Photoluminescence enhancement of Eu3+ by energy transfer from Bi2+ to Eu3+ in bismuth glass nanocomposites
Shiv Prakash
Singh
and
Basudeb
Karmakar
*
Glass Science and Technology Section, Glass Division, CSIR-Central Glass and Ceramic Research Institute, 196 Raja S.C. Mullick Road Kolkata, 700 032, West Bengal, India. E-mail: basudebk@cgcri.res.in
First published on 5th September 2011
Abstract
Bi2+-enhanced photoluminescence of Eu3+ doped Bi0-bismuth glass nanocomposites are demonstrated here. The generation of bismuth nanoparticles (NPs) and its typical surface plasmon resonance at 460 nm are controlled by the oxidative method instead of the conventional reduction technique during melt-quench processing. TEM images evidence the presence of spherical Bi0 NPs of 10–15 nm sizes and SAED pattern reveals their crystalline rhombohedral phase formation. The enhanced photoluminescence of Eu3+ is found to occur at 613 and 703 nm due to 5D0→7F2 and 5D0→7F4 transitions respectively. We believe that it has happened due to energy transfer from Bi2+ to Eu3+.
Introduction
Rare earth doped glasses have attracted much attention world wide for their extensive applications such as in glass lasers, optical fiber amplifiers, optoelectronic and memory devices, flat-panel displays, etc. The luminescent properties of Eu3+ have fascinated researchers as a dopant since they can emit red fluorescence with high luminescence efficiency under UV light excitation.1 Many researchers have activated it to enhance the europium emission intensity by co-doping with either another rare earth or transition metal ions in different matrices. Moreover, enhancement of rare earth luminescence in presence of metal nanoparticles is again a very fascinating area for researchers.2,3 In this context, bismuth ions are one of efficient luminescent activator with applications in lasers and a sensitizer for some rare earth ions, and are nowadays being investigated.4 However, the luminescence origin from bismuth ions is still unclear and hence is highly debated. Many reports are available for the enhancement of luminescence of Eu3+ in presence of Bi3+ as a sensitizer in various matrices.5 But the enhancement of luminescence of Eu3+ in the presence of bismuth ions of other valance states has not been reported previously.In view of above, bismuth glasses are considered as one of the most important amongst the heavy metal oxide glasses as a host matrix for rare earths.6 But the problem associated with these glasses is their dark-brown or black coloration due to formation of metallic bismuth (Bi0) during melting.7 These glasses are, therefore, restricted to a great extent for optical and photonic applications. Consequently, synthesis of transparent bismuth glasses by suppressing the formation of Bi0 nanoparticles (NPs) in bismuth glasses is very important for both scientific as well as technological points of view. Recently, we have reported the suppression of metallic bismuth formation in bismuth glasses by the oxidative process using KNO3 and KClO4.8
In this communication, we demonstrate the enhancement of the emission intensity of Eu3+ ion by the Bi2+ ions during controlled formation of Bi0 using K2S2O8 as oxidizing agent in the B2O3–ZnO–Bi2O3–SiO2–K2O glass system.
Experimental
Glasses were prepared by the melt-quench technique with the composition (wt%) 19B2O3–23ZnO–45Bi2O3–9SiO2–(4−x)K2O–xK2S2O8 (where x is 0, 0.2, 1.0 and 3.0, and named as a, b, c, and d respectively). All the glasses were doped with 0.5 wt% of Eu2O3 and designated as e, f, g and h respectively. The composition of glass has also been provided in Table 1. Bi2O3, H3BO3, ZnO and K2CO3 from Loba Chemie; SiO2 (Bremthaler/Quarzitwerk), K2S2O8 (Merck) and Eu2O3 (Alfe Aesar) were used as raw materials. The batch for 25 g glass was melted in a silica crucible at 1100 °C for 30 min followed by casting and annealing at 420 °C for 2 h in air.| Glass identity | Oxidant, K2S2O8 content, x, wt% | Concentration of Eu2O3 in excess, wt% | Emission center, nm |
|---|---|---|---|
| a | 0.0 | — | 703 |
| b | 0.2 | — | 703 |
| c | 1.0 | — | 703 |
| d | 3.0 | — | 703 |
| e | 0.0 | 0.5 | 587, 613, 652, 703 |
| f | 0.2 | 0.5 | 587, 613, 652, 703 |
| g | 1.0 | 0.5 | 587, 613, 652, 703 |
| h | 3.0 | 0.5 | 587, 613, 652, 703 |
The UV-Vis absorption spectra were recorded using a Perkin-Elmer (Lambda 20) double beam UV-visible spectrophotometer. The X-ray diffraction patterns of the bulk samples were recorded in an X'pert Pro MPD diffractometer (PANalytical) operating at 40 kV and 30 mA using Ni-filtered Cu-Kα radiation with the X'celerator with step size 0.05° (2θ) step time 0.5 s, from 5° to 90°. The TEM and SAED images were taken using a FEI instrument (Tehnai-30, ST G2) operating at an accelerating voltage of 300 kV. The fluorescence spectra were measured with a Perkin Elmer Luminescence Spectrophotometer (Model LS55) exciting at 393 nm using a Xenon lamp as the excitation source.
