Drip and evaporation method for dye-TiO2 nano junction formation in dye sensitized solar cells
Sang-Hoon
Lim
and
Shi-Woo
Rhee
*
System on Chip Chemical Process Research Center, Department of Chemical Engineering, Pohang University of Science and Technology (POSTECH), Pohang, 790-784, Korea. E-mail: srhee@postech.ac.kr; Fax: +82-54-279-8619; Tel: +82-54-279-2265
First published on 18th August 2011
Abstract
The dripping of dye solution and solvent evaporation method for faster dye adsorption in dye-sensitized solar cells (DSCs) was developed and its effect on the adsorption time and the cell performance was studied. N719 dye solution was dripped on to the TiO2 porous layer on the FTO glass substrate with heating. The solvent and water generated from the adsorption reaction quickly evaporated and the dye adsorption rate on the TiO2 porous layer was substantially enhanced so that the dye adsorption time was significantly shortened, from 24 h for the conventional dipping method to 4 min. With the dripping and evaporation method, the DSC efficiency was slightly higher than the dipping method due to the higher dye adsorption. Electron transport properties and characteristics of the DSCs prepared with the dripping and evaporation method and the dipping method were investigated with electrochemical impedance spectroscopy (EIS) measurements.
1. Introduction
Since the dye-sensitized solar cell (DSC) was developed by Grätzel and co-workers in 1991, it has drawn much attention due to its high efficiency, low cost and easy fabrication.1 The DSCs are composed of a photo electrode, dye, electrolyte and counter electrode, and each part of the DSCs has been studied by many research groups. The important key components for DSC efficiency are the mesoporous TiO2 layer and the dye.2 The dye must have a high conversion yield from photon energy to electrons and long term stability, the Ru(II) complexes (N3, N621, N712, N719, N749) have been widely used and studied.2–5 Currently, the highest certified efficiency of DSCs using N719 is 11.1%.6Many research groups and companies have explored the commercialization of DSCs7–9 with an effort to decrease the production cost and processing time. DSCs with the N719 dye required a dipping time of 12–24 h in a dye solution of 0.3–0.5 mM (1 mM = 1 × 10−3 M) which is too long for commercial production.2
Three methods have been tested to reduce the production time. One method was to use a highly concentrated dye solution of about 20 mM.2 In this method, dye adsorption was finished in a few minutes but much of the expensive dye was unused and wasted. Another method was applying an electric field to the dye solution to enhance the negative dye ion movement towards the TiO2 layer and the adsorption time was decreased down to 5 h at a concentration of 0.2 mM9. However, the dye adsorption time was still long to be practical.
The other method was to increase the temperature of the dye solution to 80 °C, which could decrease the dye adsorption time down to 2 h at a concentration of 0.3 mM.10
Fig. 1 shows the concept of the new drip and evaporation method for dye adsorption. N719 dye was adsorbed on the TiO2 surface through a reaction between the N719 dye and Ti-OH formed on the TiO2 nanoparticle layer, and H2O was generated from the reaction.10–12 The N719 dye is nonvolatile but H2O and the solvent of the dye solution are volatile. The N719 dye solution contacts the surface of the 10 micron (μm) thick TiO2 nanoparticle layer with a 100 μm thick masking film at both ends. The glass substrate with the TiO2 nanoparticle layer on it is heated. The contact between the N719 dye molecule and the surface of the TiO2 layer increases with solvent evaporation and all the solvent is evaporated as time goes on. The dye molecules adsorb onto the surface of the TiO2 layer and the adsorption rate is fast on the heated surface. Simultaneously, the generated H2O is evaporated. The drip and evaporation method could decrease the dye adsorption time with a starting dye concentration below 0.3 mM without any loss of dye molecules and produce DSCs with efficiencies slightly higher than those of the dipping method.
 | ||
| Fig. 1 The schematic diagram of the drip and evaporation method. | ||
In this work, DSCs made with the drip and evaporation method and the dipping method were analyzed with electrochemical impedance spectroscopy (EIS) and the cell efficiency was compared.
