| n-Decane | 2.0 | n | y | n | n.d. | n |
| Toluene | 2.38 | n | y | n | n | n |
| Ethylacetate | 6.02 | y | y | n | n.d. | n |
| Tetrahydrofuran | 7.58 | y | y | n | n.d. | * |
| Dichloromethane | 8.93 | y | y | n | n.d. | y |
| Acetone | 20.56 | y | y | n | * | * |
| Ethanol | 24.55 | y | y | y | y | y |
| Methanol | 32.66 | y | y | y | y | y |
| Acetonitrile | 35.94 | y | y | n | n.d. | y |
| Water | 78.30 | y | y | y | y | y |
The results from miscibility tests with organic solvents are presented in Table 4. The SILs were mainly miscible with polar solvents such as water and methanol. In general, SIL1 is miscible with solvents having dielectric constant over 8 at 25 °C. However, this was not the case with acetone, which has the dielectric coefficient of 20.56 at 25 °C and is a polar solvent. Instead, some reaction occurred, since white crystals were formed when acetone was mixed with the SIL1. The reason for the formation of white crystals is not yet known. The same reaction occurred also with tetrahydrofuran. SIL2 was not miscible with hexane and acetone, but was miscible with ethanol and water. The SILs were immiscible with the solvents exhibiting a value of dielectric coefficient less than 8 (Table 4).
The change in polarity was studied using Nile Red as a solvatochromic dye. The mixture of DBU and glycerol with Nile Red has a red color which is common for low polarity solvents (Fig. 8a).1–3 However, when CO2 was bubbled through the mixture, the color changed from red to milky as the polarity of the liquid increases, as can be seen in Fig. 8b. The same color change occurred to the other direction when nitrogen was bubbled through the liquid. It can be concluded that CO2 can be used as a trigger for switching the DBU-glycerol mixture from molecular to ionic form and N2 for switching the ionic form back to the molecular mixture.
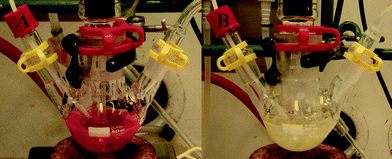 | ||
| Fig. 8 The mixture of DBU and glycerol with Nile red, (A) before and (B) after CO2 was bubbled through the liquid until there was no weight increase. | ||
Conclusions
Switchable ionic liquids that were thermally stable up to 120 °C were synthesized from a cheap and renewable feedstock, glycerol, together with DBU and an acid gas. Glycerol is a typical byproduct from biodiesel production, whereas CO2 or SO2 are acid gases formed during combustion of fossil fuels. The mixture of glycerol and DBU was successfully converted from a molecular liquid mixture to an ionic liquid by bubbling CO2 or SO2 through it. The switching process is readily reversible since CO2 can be decoupled out with a flow of N2 and this process can be enhanced by heating the SIL to its decomposition temperature. The structures of the SILs were confirmed by FTIR and NMR.FTIR spectra indicated that DBU was protonated via formation of N–H and C=NH+ bands. Furthermore, deprotonation of glycerol was confirmed by FTIR. NMR spectra of the SIL showed the formation of glyceryl carbonate.
The SIL synthesized by bubbling an acid gas through a mixture of DBU and glycerol exhibited viscosity increase from 0.96 PaS in their molecular form to 160 PaS in the ionic form, when no more weight increase was observed during CO2 bubbling. They also exhibited different solubility properties as in ionic form compared to their molecular version. The polarity change of DBU-glycerol-CO2 system was visually demonstrated by the addition of Nile red dye, when the color of the low polar molecular solvent changed from red to light yellow as the ionic liquid was formed.
Experimental section
Chemicals
DBU (Aldrich, 99% grade) was distilled in vacuum under a nitrogen atmosphere and dried over 4 Å molecular sieves. Glycerol (Aldrich, 99+ %) was used as received. Supercritical grade CO2 (AGA, 99.999%, H2O < 0.5 ppm), nitrogen (AGA, 99.998%, H2O < 3 ppm) and SO2 (AGA, 99.98%, H2O < 200 ppm, N2 < 14 ppm) were used as received. Solvents used for miscibility tests were as follows; Acetone (J.T. Baker, 99.5%), acetonitrile (Sigma Aldrich, 99.8%), n-decane (Fluka, 95%), dichloromethane (Sigma Aldrich, 99.9%), ethanol (Altia, 99.5%), ethyl acetate (Fluka, 99.5%), hexane (Merck, 99%), tetrahydrofuran (Lab-Scan, 99.8%) and toluene (Merck, 99.9%).Preparation and characterization of switchable ionic liquids (SILs)
The DBU glyceryl carbonate10 was prepared via bubbling of CO2 through a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio solution of dried DBU and glycerol, whereas the corresponding sulfonate was prepared by bubbling SO2 through a similar mixture as in the CO2 version. DBU (43.843 g) and glycerol (8.848 g) were used when preparing a 3
1 molar ratio solution of dried DBU and glycerol, whereas the corresponding sulfonate was prepared by bubbling SO2 through a similar mixture as in the CO2 version. DBU (43.843 g) and glycerol (8.848 g) were used when preparing a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of the mixture. Thereafter, the mixture was charged into a dry flask in a glove box under N2 atmosphere. A narrow-gauge glass tube was inserted and either CO2 or SO2 was bubbled through the liquid at a constant rate until there was no more weight increase. The reaction was notably exothermic and the solution was stirred mechanically throughout the bubbling cycle. Also, during the course of the reaction the liquid became viscous.