Results and discussion
Fig. 1 (A) shows the UV-Vis absorption spectra of samples a, b, c and d with an increase in concentration of oxidant K2S2O8 (0 to 3 wt%) in the glasses. The base glass is deep black in color, but the transparency increases with addition of K2S2O8. The glass melted using 3 wt% of K2S2O8 is highly transparent. The base glass reveals a very weak surface plasmon resonance (SPR) band (shown in the inset) due to the presence of a huge amount of Bi0 NPs. When 0.2 wt% K2S2O8 has added to the base glass, the surface plasmon band has visualized very distinctly. The intensity of SPR band gradually decreases with addition of further amount of K2S2O8 (up to 3.0 wt%). The observed broad absorption band at 460 nm is appeared due to the SPR of Bi0 NPs.7–9 The SPR comes up due to collective oscillation of conduction band electrons of metallic nanoparticles embedded in dielectric host in response to optical excitation. Peng et al.7 have found a broad absorption peak around 465 nm for Bi2O3 contained glasses and assigned the peak as surface plasmon resonance (SPR) of metallic bismuth. Singh and Karmakar8 and Khonthon et al.9 have also described such absorption band of Bi0 NPs at 460 nm in the different glass matrices, which correlates well with the present result. The UV-Vis absorption spectra of the glasses containing a constant amount of Eu2O3 (0.5 wt%) are also shown in the Fig. 1 (B). They exhibit characteristic absorption bands of Eu3+ at 393 and 464 nm which are attributed to transitions from 7F0 to 5L6 and 5D2 states respectively as well as broad SPR band at 460 nm.1 The intensity of SPR band gradually decreases with addition of oxidant K2S2O8, but the characteristic absorption bands of Eu3+ remain unchanged in all the glasses (e–h).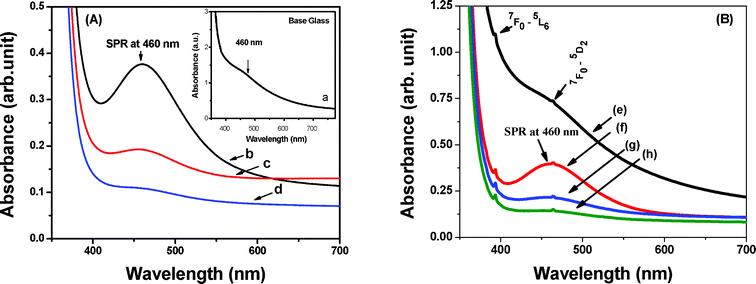 | ||
| Fig. 1 Absorption spectra of glasses (A) without and (B) with Eu2O3 contained glasses. For composition, see Table 1. | ||
The significant effects of strong oxidizing agent K2S2O8 are, therefore, the controlled generation of Bi0 NPs and its SPR which results in the elimination of blackening color of bismuth glasses. By comparing the standard reduction potentials,10 it is clear that the standard reduction potential of S2O82−/SO42− (2.01 V) is much higher than that of Bi+/Bi0 (0.50 V), Bi3+/Bi0 (0.31 V), Bi3+/Bi+ (0.20 V) and Bi3+/Bi2+ (< 0.20 V) species. Consequently, it easily favors the suppression of the formation of Bi0 NPs and the glasses gradually become more transparent with increasing K2S2O8 content.
Fig. 2 shows the XRD pattern of the glass obtained by using 0.2 wt.% of K2S2O8 (Glass b) as a representative. Although the glasses have not shown very distinct XRD patterns, the unresolved peaks depict around 27.63 and 28.78 degrees 2θ indicate the existence of d116 and d122 Miller planes of rhombohedral metallic bismuth (JCPDS file no. 01-0699). However, TEM image (Fig. 3) of the same glass (Glass b) confirms the clear existence of bismuth nanoparticles. The TEM image shows 10–15 nm size of bismuth nanoparticles. The selected area electron diffraction (SAED) patterns inserted in the inset of Fig. 3, show d104 and d012 planes of bismuth nanoparticles correspond to rhombohedral crystal system (JCPDS file no. 85-1330). These results of TEM and SAED pattern again support that the glass contained Bi0 NPs and correlated well with the SPR of Bi0 NPs.
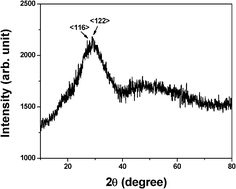 | ||
| Fig. 2 X-ray diffraction pattern of the glass obtained by using 0.2 wt% of K2S2O8. | ||
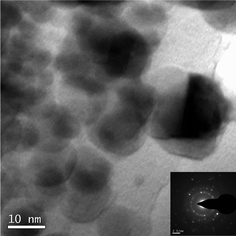 | ||
| Fig. 3 TEM image of the glass containing 0.2 wt% of K2S2O8. Its SAED pattern (inset) shows <104> and <012> <hkl> planes of rhombohedral bismuth (JCPDS file 85-1330). | ||
In Fig. 4 (A), emission spectra were obtained at 587, 613, 652 and 703 nm due to 5D0→7F1, 5D0→7F2, 5D0→7F3, and 5D0→7F4 transitions respectively of Eu3+ when excited by 393 nm at a fixed concentration of Eu3+ for all samples (e, f, g and h). These emission spectra compared with the base glass, it is observed that a peak has appeared at 703 nm in the base glass. This peak is also present in the glasses containing Eu3+ ions. To examine the origin of this peak, the emission spectra of all the glasses without containing Eu3+ has been analyzed. In Fig. 4 (B), the emission spectra at 703 nm of the glasses a, b, c and d has been recorded by excitation at 393 nm. This emission intensity at 703 nm increases with decrease in the intensity of SPR band of bismuth nanoparticles. The excitation spectra at 393 nm for the glasses a, and d have been shown in Fig. 5 to examine the origin of the emission center at 703 nm. The composition and emission centers have also been listed in Table 1. Here, the emission intensity significantly increases with the concentration of K2S2O8. Therefore, it is reasonable to believe that some emission center of bismuth gradually formed due to oxidation process during suppression of Bi0 formation. Various researchers have reported about the different emission centers for the different valence state of bismuth such as Bi3+, Bi2+ and Bi+.11–18 However, it is difficult to predict the exact valence state of Bi ion formed during melting process as their reduction potentials are very close to each other as indicated earlier.
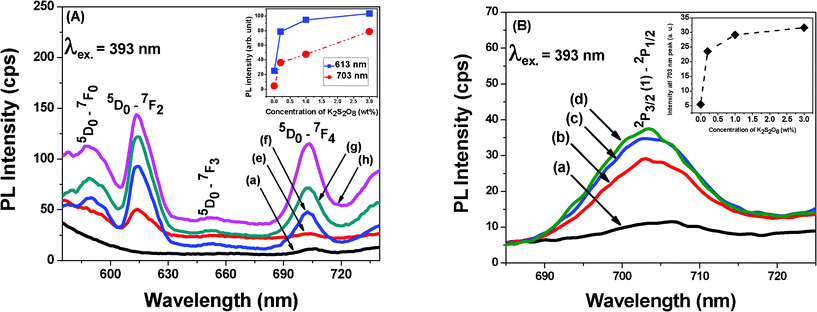 | ||
| Fig. 4 Photoluminescence (PL) spectra for excitation at 393 nm of (A) with and (B) without Eu2O3 contained glasses. In insets of (A) and (B), the PL intensities have shown as a function of concentration of K2S2O8. For composition, see Table 1. | ||
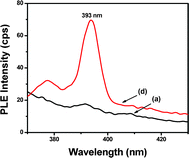 | ||
| Fig. 5 Photoluminescence excitation (PLE) spectra for emission at 703 nm. For composition, see Table 1. | ||
The typical luminescence spectra of Bi3+ centered in the blue to green region when excited in the UV region has been reported by some researchers.11,12 The emission due to Bi+ has been reported in NIR region.12–14 Zhou et al.12 have found emission due to Bi+ ions in the NIR region centered at 1100 and 1400 nm when exited at 532 and 800 nm respectively. Peng et al.13 have demonstrated NIR emission centered at 1250 nm in the BBSPS glasses, upon optical excitation at 785 nm and this emission center ascribed to Bi+ species. The energy levels of Bi0 was explained by Peng et al.15 and they assigned red and NIR emission bands due to Bi0 at 630 nm and 843 nm to electron transition 2D5/2→4S3/2 and 2D3/2→4S3/2 respectively. But, in this study, the luminescence due to Eu3+ increases instead of decreasing with the decrease in Bi0 concentration. Therefore, here the emission band at 703 nm could not be assigned to Bi3+, Bi+ or Bi0 species.