2. Experiments
A TiO2 photo electrode was prepared with screen printing using TiO2 nano-paste (Ti-Nanoxide T/SP from Solaronix) on a cleaned fluorine doped tin oxide (FTO, 8Ω/□) coated glass substrate. The area of the TiO2 nanoparticle layer was 0.25 cm2. After screen printing, the sample was dried at 120 °C and annealed at 500 °C for 30 min in air. N719 dye was adsorbed by two methods, the dipping and the drip and evaporation method. In the dipping method, the porous layer of the TiO2 substrate was immersed in a 0.3 mM solution of N719 dye in acetonitrile/tert-butanol (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 24 h. Then, the substrate was rinsed with ethanol and dried with nitrogen gas blowing. In the drip and evaporation method, the dye solution was dripped on the TiO2 nanoparticle layer with a masking adhesive film at the edges. The TiO2 nanoparticle layer on the FTO glass was heated on a hot plate at temperatures of 60 °C, 80 °C, 100 °C, 120 °C and 140 °C. The amount of the dye solution dripped with a micro-pipette on the TiO2 nanoparticle layer was 50 μl, 100 μl, 150 μl, 200 μl, 250 μl and 300 μl each and the amount was controlled with the drip time. Various concentrations of the N719 dye solution (0.05 mM, 0.1 mM and 0.3 mM) were used and after dripping, the mask was removed. The counter electrode was prepared by depositing a platinum (Pt) precursor solution (Plastisol from Solaronix) on the cleaned FTO coated glass substrate. After drying at room temperature for 5 min, the electrode was annealed at 400 °C for 5 min in air. A 60 μm thick hot melt ionomer film (Surlyn, SX1170-60 from Solarnix) was sandwiched between the TiO2 photo electrode and Pt counter electrode by heating at 120 °C for a few seconds. The space between the electrodes was filled with liquid; the electrolyte consisting of 0.6 M 1-butyl-3-methyl imidazolium iodide, 0.03 M I2, 0.1 M guanidinium thiocyanate and 0.5 M 4-tertbutylpyridine in a solvent mixture of acetonitrile and valeronitrile (volume ratio 85
1) for 24 h. Then, the substrate was rinsed with ethanol and dried with nitrogen gas blowing. In the drip and evaporation method, the dye solution was dripped on the TiO2 nanoparticle layer with a masking adhesive film at the edges. The TiO2 nanoparticle layer on the FTO glass was heated on a hot plate at temperatures of 60 °C, 80 °C, 100 °C, 120 °C and 140 °C. The amount of the dye solution dripped with a micro-pipette on the TiO2 nanoparticle layer was 50 μl, 100 μl, 150 μl, 200 μl, 250 μl and 300 μl each and the amount was controlled with the drip time. Various concentrations of the N719 dye solution (0.05 mM, 0.1 mM and 0.3 mM) were used and after dripping, the mask was removed. The counter electrode was prepared by depositing a platinum (Pt) precursor solution (Plastisol from Solaronix) on the cleaned FTO coated glass substrate. After drying at room temperature for 5 min, the electrode was annealed at 400 °C for 5 min in air. A 60 μm thick hot melt ionomer film (Surlyn, SX1170-60 from Solarnix) was sandwiched between the TiO2 photo electrode and Pt counter electrode by heating at 120 °C for a few seconds. The space between the electrodes was filled with liquid; the electrolyte consisting of 0.6 M 1-butyl-3-methyl imidazolium iodide, 0.03 M I2, 0.1 M guanidinium thiocyanate and 0.5 M 4-tertbutylpyridine in a solvent mixture of acetonitrile and valeronitrile (volume ratio 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). After the electrolyte injection, the hole was sealed with Surlyn and a thin glass cover.
15). After the electrolyte injection, the hole was sealed with Surlyn and a thin glass cover.
