The chemical value of wheat straw combustion residues
Jennifer R.
Dodson
,
Andrew J.
Hunt
,
Vitaly L.
Budarin
,
Avtar S.
Matharu
and
James H.
Clark
*
Green Chemistry Centre of Excellence, Department of Chemistry, The University of York, Heslington, York, UK. E-mail: james.clark@york.ac.uk; Fax: +44 (0)1904 322705; Tel: +44 (0)1904 322559
First published on 23rd August 2011
Abstract
Considerable mineralogical changes occur during the combustion of biomass such as wheat straw, which lead to variation in the physical, chemical and textural properties of the ashes formed. Mineralogical and chemical changes occur above combustion temperatures of 500 °C causing reductions in the solubility of potassium, chloride, silica and sulfate. This can be directly correlated to the evaporation of KCl, dissociation of K2SO4, softening of silica and formation of alkali silicates. Calcium extraction increased above combustion temperatures of 700 °C due to the decomposition of CaCO3. We have discovered, for the first time, that the inherent alkali in unleached wheat straw is sufficient to solubilise up to 30% of the silica in the ash at room temperature. This could be used to produce potassium silicate solution as a new valuable by-product of wheat straw combustion. It was also found that incomplete combustion at high temperatures, following leaching of ashes can lead to the formation of porous activated carbons and inorganic materials, demonstrating yet another valuable use for this waste material.
Introduction
With increasing legislative measures being applied to carbon emissions, countries around the world are looking to the potential of indigenous biomass to help reach renewable energy targets. The UK Government predicts that 5% of the nation's heat and power (70 TWh) will be generated from biomass by 2020.1 Wheat straw is of particular interest in agricultural regions of the world as an abundant waste biomass source with an approximate surplus of 4 million tonnes available in the UK annually.2The pyrolysis and combustion of wheat straw differs to coal and woody biomass sources. This is primarily due to its distinct ash chemistry, including higher total ash content, alkalinity, chlorine and silica content, resulting in lower ash melting points.3 The large-scale utilisation of this feedstock in combustion systems will therefore result in the formation of a significant quantity of new waste: biomass fly ash and slag. The valorisation of these waste materials is vital to ensure recovery and reuse of the inorganic species, in line with an elemental sustainability and biorefinery vision, and to add economic value.
The most frequently cited applications for biomass combustion residues are in: 1) agriculture, as a fertiliser or soil amendment; and 2) construction, by partly replacing cement or as an aggregate in road building.4 However, few studies have investigated the physical, chemical or textural changes occurring during the combustion of wheat straw and how these impact on the potential applications of the unique waste ashes formed.5
Herein, this work investigates the mineralogical changes occurring during the combustion of wheat straw, including the physical, chemical and textural properties of the ashes formed. This leads to proposals for the future application of these residues. Particular focus is placed on the effects on potassium, silica and chloride as the major species in the residues. The high silica and alkali content of wheat straw combustion residues indicates that they could be directly applicable for the formation of alkali silicate solutions. These are widely used with applications including the formation of zeolites, use as binders, in water treatment and pulp bleaching.6 This could be a novel approach if the need for additional chemicals can be eliminated.
Experimental
Wheat straw (Claire, 2007) was obtained from G & H Cholmley, Wintringham. Straw was milled to <2 mm and stored at room temperature prior to use. Moisture content was measured at 120 °C and ash content at 550 °C to constant weight in triplicate and the average taken. The calorific value was analysed using a Parr 6200 Bomb Calorimeter.Ash formation
Typically, milled wheat straw (10.0 g) was combusted in a porcelain crucible in a muffle furnace at the desired temperature (400 °C to 800 °C) and for a set time (10 min or 2 h).Extraction
On removal from the furnace the ash was directly quenched in distilled water. The resulting solutions were stirred continuously for 24 h at room temperature in sealed bottles before filtering. The ashes were dried at 60 °C under vacuum for 48 h and the solutions were stored for analysis. Extractions were repeated in triplicate at 400, 600 and 800 °C. Polypropylene equipment was used for all extraction, storage and analytical processes.Analyses of solutions
pH measurements were performed at the beginning and end of the extraction using a Jenway 3505 pH meter attached with a glass Thermo Scientific Russell Ultra high accuracy K-series polymer reference electrode.Silicon and potassium content were analysed by atomic absorption spectroscopy (AAS). Silicon concentrations were analysed using the 251.6 nm line by a hollow cathode Si lamp with a high temperature burner and a combined nitrous oxide-acetylene flame under stoichiometric conditions with addition of 0.2 wt% KCl. Potassium concentrations were analysed from the 766.5 nm line using a hollow cathode potassium lamp and a low temperature burner with an air-acetylene flame with addition of 0.5% w/v NaCl in all solutions to reduce ionisation.
Minor elements were analysed by Inductively Coupled Plasma Atomic Emissions Spectroscopy (ICP-AES) for 39 inorganic elements. Anions were analysed by ion chromatography (IC).
Analyses of ashes
Elemental analysis based on carbon, hydrogen and nitrogen content was carried out using an Exeter Analytical (Warwick, UK) CE440 Elemental Analyser, calibrated against acetanilide with an S-benzyl-thiouronium chloride internal standard. CHN wt% of the starting material was 42.3, 5.62 and 0.36 respectively.Quantitative X-Ray Fluorescence (XRF) analysis of all major oxides in wheat straw was carried out by fusing ground ashes with lithium tetraborate (Li2B4O7). Elemental analysis of ashes before and after extraction under different combustion conditions was performed on a Horiba Micro-analytical XRF on finely ground compressed ashes.
X-Ray Diffraction (XRD) was used to study the crystalline species present in the ashes. A Bruker-AXS D8 Advance diffractometer with a Kristalloflex 760 X-Ray generator which produces monochromatic Kα X-Rays from a copper source was employed. Scans were taken across the range 5–80° 2θ over 30 min with a 45 kV voltage and 20 mA current. The evaluation programme EVA and the Bruker CDS database were used to identify the phases present in the samples.
Solid state 29Si NMR was carried out using a Varian Unity Inova spectrometer operating at 59.56 MHz for 29Si. Spectral referencing was with respect to neat, external tetramethylsilane. The spectra were recorded with direct excitation and a 30 s recycle delay.
Nitrogen adsorption measurements were carried out at 77 K using a Micromeritics ASAP 2010 volumetric adsorption analyser. Before measurement the samples were heated under vacuum for 1–2 h at 120 °C. The BET specific surface areas were evaluated using adsorption data in the relative pressure range from 0.06 to 0.15. A micropore analysis method (MP method) was used to evaluate the micropore volume of the samples and the pore size distribution of the materials.
Results
In comparison to other materials frequently used in combustion systems7 (Table 1) wheat straw has a distinctive composition. The ash content is higher than for woody biomass, with higher potassium and chlorine levels and lower concentrations of other elements. The silica and potassium content of the wheat straw used in this study are typical of high alkali wheat straw harvested in various countries throughout Europe.3,8,9,10,11,12,13 Lower alkali and chlorine contents are generally found only when the wheat straw has been pre-treated by water washing14 or leached by rain water in the field before harvesting.15 These variations fundamentally affect the combustion of the material, the nature of the ash formed and therefore its applications.| Wheat strawa | Coalb | Woodb | |
|---|---|---|---|
| a This study. b Ref. 7. c As received. | |||
| Moisture (wt%) | 7.10 | 9.15 | 9.08 |
| Ash (wt%)c | 5.60 | 12.5 | 1.8 |
| CV (MJ/kg)c | 17.1 | 25.0 | 16.7 |
| Ash content (dry wt %) | |||
| SiO2 | 44.25 | 53.49 | 22.8 |
| K2O | 28.33 | 0.46 | 10.4 |
| CaO | 12.64 | 2.65 | 24.2 |
| MgO | 1.37 | 0.42 | 5.1 |
| Na2O | 0.11 | 0.51 | 2.7 |
| Al2O3 | 0.19 | 33.92 | 3.7 |
| P2O3 | 1.54 | 0.65 | 10.2 |
| SO3 | 2.84 | n.a. | 5.3 |
| Cl | 8.19 | ||
| LOI | 8.2 | ||
Mineralogical changes
The decomposition profile (Fig. 1) is typical of a lignocellulosic material, showing the onset of an initial volatilisation stage around 220 °C followed by a char oxidation stage at higher temperatures.16 The wheat straw was combusted between 400 °C and 800 °C for a set time (10 min or 2 h). The combustion conditions chosen ranged from the onset of char combustion of the wheat straw (400 °C, 10 min) to complete combustion and ash melting (800 °C, 2 h). This enabled a complete exploration of the interaction between the physical, chemical and textural properties of the residues formed throughout the combustion range. | ||
| Fig. 1 Thermogravimetric profile of wheat straw combustion. Heating rate 10 °C/min. | ||
After two hours combustion the percentage solid remaining up to 500 °C (Table 2) was similar to the ash content found in the initial wheat straw, indicating that complete combustion had occurred. At higher combustion temperatures there was a small but continued mass loss due to the volatilisation of inorganic species, consistent with previously reported data.17 After only 10 min combustion significant proportions of organic material still remain, although combustion rates increase with temperature. This would obviously not be ideal in a commercial combustion system, however, it will give insight into the changes occurring in the inorganics during the char oxidation combustion stage.
| Temperature (°C) | Time (min) | Mass remaining (%) | C (%)a | H (%)a | N (%)a |
|---|---|---|---|---|---|
| a Average of duplicate. | |||||
| 400 | 10 | 26.4 | 65.10 | 2.83 | 0.60 |
| 500 | 10 | 24.4 | 67.27 | 2.33 | 0.57 |
| 600 | 10 | 19.3 | 65.52 | 1.57 | 0.56 |
| 700 | 10 | 14.1 | 60.87 | 1.19 | 0.50 |
| 800 | 10 | 14.3 | 58.12 | 1.01 | 0.60 |
| 400 | 120 | 5.1 | 4.18 | 0.76 | 0.00 |
| 500 | 120 | 5.1 | 5.99 | 0.78 | 0.00 |
| 600 | 120 | 4.7 | 3.93 | 0.60 | 0.00 |
| 700 | 120 | 4.5 | 0.54 | 0.24 | 0.00 |
| 800 | 120 | 4.3 | 0.89 | 0.18 | 0.00 |
The mineralogical changes occurring in the ashes as a function of time and temperature were identified by XRD (Fig. 2) and XRF. Fig. 2a illustrates a broad background region between 20–30° 2θ in the X-Ray Diffraction patterns of wheat straw ashes combusted for 10 min. This feature corresponds to amorphous silica, as previously identified in rice hull ash.18 Sylvite (KCl) is the main crystalline phase observed, present at all temperatures. Calcite (CaCO3) increases in intensity with increasing combustion temperature due to the increasing relative inorganic concentration of the ashes. The other intense peaks present, due to the aluminium sample holder, reduce in intensity with increasing temperature. This could indicate increasing crystallinity of the combustion residue.
 | ||
| Fig. 2 XRD patterns of wheat straw ashes after (a) 10 min combustion and (b) 2 h combustion from 400 °C to 800 °C. The phases identified are: 1: sylvite; 2: calcite; 3: K2SO4; 4: quartz; 5: CaSiO3; 6: aluminium sample holder. | ||
Similar crystalline phases are observed after 2 h combustion (Fig. 2b). The intensity of the sylvite peaks decrease at 700 °C and are absent at 800 °C, in agreement with previous observations regarding the loss of KCl during the combustion of wheat straw.19 Above 500 °C calcite is no longer detected. This is at a lower temperature than would be predicted by its decomposition temperature (848 °C), probably due to an initial fast combustion rate resulting in hot spots or locally elevated temperatures within the ashes, higher than the actual furnace temperature. The breakdown of calcite and loss of sylvite correlate well with the minor mass losses observed during combustion at 600 °C and above (Table 2). At all temperatures the minor presence of quartz is discerned, peaking in intensity at 700 °C, whilst calcium silicate appears at 700 °C, indicating the transformation of the silica phase. Arcanite (K2SO4) is present in the ashes at all temperatures. Cristobalite is not observed, contrasting with previous research in which this was the major silica phase at higher temperatures.17 This observation is linked to the higher potassium, chlorine and calcium content of the wheat straw used in this study, causing the formation of alkali silicates rather than the conversion of amorphous silica into other silica polymorphs. This highlights the variability in mineralogical changes dependent on the initial inorganic content of the straw.
Chemical changes
The chemical changes within the ashes (including solubility of the elements) during combustion were studied by ambient aqueous extraction, using a similar method to a Swedish standard ash leaching technique.20The total aqueous solubility of inorganic elements (Fig. 3) was consistent based on both solution (AAS, ICP, IC) and solid analyses (XRF). A gradual decline in total solubility is observed with increasing combustion temperature after only 10 min combustion, indicating that immediate chemical changes are occurring. Above 500 °C there is a sharp decline in the aqueous solubility of inorganic species following 2 h combustion. Studying the solubility of the five major elements present in the ash: K, Si, Cl, Ca and S; indicates that the majority of this reduction is due to decreasing extraction of potassium, chlorine and silicon (Fig. 3).
 | ||
| Fig. 3 Total inorganics extracted and concentration of major elements in solution following extraction after (a) 10 min combustion and (b) 2 h combustion. Faint dashed lines indicate the maximum potential extraction for each element. | ||
Potassium and chloride
Up to 500 °C the solubility of both potassium and chloride remains constant irrespective of the extent of combustion. Potassium extraction levels are between 65–70%, whilst chloride is close to 100%, similar to the extraction found for these elements during the leaching of pyrolysis chars formed at 500 °C.8Potassium extraction is lower than that of uncombusted wheat straw.10 The lack of complete potassium extraction may be due to partial volatilisation of potassium bound to the organic matrix at temperatures <400 °C,21 although this would also affect the initial elemental analysis of the wheat straw. Previous researchers have suggested that potassium release is controlled by diffusion of KCl and K2CO3 from the ash surface, with some fixed insoluble potassium bound in the char or ash.8 There is a direct correlation between the potassium concentration and levels of chloride ions in solution (Fig. 4) and KCl could no longer be identified in XRD spectra following extraction. However, the solubilisation of potassium as either KCl or K2SO4 only accounts for 60–70% of the potassium dissolved. The high pH of the solutions formed suggests that the remainder is in the form of K2CO3 or KOH. | ||
| Fig. 4 Correlation between potassium concentration and chloride and sulfate concentration. | ||
At 600 °C and above, the availability of potassium and chloride for extraction decreases substantially with increasing combustion time and temperature, down to 7–9% for potassium and 1–3% for chloride following 2 h combustion at 800 °C (Fig. 3b). Surface ionisation probe studies of wheat straw pyrolysis showed a dramatic increase in alkali emissions above 500 °C from the ash component.19 Molecular beam mass spectrometry of switchgrass,22 another high potassium, high chloride grass, identified the predominant alkali metal containing species released at 800 °C during the char combustion stage as KCl. Higher temperatures did not increase the volatilisation of sylvite; this correlates with XRD observations in this study, which indicated a reduction in KCl content in the ashes at 700 °C and above. The potassium and chloride concentrations in the solutions are therefore reducing at higher combustion temperatures due to KCl evaporation.
Nevertheless, the data also suggests that not all of the potassium is volatilised as KCl. XRF analysis (Fig. 5) shows that 67% of the initial potassium remains in the ashes at 800 °C after 2 h combustion. Following extraction, potassium levels remaining in the ash increase with combustion temperature. Therefore the levels of potassium available for extraction are not only affected by evaporation of potassium during combustion but, are also due to the formation of less soluble potassium species. The broad amorphous background in the XRD spectra (Fig. 2b) indicates the formation of glass-like potassium or mixed metal silicate species which would be insoluble under ambient conditions.
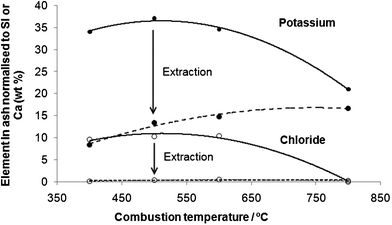 | ||
| Fig. 5 Change in potassium and chloride in ashes before and after aqueous extraction. | ||
Silica extraction
The extraction of silica varies significantly with combustion conditions. It is possible to solubilise up to 30% of the silica present in the wheat straw ash under ambient conditions by utilising its inherent alkalinity.Generally silica extraction decreases with increasing combustion temperature. The silica solubility decreases most dramatically with both temperature and time above 500 °C, with combustion at 400 °C giving almost constant extraction levels from 10 min to 2 h of combustion. Analogous results were found for rice hull ashes, with the most reactive silica formed with combustion temperatures of 400 and 500 °C, although for these studies additional alkali was added.23
Silica extraction shows a complex relationship with the pH of the solution (Fig. 6). Below 600 °C combustion, the initial pH of the solution is constant independent of combustion condition. This suggests that the alkaline species solubility is high and occurs quickly, within the first 5–10 min of addition of the ash to water. For these solutions, after 24 h extraction, the solutions show an inverse correlation between the pH of the solution following extraction and the percentage of silica dissolved. This is due to the dissolution of silica neutralising the hydroxide present as silicate ions are formed, resulting in a lowering of the pH. This trend only breaks down above 600 °C for 2 h combustion. For these solutions the pH of the initial solution decreases with increasing combustion temperature, which can be linked to mineralogical changes in the ashes decreasing the extraction of alkaline potassium species (Fig. 5). This lowers the maximum dissolution of silica since fewer silicate ions are formed, causing saturation of the solution with monosilicic acid to occur more quickly. However, for these solutions the pH actually increases during extraction, due to the slower solubilisation of calcium species.
 | ||
| Fig. 6 Relationship between pH of solution and silica extraction. Hollow circles indicate initial pH, filled circles indicate final pH after 24 h extraction. | ||
Calcium concentrations in the extracts are almost zero for all solutions, but increase marginally above 700 °C for ashes combusted for 2 h (Fig. 3). This is associated with the decomposition of calcium carbonate at higher temperatures and longer times of combustion, as observed by XRD (Fig. 2), forming soluble calcium oxide. However, despite the absence of calcite in the XRD pattern above 600 °C, the low concentration of calcium in the ash solutions at this temperature could be caused by the reaction of calcium with silica in solution. Insoluble calcium silicate precipitates would be formed, removing both calcium and silicon from the solution and thereby contributing to the lowering of the silica concentration found in the solution at these temperatures.
Si NMR
The decreases in the solubility of silica can also be linked to changes in the silica structure during combustion. As the temperature of combustion increases amorphous silica becomes more crystalline. Quartz peaks were observed in the XRD patterns above 500 °C (Fig. 2). This is much less soluble than amorphous silica owing to its more tightly packed structure, making it more resistant to attack by hydroxide ions.2429Si MAS NMR can show in much more detail changes occurring in the silica structure during combustion and following extraction (Fig. 7). It was only possible to analyse the ashes after 2 h combustion for 29Si during to the high carbon content remaining in the ashes combusted for 10 min. At 400 °C the spectra are broad, with silica species distributed between Q2, Q3 and Q4 sites (Fig. 7a). This suggests an amorphous silica structure with large variation in Si-O-Si bond angles and large numbers of terminal silanol groups with a central tightly packed silica network. The silanol groups are the only reactive sites in the silica, enabling dissolution to occur with relative ease. As the temperature of combustion increases the Q4 peak disappears and a broad uniform peak remains at a lower chemical shift with majority Q3 character. This is in contrast to rice hull ashes.2529Si NMR of these ashes shows an initial similar broad spectra that narrows and shows increasing Q4 character with higher temperature of combustion. The XRD data obtained in this study suggests that this variation is due to the formation of glass-like alkali silicates at higher heating temperatures with either K or Ca as the counter ion at the terminating group. | ||
| Fig. 7 Solid state 29Si NMR (a) before and (b) after extraction. | ||
Following extraction (Fig. 7b) there is an increase in the concentration of Q4 sites with reducing variation between the initial and extracted ash with higher temperature of combustion, which correlates with the percentage of silica dissolved. This implies the extraction of silica occurring with attack of hydroxide ions at the terminal silanol sites in agreement with the mechanism of silica dissolution.23 The continued presence of Q1-Q3 sites suggests that further silica is available for easy dissolution although the silica at Q4 sites may need more aggressive conditions and longer time-frames to dissolve.
Other elements
The concentration of sulphur in the solutions is extremely high, reducing from a maximum extraction of 80% after 2 h combustion at 400 °C, similar to the extraction observed from uncombusted wheat straw by water leaching.11 Soluble potassium sulfate was present in the XRD patterns up to 800 °C and has previously been identified as the most stable sulphur compound under oxidising conditions for wheat straw in equilibrium calculations.26 The decreasing solubilisation of sulphur with temperature has been observed previously and was attributed to the dissociation of K2SO4 and release of SO2 to the gas phase due to the preference of K and Ca to form alkali silicates at higher temperatures.26 However, in this case the capture of sulphur within the ashes and extraction from ashes formed at 800 °C remains high, potentially due to melting of the ash particles around the sulfate particles preventing its dissociation.Only traces of Al, Mg, Fe, Mn, and P were released during extraction. Similar observations were found for wood ashes and attributed to the high pH of the solutions lowering their solubility. The remaining ashes are therefore enriched in these elements. Of the heavy metals neither cadmium nor nickel were present in the solutions formed, both of these have been of concern in the utilisation of biomass waste ashes in Sweden.27 Minor trace concentrations of As, Pb, Tl and Zn were found in all solutions, although As, Pb, Zn are generally found to be released to a significant extent during combustion processes and so might be found in higher concentrations in fly ashes formed.28
Textural properties
To further understand textural changes occurring in the ashes during combustion and following extraction the porosity of the ashes was studied (Fig. 8).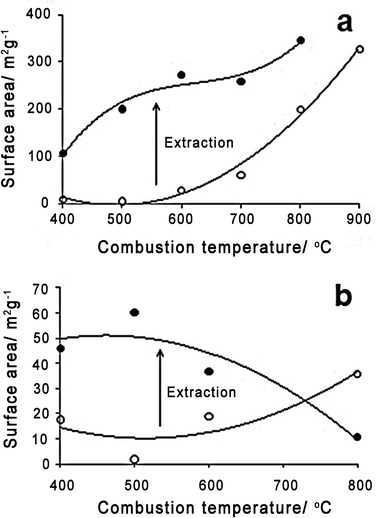 | ||
| Fig. 8 Surface area of ashes with (a) 10 min and (b) 2 h combustion. | ||
After only 10 min combustion the surface area of the ashes rises dramatically with increasing temperature of combustion above 600 °C. This coincides with the melting point and liberation to the gas phase of KCl and the softening of silica and formation of alkali silica species. Therefore, it could be related to the movement of molten or gaseous salts through the carbon structure creating pores. This is supported by elemental analysis of melted wheat straw particles showing increased levels of potassium and chlorine on the external surfaces due to evaporation at these temperatures.29TEM images of the ashes at 400 °C and 800 °C appear to show changes in the inorganic species from discrete particles to fractured platelets, although EDX analysis would be required to confirm this (Fig. 9). Pore size distribution analysis shows a highly microporous material developing at 700 °C and above (Fig. 10). The surface area at 800 and 900 °C are similar to the lower end of those found during the physical activation of pyrolysis chars for the formation of activated carbons from agricultural residues.30 During this process steam, air or CO2 are added to the char at temperatures of 800 °C or greater for between 30 min to 12 h causing burn-off and the development of micropores.31 After 10 min combustion similar processes are occurring with partial oxidation of the char surface. This could also account for the high surface area of the materials.
 | ||
| Fig. 9 TEM images of ashes after 10 min combustion at (a) 400 °C and (b) 800 °C. | ||
 | ||
| Fig. 10 Pore distribution of ashes after 10 min combustion before (dashed) and after (solid) extraction. | ||
Following complete combustion at these temperatures the internal surface area is considerably reduced as the organic content burns out and the inorganic species begin to melt.
After water leaching both the 10 min and 2 h ashes show an increase in surface area, pore volume and surface energy compared to the initial ashes, directly correlating with the total inorganic content extracted (Fig. 3). The surface area and the pore size distribution (Fig. 10) present after 10 min combustion from 500–700 °C are almost identical to that present at 800 °C before extraction. This supports the proposition that soluble inorganic species are causing the increase in pore volume and surface area in the initial ash as they become mobile at higher combustion temperatures.
This suggests that the inorganics are distributed initially as a nano-network throughout the wheat straw material. The removal of this network by leaching after partial combustion produces high surface area microporous carbonaceous materials at much lower temperatures than traditionally used for activated carbon production. The solid formed following extraction could act as a template to produce nano-structures or as an adsorbent for wastewater treatment, for example.
Applications
These results suggest several possible applications for wheat straw combustion ashes. The high potassium solubility and alkalinity of the ashes indicates their suitability as a fertiliser to recycle nutrients, reduce the need for new potassium fertiliser production and neutralise soil acidity. These experimental results indicate that the highest potassium solubility is found for residues combusted below 600 °C.It is also possible to form alkali silicates directly from the ashes without the addition of additional alkali at ambient conditions. Combustion at temperatures below 600 °C also gives the highest silica extraction by producing the most alkaline solutions, preventing calcium solubility and keeping the silica in a more soluble amorphous form. This could be an excellent use for the ashes as potassium and silica are the two most prevalent inorganic components. This would also reduce the need for the current energy intensive methods of production involving the fusion of sand and sodium carbonate at 1100 °C followed by hydrothermal dissolution.
Although straw powered furnaces often operate at lower temperatures than coal powered boilers to reduce the impacts of fouling and slagging, 500–600 °C is below the usual operating temperatures of 700–900 °C.32 However, this data is usually based on the gas temperature rather than the grate temperature, which may be lower. At these higher combustion temperatures potassium will be volatilised as KCl and can be captured in the fly ash which should also therefore act as a good soil amendment. However, increasing levels of potassium may be captured in the bottom ashes as alkali silicates reducing the total recoverable amount. The formation of alkali silicate solutions from this material would still be possible, potentially using the alkaline fly ash, but harsher extraction conditions would be required to dissolve the crystalline or glassy material. Fluidised-bed combustion could provide an alternative technological route to lower temperature combustion residues since secondary char combustion occurs around 650 °C, substantially below the flame temperature.33 The impacts of the bed material on the ash formed and its utilisation would require further study.
A different approach would be the extraction of the inorganic species following pyrolysis at 500 °C. Previous work into the extraction of inorganic species from microwave pyrolysis chars shows that this can reduce slag formation on combustion and increase the calorific value of the chars.34
The textural properties show the development of porous materials following the extraction of inorganics. Partial combustion of biomass materials at low temperatures followed by leaching could be a viable route to lower cost nano-structured porous templates or adsorbents when combined with recovery of the inorganics for recycling.
For each of these potential applications further studies would need to be undertaken. In particular it would be necessary to consider the influence of minor contaminants, especially toxic, on the final application and whether further purification steps were necessary.
Conclusion
We have studied the complete impact of combustion conditions on the major inorganic species within wheat straw and the mineralogical, chemical and textural changes that occur. Mineralogical and chemical changes occur above combustion temperatures of 500 °C causing reductions in the solubility of potassium, chloride, silica and sulfate. This can be correlated to evaporation of KCl, dissociation of K2SO4, softening of silica and formation of alkali silicates. Calcium extraction increased above combustion temperatures of 700 °C due to the decomposition of CaCO3.We have discovered, for the first time, that the inherent alkali in unleached wheat straw is sufficient to solubilise up to 30% of the silica in the ash at room temperature. This could be used to produce potassium silicate solution as a new valuable by-product of wheat straw combustion. Current and on-going research is demonstrating the use of such silicates to be effective replacements for traditional formaldehyde binders in construction boards.35
Incomplete combustion at low temperatures (from 500 °C), following leaching of ashes can lead to the formation of porous activated carbons and inorganic materials, which demonstrate another valuable application for this waste material. This could be a new route to low cost nano-structured porous templates or adsorbents.
Acknowledgements
We thank DEFRA who sponsored this research through the Renewable Materials LINK Programme. We are grateful to Durham University Solid State NMR Service, Yara Analytical Services, Sheffield Hallam University for XRF analyses, Meg Stark for taking the TEM images and Rebecca Sutton for help with ion chromatography analysis.We would also like to acknowledge the comments of the referees in helping to improve this paper.
References
- HM Government. The UK Renewable Energy Strategy, 2009 Search PubMed.
- J. Copeland and D. Turley, National and Regional Supply/Demand balance for agricultural straw in Great Britain,CSL, 2008 Search PubMed.
- M. J. F. Llorente and J. E. Garcia, Fuel, 2005, 84, 1893 CrossRef.
- A. Gómez-Barea, L. F. Vilches, C. Leiva, M. Campoy and C. Fernández Pereira, Chemical Engineering Journal, 2009, 146, 227 CrossRef CAS.
- H. Biricik, F. Aköz, I. Berktay and A. N. Tulgar, Cement and Concrete Research, 1999, 29, 637 Search PubMed.
- PQ Corporation, Soluble Silicates and their Applications, PQ Corporation, Warrington, 2002, http://www.pqcorp.com/technicalservice/literaturelist.asp?Contact_Us=CORP, 18/01/11 Search PubMed.
- G. Di Nola, W. de Jong and H. Spliethoff, Fuel Processing Technology, 2010, 91, 103 CrossRef CAS.
- P. A. Jensen, B. Sander and K. Dam-Johansen, Biomass Bioenergy, 2001, 20, 447 CrossRef CAS.
- C. Gilbe, M. Ohman, E. Lindstrom, D. Bostrom, R. Backman, R. Samuelsson and J. Burvall, Energy Fuels, 2008, 22, 3536 CrossRef CAS.
- T. R. Miles, L. L. Baxter, R. W. Bryers, B. M. Jenkins and L. L. Oden, Biomass Bioenergy, 1996, 10, 125 CrossRef CAS.