Hamstra et al.16 have suggested the red luminescence due to Bi2+ ions, varying in the range of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–16
100–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 (585–625 nm) in the alkaline earth metal sulfates. Ren et al.17 have exposed the visible luminescence spectra excited in the range 400–650 nm. The observed luminescence, when excited within this range, consists of two bands centered between 650 and 760 nm respectively. They assigned this red luminescence as characteristic spectra of Bi2+. Such red luminescence bands arise from the electron transition between the first excited level 2P3/2 (1) and ground level 2P1/2 of Bi2+. Because the absorption band observed by them is about 500 nm and the red luminescence from the glass are almost the same with the absorption and luminescence from Bi2+ activated crystals. They have ascribed this peak to the electron transition 2P1/2→2P3/2 and 2P3/2→2P1/2, respectively. Zhou et al.12 have also reported red emission due to Bi2+ ions. Taking into account all the above arguments, it is reasonable to assign the observed emission band at 703 nm to the emission of Bi2+ ions.
000 cm−1 (585–625 nm) in the alkaline earth metal sulfates. Ren et al.17 have exposed the visible luminescence spectra excited in the range 400–650 nm. The observed luminescence, when excited within this range, consists of two bands centered between 650 and 760 nm respectively. They assigned this red luminescence as characteristic spectra of Bi2+. Such red luminescence bands arise from the electron transition between the first excited level 2P3/2 (1) and ground level 2P1/2 of Bi2+. Because the absorption band observed by them is about 500 nm and the red luminescence from the glass are almost the same with the absorption and luminescence from Bi2+ activated crystals. They have ascribed this peak to the electron transition 2P1/2→2P3/2 and 2P3/2→2P1/2, respectively. Zhou et al.12 have also reported red emission due to Bi2+ ions. Taking into account all the above arguments, it is reasonable to assign the observed emission band at 703 nm to the emission of Bi2+ ions.
Emission intensity of Eu3+ ions in Fig. 4 (A) increased monotonically with the concentration of K2S2O8 as shown in the inset, evidencing the energy transfer (ET) mechanism from Bi2+ to Eu3+ ions. Such energy transfer mechanism can also be well understood from the excitation spectra of the glass a, and d as shown in Fig. 5. The glasses a, and d revealed the excitation center at 393 nm for the emission at 703 nm which also matched well with the excitation wavelength of Eu3+. Hence, upon excitation at 393 nm, both Bi2+ and Eu3+ ions are simultaneously excited from their ground levels to the respective higher energy levels 2P3/2(2) and 5L6. The energy level diagram of Bi2+ and Eu3+ ions and possible energy transfer process are schematically presented in Fig. 6. An excited Bi2+ ion relaxes from 2P3/2 (2) to 2P3/2 (1) state nonradiatively and transfers the energy to a neighboring 5D0 level of Eu3+ ions, which is energetically close to each other. Thus, it (Bi2+) enhances the emission intensities of 5D0→7F2 and 5D0→7F4 transitions of Eu3+ ions at 613 and 703 nm respectively. A similar study has been carried out by Guo et al..19 They have systematically investigated the energy transfer mechanism between luminescent centers of molecule-like, non-plasmonic Ag particles (ML-Ag-particles) and Eu3+. They have demonstrated that upon 347 nm excitation, ML-Ag-particles are excited from the ground state to the excited state. Because the excited state of ML-Ag particles and the 5L6 level of Eu3+ ions are energetically close to each other, energy transfer from ML-Ag-particles to Eu3+ ions can easily proceed.