Current–voltage (I–V) characteristics of the DSCs were measured employing a solar simulator equipped with a 300 W xenon lamp. The power of the simulated light was calibrated to AM 1.5 (100 mW cm−2), using a standard Si solar cell. I–V curves were obtained by applying an external bias to the cell and measuring the generated photocurrent with a Keithley model 2400 digital source meter. To measure the amount of dye adsorbed on the TiO2 surface, 1 mM KOH solution was used to dissolve the attached dye molecules and a UV-VIS spectrometer (OPTIZEN POP) was used to measure the absorption peak. The incident photon-to-current conversion efficiency (IPCE) was measured as a function of the wavelength using photomodulation spectroscopy (model Merlin, Oriel). The thickness of the TiO2 nanoparticle layer was measured with a scanning electron microscope (SEM, JEOL JSM-7401F in NCNT). The penetration depth of the dye solution and the amount of dye adsorption as a function of the depth of the TiO2 nanoparticle layer were measured by energy-dispersive X-ray spectroscopy (EDS, JEOL JSM-7401F in NCNT). Electrochemical impedance spectroscopy (EIS) measurements were carried out under the illumination of an AM 1.5 solar simulator (100 mW cm−2) by applying a 10 mV AC on top of the Voc of the DSC signal over the frequency range of 100 mHz to 100 kHz using a potentiostat (SP-200, Bio Logic Science Instruments). Electrochemical parameters were derived from the impedance spectra by EC-Lab software impedance analysis software.
3. Results and discussion
For the optimization of the drip and evaporation method, we compared it with the dipping method. Table 1 shows the effect of the dye solution volume dripped on the porous TiO2 layer. It was known that optimal temperature of the N719 dye for faster adsorption was about 75 °C at a concentration of 0.3 mM, which was also used in this experiment.10 The dye adsorption time of the DSCs was increased when the solution volume was increased because it took more time for the solvent to evaporate. Above 200 μl, efficiency was not increased and it was confirmed that the optimum amount of the dye solution was about 200 μl and 4 min was required for the evaporation.| Method | Time | Drip amount | J sc (mA cm−2) | V oc (V) | FF (%) | η (%) |
|---|---|---|---|---|---|---|
| Dipping | 24 h | 11.9 | 0.841 | 66.1 | 6.6 | |
| Drip and evaporation | 1 min | 50 μl | 7.0 | 0835 | 65.5 | 4.1 |
| 2 min | 100 μl | 9.7 | 0.835 | 70.2 | 5.8 | |
| 3 min | 150 μl | 11.1 | 0.835 | 69.3 | 6.4 | |
| 4 min | 200 μl | 12.0 | 0.820 | 70.1 | 6.9 | |
| 5 min | 250 μl | 12.5 | 0.793 | 67.8 | 6.8 | |
| 6 min | 300 μl | 13.0 | 0.841 | 66.2 | 6.8 |
Table 2 shows the effect of the dye solution concentration on the efficiency of the DSC and it increased as the dye concentration was increased from 0.05 mM to 0.3 mM. In the drip and evaporation method, the same level of dye concentration is required (about 3 mM) and the efficiency is comparable or even higher than the dipping method.
| Method | Conc. (mM) | J sc (mA cm−2) | V oc (V) | FF (%) | η (%) |
|---|---|---|---|---|---|
| Dipping | 0.3 | 11.9 | 0.841 | 66.1 | 6.6 |
| Drip and evaporation | 0.05 | 5.1 | 0.805 | 71.9 | 3.0 |
| 0.1 | 8.7 | 0.841 | 69.2 | 5.0 | |
| 0.3 | 12.0 | 0.820 | 70.1 | 6.9 |
Table 3 shows the effect of the substrate temperature and the dye adsorption time was decreased with the increase in the substrate temperature from 80 °C to 140 °C. The efficiency of the DSC was substantially decreased at temperatures over 100 °C due to the boiling of the solution (the boiling point of acetonitrile is 81–82 °C and 4-tert-butanol is 82–83 °C). It was confirmed that the dripping of 200 μl of 0.3 mM solution on the substrate heated at 80 °C showed the best cell performance and the dye adsorption time was decreased down to 4 min.
| Method | T/°C | Time | J sc (mA cm−2) | V oc (V) | FF (%) | η (%) |
|---|---|---|---|---|---|---|
| Dipping | 25 | 24 h | 11.9 | 0.841 | 66.1 | 6.6 |
| Drip and evaporation | 60 | 6 min | 12.0 | 0.823 | 68.8 | 6.8 |
| 80 | 4 min | 12.0 | 0.820 | 70.1 | 6.9 | |
| 100 | 3 min 40 s | 12.0 | 0.811 | 66.0 | 6.4 | |
| 120 | 3 min 20 s | 10.5 | 0.783 | 67.3 | 5.5 | |
| 140 | 3 min | 9.3 | 0.795 | 67.8 | 5.0 |
Fig. 2 shows the cross sectional SEM image of the TiO2 nanoparticle layer. Huang et al. studied the thickness effects of the TiO2 nanoparticle layer in the DSCs. The efficiency of the DSCs increased with the increase in the thickness of the TiO2 nanoparticle layer but the efficiency did not increase past 10 μm.13 The thickness of our TiO2 nanoparticle layer was 10.2 μm.