- D. C. Dayton, B. M. Jenkins, S. Q. Turn, R. R. Bakker, R. B. Williams, D. Belle-Oudry and L. M. Hill, Energy Fuels, 1999, 13, 860 CrossRef CAS.
- S. Arvelakis, P. A. Jensen and M. Dam-Johansen, Energy Fuels, 2004, 18, 1066 CrossRef CAS.
- D. Nutalapati, R. Gupta, B. Moghtaderi and T. F. Wall, Fuel Processing Technology, 2007, 88, 1044–1052 CrossRef CAS.
- B. M. Jenkins, R. R. Bakker and J. B. Wei, Biomass Bioenergy, 1996, 10, 177 CrossRef CAS.
- P. Hald, Alkali Metals at Combustion and Gasification - Equilibrium Calculations and Gas-Phase Measurings. DPhil Thesis, Technical University of Denmark, 1994 Search PubMed.
- A. Demirbas, Prog. Energy Combust. Sci., 2004, 30, 219 CrossRef CAS.
- P. Thy, B. M. Jenkins, S. Grundvig, R. Shiraki and C. E. Lesher, Fuel, 2006, 85, 783 CrossRef CAS.
- A. Proctor, Journal of the American Oil Chemists Society, 1990, 67, 576 Search PubMed.
- B. Olanders and B.-M. Steenari, Biomass Bioenergy, 1995, 8, 105 CrossRef CAS.
- P. Mellbo, S. Sarenbo, O. Stalnacke and T. Claesson, Waste Management, 2008, 28, 2235 CrossRef CAS.
- J. G. Olsson, U. Jaglid, J. B. C. Pettersson and P. Hald, Energy Fuels, 1997, 11, 779 CrossRef CAS.
- D. C. Dayton, R. J. French and T. A. Milne, Energy Fuels, 1995, 9, 855 CrossRef CAS.
- G. R. Rao, A. R. K. Satry and P. K. Rohatgi, Bulletin of Materials Science, 1989, 12, 469 Search PubMed.
- R. K. Iler, The Chemistry of Silica. 1979, New York, Wiley & Sons Search PubMed.
- D. G. Nair and A. Fraaij, Cement and Concrete Research, 2008, 38, 861 Search PubMed.
- J. N. Knudsen, P. A. Jensen, W. G. Lin, F. J. Frandsen and K. Dam-Johansen, Energy Fuels, 2004, 18, 810 CrossRef CAS.
- M-L Sander and O. Andren, Water Air and Soil Pollution, 1995, 93, 93.
- A. J. Pedersen, S. C. van Lith, F. J. Frandsen, S. D. Steinsen and L. B. Holgersen, Fuel Processing Technology, 2010, 91, 1062 CrossRef CAS.
- Y. Wu, S. Wu, Y. Li and J. Gao, Energy Fuels, 2009, 23, 5144–5150 CrossRef CAS.
- P.D. Paraskeva, D. Kaldens and E. Diamadopoulos, Journal of Chemical Technology and Biotechnology, 2008, 83, 581 Search PubMed.
- T. Zhang, W. P. Walawender, L.T. Fan, M. Fan, D. Daugaard and R. C. Brown, Chemical Engineering Journal, 2004, 105, 53 CrossRef CAS.
- F. J. Frandson, H. P. Nielson, P. A. Jensen, L. A. Hansen, H. Livberg, J. Dam-Johansen, P. F. B. Hansen, K. H. Andersen, H. S. Sorensen, O. H. Larsen, B. Sander, N. Henriksen, P. Simonsen, in Impact of Mineral Impurities in Solid Fuel Combustion, ed R. Gupta, T. Wall and L. Baxter, Kluwer Academic / Plenum Publishers, New York, 1999, pp. 271-283 Search PubMed.
- D. L. Klass, Biomass for Renewable Energy, Fuels and Chemicals, Academic Press, California, 1998. Pp. 209 Search PubMed.
- V. L. Budarin, P. S. Shuttleworth, J. R. Dodson, A. J. Hunt, B. Lanigan, R. Marriott, K. J. Milkowski, A. J. Wilson, S. W. Breeden, J. Fan, E. K. H. Sin and J. H. Clark, Energy Environ. Sci., 2011, 4, 471–479 RSC.
- V. L. Budarin, J. R. Dodson, K. J. Milkowski, and J. H. Clark, Biorefinery Products in Structural Materials, Patent application WO/2009/087360, 2009 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