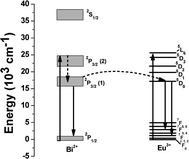 | ||
| Fig. 6 Schematic representation of energy transfer from Bi2+ to Eu3+ in bismuth glass. | ||
Conclusions
Eu3+ doped Bi0-bismuth glasses nanocomposites containing Bi2+ were prepared. Luminescence investigations revealed the enhancement of emission intensity of Eu3+ ion. This enhancement in the emission intensity is supposed to be due to energy transfer from Bi2+ to Eu3+ ion. This is the first demonstration of energy transfer from Bi2+ to Eu3+ ions in bismuth glass. We believe that these findings would certainly facilitate more study on the luminescence properties of bismuth active centers and their application in photonics.Acknowledgements
SPS expresses his sincere gratitude for the financial support of the CSIR, New Delhi, India in the form of CSIR-SRF under the sanction number 31/15(78)/2010-EMR-I. The authors thank Director of the institute for his kind permission to publish this paper. They also thankfully acknowledge the Electron Microscope Division of this institute for recording the TEM and SAED images.References
- C. A. Kodaira, H. F. Brito, O. L. Malta and O. A. Serra, J. Lumin., 2003, 101, 11 CrossRef CAS.
- M. Wu, J. R. Lakowicz and C. D. Geddes, J. Fluoresc., 2005, 15, 53 CrossRef CAS.
- Q. Wang, F. Song, C. Ming, H. Zhao, J. Liu, C. Zhang, S. Lin and E. Y. B. Pun, J. Opt. Soc. Am. B, 2011, 28, 220 CrossRef CAS.
- J. Ruan, E. Wu, H. Zeng, S. Zhou, G. Lakshminarayana and J. Qiu, Appl. Phys. Lett., 2008, 92, 101121 CrossRef.
- L. Chen, Y. Jiang, Y. Yang, J. Huang, J. Shi and S. Chen, J. Phys. D: Appl. Phys., 2009, 42, 215104 CrossRef.
- G. F. Yang, D. M. Shi, Q. Y. Zhang and Z. H. Jiang, J. Fluoresc., 2008, 18, 131 CrossRef CAS.
- M. Peng, C. Zollfrank and L. Wondraczek, J. Phys.: Condens. Matter, 2009, 21, 285106 CrossRef.
- S. P. Singh and B. Karmakar, Mater. Chem. Phys., 2010, 119, 355 CrossRef CAS.
- S. Khonthon, S. Morimoto, Y. Arai and Y. Ohishi, Opt. Mater., 2009, 31, 1262 CrossRef CAS.
- P. Vanýsek, Electrochemical Series, in CRC Hand Book of Chemistry and Physics, CRC Press, London, p. 22, 1994. Search PubMed.
- X. T. Wei, Y. H. Chen, X. R. Cheng, M. Yin and W. Xu, Appl. Phys. B: Lasers Opt., 2010, 99, 763 CrossRef CAS.
- S. Zhou, N. Jiang, B. Zhu, H. Yang, S. Ye, G. Laksminarayana, J. Hao and J. Qiu, Adv. Funct. Mater., 2008, 18, 1407 CrossRef CAS.
- M. Peng, Q. Zhao, J. Qiu and L. Wondraczek, J. Am. Ceram. Soc., 2009, 92, 542 CrossRef CAS.
- Y. Arai, T. Suzuki, Y. Ohishi, S. Morimoto and S. Khonthon, Appl. Phys. Lett., 2007, 90, 261110 CrossRef.
- M. Peng, C. Zollfrank and L. Wondraczek, J. Phys.: Condens. Matter, 2009, 21, 285106 CrossRef.
- M. A. Hamstra, H. F. Folkerts and G. Blasse, J. Mater. Chem., 1994, 4, 1349 RSC.
- J. Ren, G. Dong, S. Xu, R. Bao and J. Qiu, J. Phys. Chem. A, 2008, 112, 3036 CrossRef CAS.
- H. Sun, Y. Sakka, M. Fujii, N. Shirahata and H. Gao, Opt. Lett., 2011, 36, 100 CrossRef CAS.
- H. Guo, X. F. Wang, J. D. Chen and F. Li, Opt. Express, 2010, 18, 18900 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