 | ||
| Fig. 2 The cross sectional SEM image of the TiO2 nanoparticle layer. | ||
Fig. 3 (a) shows the EDS image and the ruthenium (Ru) atom depth profile in the TiO2 nanoparticle layer prepared with the drip and evaporation method and (b) shows the EDS image with the dipping method. The penetration of the dye solution with the drip and evaporation method was similar to the dipping method and it took about 4 min instead of 24 h with dipping method.
 | ||
| Fig. 3 The EDS images and ruthenium (Ru) atom depth profile (red line) in the TiO2 nanoparticle layer prepared (a) with the drip and evaporation method and (b) with the dipping method. | ||
Fig. 4 (a) shows the UV absorption spectra of the KOH solution after dye dissolution from the sample prepared with the drip and evaporation method and the dipping method. The two broad peaks at 500 nm and 368 nm in N719 were assigned to the metal to ligand charge transfer (MLCT).4 The normalized relative amount of adsorbed dye was calculated from the two peaks,14–15 and the normalized relative amount of adsorbed dye of the drip and evaporation method was 1 and the dipping method was 0.66. The overall dye adsorption amount on the TiO2 nanoparticle layer was higher with drip and evaporation method. Fig. 4 (b) shows the IPCE curves of the DSC prepared with each method and it is seen that the power conversion efficiency was higher with the drip and evaporation method which is consistent with the higher Jsc in the I–V characteristics.
 | ||
| Fig. 4 (a) Absorption spectra of the KOH solution with dissolved dye and (b) IPCE curve of the samples prepared with each method. | ||
Fig. 5 shows the I–V curve of the DSCs with the dipping method and the drip and evaporation method at optimum conditions. In the I–V characteristics, it was known that the decrease in Jsc and increase in Voc were from adsorbed H2O on the TiO2 nanoparticle layer.16 It is believed that H2O formed from the dye adsorption reaction was evaporated with the drip and evaporation method, while some of the H2O adsorbed onto the TiO2 nanoparticle layer remained in the dipping method so that Jsc was increased and Voc was decreased in the drip and evaporation method. The higher fill factor (FF) of the DSC prepared with the drip and evaporation method was due to the higher dye adsorption amount and the higher power conversion efficiency.
 | ||
| Fig. 5 The I–V curve of the DSC made with the drip and evaporation method at optimal conditions (80 °C, 200 μl, 0.3 mM) and the DSC made with the dipping method. | ||
The EIS measurement was carried out for the electrochemical analysis of the electron transport properties in the DSCs prepared with the drip and evaporation method and the dipping method. Fig. 6 shows the Nyquist plot for the DSCs prepared with each method, and inset is the equivalent circuit for the DSCs.
 | ||
| Fig. 6 Nyquist plot of the impedance data of the DSC prepared with the drip and evaporation method at optimal conditions and the dipping method. The fitting lines are based on the equivalent circuit model shown in the inset. | ||
The equivalent circuit for the DSCs based on the diffusion-recombination model proposed by Bisquert and many research groups was used as the model.17–20 The estimation of the electron transport parameters of the DSCs was conducted from the Nyquist plot according to the procedure demonstrated by Adachi et al.21 The electron transport resistance in TiO2 (Rw), the charge transport resistance related to the recombination of electrons at the TiO2/electrolyte interface (Rk), the TiO2 nanoparticle layer thickness (L), the reaction rate constant for recombination (keff), the effective electron lifetime (τeff = 1/keff) and the effective diffusion coefficient (Deff) of electrons in the TiO2 nanoparticle layer prepared with each method from the impedance analysis are listed in Table 4.
| Method | R w (Ω) | R k (Ω) | L (μm) | k eff (s−1) | τ eff (s) | D eff (cm2s−1) |
|---|---|---|---|---|---|---|
| Dipping | 25.75 | 34.48 | 10.19 | 10.53 | 0.095 | 1.48×10−5 |
| Drip and evaporation | 17.31 | 19.93 | 10.19 | 14.36 | 0.070 | 1.72×10−5 |
D eff was calculated using the following equation,21
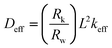 | (1) |
4. Conclusions
In summary, the drip and evaporation method for the dye-TiO2 nano junction formation in the DSCs is simple and more suitable for a continuous process. The dye adsorption time, even with a low concentration of dye solution (below 0.3 mM) was about 4 min which is substantially lower than the conventional dipping method of 12–24 h. The DSC efficiency was also higher due to the higher dye adsorption amount.Acknowledgements
This research was supported by the Korea Research Foundation (KRF) through the National Research Laboratory Project and the nano fusion program of POSCO.References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. K. Nazeeruddin, F. D. Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835 CrossRef CAS.
- M. Grätzel, J. Photochem. Photobiol., A, 2004, 164, 3 CrossRef.
- M. K. Nazeeruddin, R. Humphry-Baker, P. Liska and M. Grätzel, J. Phys. Chem. B, 2003, 107, 8981 CrossRef CAS.
- Z.-S. Wang, T. Yamaguchi, H. Sugihara and H. Arakawa, Langmuir, 2005, 21, 4272 CrossRef CAS.
- Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 2006, 45, L638 CrossRef CAS.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145 CrossRef.
- H. Lindström, A. Holmberg, E. Magnusson, S.-E. Lindquist, L. Malmqvist and A. Hagfeldt, Nano Lett., 2001, 1, 97 CrossRef.
- H. Seo, M.-K. Son, I. Shin, J.-K. Kim, K.-J. Lee, K. Prabakar and H.-J. Kim, Electrochim. Acta, 2010, 55, 4120 CrossRef CAS.
- F. Hirose, M. Shikaku, Y. Kimura and M. Niwano, J. Electrochem. Soc., 2010, 157(11), B1578 CrossRef CAS.
- W.-T. Chuang, B.-S. Chen, K.-Y. Chen, C.-C. Hsieh and P.-T. Chou, Chem. Commun., 2009, 6982 RSC.
- Y. Kim, B. J. Yoo, R. Vittal, Y. Lee, N.-G. Park and K.-J. Kim, J. Power Sources, 2008, 175, 814 CrossRef.
- C.-Y. Huang, Y.-C. Hsu, J.-G. Chen, V. Suryanarayanan, K.-M. Lee and K.-C. Ho, Sol. Energy Mater. Sol. Cells, 2006, 90, 2391 CrossRef CAS.
- M. K. Nazeeruddin, S. M. Zakeeruddin, R. Humphry-Baker, M. Jirousek, P. Liska, N. Valchopoulos, V. Shklover, C.-H. Fischer and M. Grätzel, Inorg. Chem., 1999, 38, 6298 CrossRef CAS.
- N. R. Neale, N. Kopidakis, J. van de Lagemaat, M. Grätzel and A. J. Frank, J. Phys. Chem. B, 2005, 109, 23183 CrossRef CAS.
- K. Zhu, T. B. Vinzant, N. R. Neale and A. J. Frank, Nano Lett., 2007, 7, 3739 CrossRef CAS.
- J. Bisquert, J. Phys. Chem. B, 2002, 106, 325 CrossRef CAS.
- J.-J. Wu, G.-R. Chen, H.-H. Yang, C.-H. Ku and J-Y. Lai, Appl. Phys. Lett., 2007, 90, 213109 CrossRef.
- C.-H. Ku and J.-J. Wu, Appl. Phys. Lett., 2007, 91, 093117 CrossRef.
- Y. Alivov and Z. Y. Fan, Appl. Phys. Lett., 2009, 95, 063504 CrossRef.
- M. Adachi, M. Sakamoto, J. Jiu, Y. Ogata and S. Isoda, J. Phys. Chem. B, 2006, 110, 13872 CrossRef CAS.
- S. E. Koops, P. R. F. Barnes, B. O'Regan and J. R. Durrant, J. Phys. Chem. C, 2010, 114, 8054 CAS.
- S. Nakade, Y. Saito, W. Kubo, T. Kanzaki, T. Kitamura, Y. Wada and S. Yanagida, Electrochem. Commun., 2003, 5, 804 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