1 molar ratio of the mixture. Thereafter, the mixture was charged into a dry flask in a glove box under N2 atmosphere. A narrow-gauge glass tube was inserted and either CO2 or SO2 was bubbled through the liquid at a constant rate until there was no more weight increase. The reaction was notably exothermic and the solution was stirred mechanically throughout the bubbling cycle. Also, during the course of the reaction the liquid became viscous.
The synthesized switchable ionic liquid (SIL) was characterized by means of nuclear magnetic resonance spectroscopy (NMR) Bruker AV 600 to confirm the presence of carbonate salts. 1H (600 MHz) and 13C (150 MHz) NMR spectra of the SIL was recorded neat at 60 °C. The SIL was also characterized by FTIR-ATR spectroscopy, with a Bruker IFS66/S FTIR spectrometer equipped with a mercury-cadmium-telluride (MCT) detector. A small sized cell made from Teflon® was used in the spectroscopic measurements. The cell was attached to the FTIR spectrometer using a beam condenser (Harrick, 4XF-BR3). Each spectrum consists of 64 co-added interferograms with a resolution of 4 cm−1. The DBU and glycerol spectra were related to a reference spectrum taken from an empty cell whereas in the case of SIL, spectra from a cell filled with a neat DBU-glycerol mixture were used as reference spectra. The experimental setup for the FTIR-ATR technique has been described elsewhere.11
The properties of the synthesized SILs were determined by the following methods: Thermogravimetric analysis (TGA) was carried out to determine the decomposition onset of the salt. Differential scanning calorimeter (DSC TA instrument Q1000 series) was applied for the determination of phase transitions of the salts.
The water contents of the SILs were determined by using Karl-Fisher titration using hydranal composite 2 (Fluka) as a titrant.
Miscibility tests with organic solvents were conducted under an inert atmosphere. Various solvents were mixed with the Switchable Ionic Liquids/precursor mixtures, both in ionic as well as in molecular form.
The viscosities of the solvents were measured using a VOR Bohlin Rheometer at 25 °C. The low viscosity samples were measured with the bob/cup geometry and the high viscosity samples with the cone/plate geometry. The viscosity was calculated by taking the average of ten experimental points (1 point every 30 s) at a constant shear rate of 11.6 per second.
The change in polarity of the mixture (DBU-glycerol) was tested visually, by the addition of a solvatochromic dye, Nile red (Sigma) into the mixture. The change in the color was related to increase or decrease in polarity.
Acknowledgements
This work is part of the activities at the Åbo Akademi Process Chemistry Centre (ÅA-PCC) within the Finnish Centre of Excellence Program (2000–2011) appointed by the Academy of Finland. Forest cluster Ltd and The Finnish Funding Agency for Technology and Innovation (TEKES) are gratefully acknowledged for financial support. In Sweden, the Bio4Energy program is acknowledged.References
- L. Phan, D. Chiu, D. J. Heldebrant, H. Huttenhower, E. John, L. Xiaowang, P. Pollet, R. Wang, C. A. Eckert, C. L. Liotta and P. G. Jessop, Ind. Eng. Chem. Res., 2008, 539–545 CrossRef CAS.
- P. G. Jessop, D. J. Heldebrant, L. Xiaowang, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS.
- P. G. Jessop and W. Leitner, Chemical Synthesis Using Supercritical Fluids, VCH Wiley, Weinheim, 1999 Search PubMed.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2006 Search PubMed.
- S. Varanasi, A. C. Shall, P. A. Dado, J. Anderson, K. Rao and P. Paripati, WO 2008/11291A2.
- G. D'Andola, L. Szarvas, K. Massonne and V. Stegmann, WO 2008/043837.
- DIRECTIVE 2003/30/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 8 May 2003 on the promotion of the use of biofuels or other renewable fuels for transport, Accessed 31 March, 2011.
- J. Clark and F. Deswarte, Introduction to Chemicals from Biomass, Wiley, Chichester, 2008 Search PubMed.
- Y. Pu, N. Jiang and A. Ragauskas, J. Wood Chem. Technol., 2007, 23–33 CrossRef CAS.
- J.-P. Mikkola, P. Virtanen, P. Mäki-Arvela and I. Anugwom, Patent application Filed Nov 2010, Application Number FI 20106142.
- H. Neugebauer, Macromol. Symp., 1995, 61–73 CrossRef CAS.
- D. J. Heldebrant, P. G. Jessop, C. A. Thomas, C. A. Eckert and C. L. Liotta, Journal of Organic Chemistry, 2005, 5335–5338 CrossRef CAS.
- D. Heldebrant, C. R. Yonker, P. G. Jessop and L. Phan, Chem.–Eur. J., 2009, 7619–7627 CrossRef CAS.
- R. M. Silverstein, G. C. Bassler and T. C. Morrill, Spectrometric Identification of Organic Compounds, John Wiley & Sons, 1981 Search PubMed.
- T. Yu, T. Yamada, G. C. Gaviola and R. G. Weiss, Chem. Mater., 2008, 20, 5337–5344 CrossRef CAS.
- C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, VHC, Weinheim, 1990 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |