Urchin-like Co3O4 microspherical hierarchical superstructures constructed by one-dimension nanowires toward electrochemical capacitors
Linrui
Hou
a,
Changzhou
Yuan
*a,
Long
Yang
a,
Laifa
Shen
b,
Fang
Zhang
b and
Xiaogang
Zhang
*b
aAnhui Key Laboratory of Metal Materials & Processing, School of materials Science and Engineering, Anhui University of technology, Maanshan, 243002, P.R. China
bCollege of Material Science & Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing, 210016, P.R. China. E-mail: ayuancz@163.com; azhangxg@163.com; Fax: +86 555 2311570; Tel: +86 555 2311871
First published on 11th October 2011
Abstract
We describe in this report a novel synthetic strategy for synthesizing urchin-like Co3O4 microspherical hierarchical superstructures constructed by one-dimension Co3O4 nanowires, precipitating hydroxides followed by a calcinating process. Electrochemical data reveals that the as-synthesized urchin-like Co3O4 superstructures can deliver a specific capacitance (SC) of 614 F g−1 at a current density of 1 A g−1, and remain as large as 536 F g−1 at 4 A g−1 in a 3 M KOH aqueous solution. Such unique urchin-like hierarchical superstructures facilitate the electrolyte ions and electrons to easily contact the electroactive Co3O4 nanowire building blocks at high rates, which allows for sufficient Faradaic reactions to take place at large current densities for energy storage. Moreover, a SC degradation of ca. 23% after 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous charge–discharge cycles at a large current density of 4 A g−1 demonstrates their good electrochemical stability at high rates.
000 continuous charge–discharge cycles at a large current density of 4 A g−1 demonstrates their good electrochemical stability at high rates.
1. Introduction
Electrochemical capacitors (ECs), are important energy storage devices and can offer transient but extremely high powers for time-dependent power needs of modern electronics and power systems and fill the gap between dielectric capacitors and batteries.1,2 Based on the energy storage mechanisms, ECs are divided into electrical double-layer capacitors (EDLCs) and electrochemical pseudocapacitors (EPCs). Specifically, EDLCs are mainly based on carbon materials and deliver a long cycling performance but with relatively low specific capacitance (SC). In contrast to EDLCs, EPCs have much larger SCs owing to their significant redox properties. Among these electroactive materials with good pseudocapacitance, metal oxides have drawn much attention, due to their larger SCs compared to carbon materials and better cycle life than conducting polymers (CPs).2In particular, amorphous RuO2 formed by the sol–gel method has been known as the state-of-the-art electroactive material for ECs and shows a SC as high as 720 F g−1, however, its commercial application was greatly hindered by its high cost and serious toxicity.3,4 Thus, considerable efforts have been devoted to developing other transition metal oxides with good electrochemical performance and great cost advantages over noble metal oxides such as RuO2. With favorable capacitive characteristics and environmental friendliness, Co3O4 has received much research attention and was deemed to be an ideal electroactive material for ECs applications.2,5–11
Recently, nano/micro superstructures constructed by nanometre-sized building blocks were found to be one of the best systems in the ECs field,12–16 because they can offer both advantages of nanosized building blocks and micro- or submicrometer-sized structures. Specifically, the former, with a large specific surface area (SSA) and a high surface-to-bulk ratio, can offer negligible diffusion time, favorable kinetics and large SCs. And the latter, with its desirable mechanical properties, guarantees its good stability and practical fabrication. Moreover, it would be even better if the superstructures owned oriented building blocks because such unique structures can provide optimized and convenient ion diffusion paths.12–14,16,17 Therefore, in the ECs field, it is significant to design and tailor the specific microstructure of Co3O4 with the enhanced electrochemical performance (large SC, good rate properties and high electrochemical stability at high rates) by a simple but efficient strategy.
Herein, a facile strategy was reported to synthesize urchin-like Co3O4 microspherical superstructures, which are constructed by lots of aligned Co3O4 nanowires (NWs) in a radial way. Importantly, the unique urchin-like Co3O4 superstructures possess convenient ion transport paths, and deliver large SCs and good electrochemical stability at high rates, which showcases their great potential application in ECs.
2. Experimental
2.1 Material synthesis and electrode preparation
All the chemicals used here were of analytical grade. Lysine and CoCl2·6H2O were obtained from the Nanjing Chemical Company (Nanjing, China). The saturated calomel electrode (SCE) was manufactured by Leici (Shanghai, China). All aqueous solutions were freshly prepared by using high-purity water (18 MΩ cm resistance) from an Ampeon 1810-B system (Jiangsu, China). 2 g lysine and 1.3658 g CoCl2·6H2O were dissolved in 60 mL water. The solution was kept in a Teflon-lined autoclave (80 mL) with a stainless steel shell. After heating to 100 °C, the autoclave was kept at the temperature for 24 h in an oven and then allowed to cool to room temperature naturally. The pink product of the reaction was filtered, washed repeatedly, dried at 100 °C in vacuum, and then calcinated at 250 °C for 3 h to obtain the black sample.2.2 Characterization
The samples were examined by powder X-ray diffraction (XRD) (Max 18 XCE, Japan) using a Cu Kα source (λ = 0.1542 nm) at a scanning speed of 3° min−1 over a 2θ range of 10–80°. Scanning electron microscopy (SEM) images were taken with a field-emission scanning electron microscope (FESEM, JEOL-6300F, 15 kV), and selected area electron diffraction (SAED) was performed with a transmission electron microscope (TEM, FEI, TECNAI-20) and a JEOL-2010 high-resolution (HR)TEM. N2 adsorption–desorption was performed by Brunauer-Emmett-Teller (BET) measurements using an ASAP-2010 surface area analyzer. X-ray photoelectron (XPS) measurements were performed on a VGESCALAB MKII X-ray photoelectron spectrometer with an Mg Kα excitation sources (1253.6 eV).The working electrode was prepared with the electroactive material Co3O4 with acetylene black (AB) and polytetrafluoroethylene (PTFE) in a weight ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. A small amount of water was then added to this mixture to make a more homogeneous mixture, which was pressed (ca. 107 Pa) on a nickel grid (1 cm2) for the following electrochemical tests. Cyclic voltammetry (CV) and chronopotentiometry (CP) were performed with a CHI660C electrochemical workstation, and electrochemical impedance spectroscopy (EIS) was performed with a frequency response analyzer (Solatron 1255B) interfaced with potential galvanostat (Solatron 1287) controlled by a personal computer. All experiments were carried out in a three compartment cell with a working electrode, a platinum plate counter electrode (1 cm2) and a saturated calomel electrode (SCE) reference electrode at room temperature. The electrolyte was 3 M KOH aqueous solution.
0.5. A small amount of water was then added to this mixture to make a more homogeneous mixture, which was pressed (ca. 107 Pa) on a nickel grid (1 cm2) for the following electrochemical tests. Cyclic voltammetry (CV) and chronopotentiometry (CP) were performed with a CHI660C electrochemical workstation, and electrochemical impedance spectroscopy (EIS) was performed with a frequency response analyzer (Solatron 1255B) interfaced with potential galvanostat (Solatron 1287) controlled by a personal computer. All experiments were carried out in a three compartment cell with a working electrode, a platinum plate counter electrode (1 cm2) and a saturated calomel electrode (SCE) reference electrode at room temperature. The electrolyte was 3 M KOH aqueous solution.
3. Results and discussion
3.1 Physicochemical characterization
The corresponding precursor of the Co3O4 sample obtained after thermal decomposition is β-Co(OH)2, as reported before,17 where two reactions took place as follows:| H2N(CH2)3CH(NH2)COOH + H2O ⇄ [H3N(CH2)3CH(NH2)COOH]+ + OH− | (1) |
| Co2+ + 2OH− = Co (OH)2 | (2) |
And then the black Co3O4 sample is obtained after the calcination at 250 °C for 3 h, as indicated by the following eqn (3):
| 6Co (OH)2 + O2 = 2Co3O4 + 6H2O | (3) |
The XRD pattern of the as-synthesized Co3O4 sample was illustrated in Fig. 1. Eight obvious diffraction peaks, including not only the peak positions but also their relative intensities, are identified for the (111), (220), (311), (400), (422), (511), (440) and (533) planes of the cubic phase Co3O4 crystalline structure, which is consistent with the standard spectrum (Joint Committee on Powder Diffraction Standards (JCPDS) file no. 65-3103) with a space group of FD-3m(227). It indicates that a temperature of 250 °C is high enough to drive the conversion from Co(OH)2 to the Co3O4 phase. The important information about the chemical composition of the Co3O4 sample can be further provided by the XPS measurement. Fig. 2 presents the XPS spectrum of the as-prepared Co3O4 sample. The binding energies obtained in the XPS analyses were corrected for specimen charging by referencing the C1s peak to 284.60 eV. The Co2p XPS spectrum shows two major peaks with binding energy positions at 779.3 and 794.80 eV, corresponding to Co2p3/2 and Co2p1/2, respectively, characteristic of a Co3O4 phase.
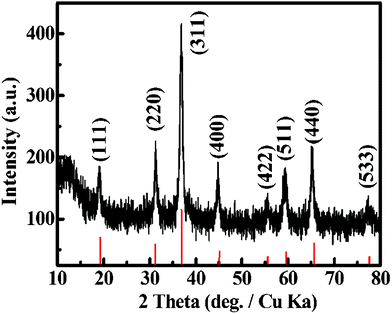 | ||
| Fig. 1 XRD and corresponding standard (indicated by the red lines) patterns of the as-synthesized Co3O4 sample. | ||
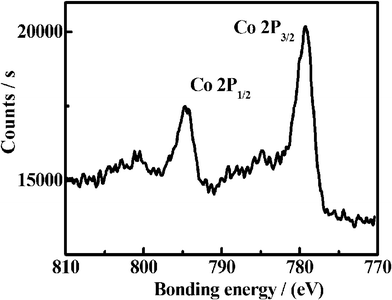 | ||
| Fig. 2 XPS spectrum of the synthesized Co3O4 samples. | ||
Fig. 3 demonstrates the FESEM images of the synthesized Co3O4 samples. Obviously, the low-magnification FESEM image (Fig. 3a) shows that the Co3O4 sample almost entirely consists of the microspherical structure with a diameter of ca. 1 to 2 μm. The magnified FESEM image (Fig. 3b) demonstrates that the surfaces of these spherical structures are fixed with many NWs and take on an urchin-like appearance. The shape of the obtained Co3O4 sample is similar to that of the precursor β-Co(OH)2,17 which possesses a unique ordered urchin-like superstructure due to the synergistic contributions from lysine and the Cl− ions. It indicates that the urchin-like configuration of the precursor is retained during the thermal treatment at 250 °C for 3 h. Moreover, the oriented Co3O4 NWs, in the radial form, create abundant “V-type” porous channels, which would facilitate the fast penetration of the electrolyte into the superstructures and contact the electroactive surface of the Co3O4 NW building blocks for energy storage.
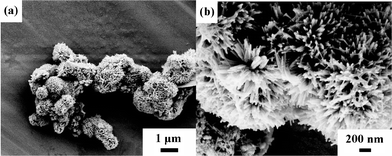 | ||
| Fig. 3 FESEM images of the as-synthesized Co3O4 sample. | ||
To demonstrate their microstructures more clearly, the (HR)TEM images of urchin-like Co3O4 microspherical hierarchical superstructures with different magnifications were shown in Fig. 4a–d. As seen from Fig. 4a–c, almost all the NWs are oriented and assembled in a radial form from the center to the surface of microspherical superstructures, which looks like an urchin (Fig. 4a). Each Co3O4 NW is found to be composed of many Co3O4 nanoparticles (NPs) with the size of ca. 10 to 50 nm, which are further assembled into one-dimension Co3O4 NWs building blocks (Fig. 4b and c). Finally, these NWs are constructed into the microsperical superstructures, i.e., the mesoporous precursor Co(OH)2 NWs turn out to be Co3O4 NWs constructed by many Co3O4 NPs after thermal treatment at 250 °C for 3 h. Interestingly, the as-obtained superstructures cannot be destroyed and broken into discrete individual Co3O4 NWs or NPs by subjecting long-time ultrasonication. This suggests that the unique superstructures are not a random aggregate of Co3O4 NPs and NWs but the ordered assembly of Co3O4 NPs and NWs. Fig. 4d clearly shows HR-lattice TEM images of the Co3O4 NPs. The interplanar distance between adjacent lattice planes is 0.24 nm, which is indexed as the (311) plane of Co3O4 crystals. It reveals that the Co3O4 microspherical superstructures possess good crystallinity, which can also be verified by their corresponding SAED pattern (the inset in Fig. 4d). The primary ring pattern suggests they are polycrystalline in nature for these NP and NW building blocks.
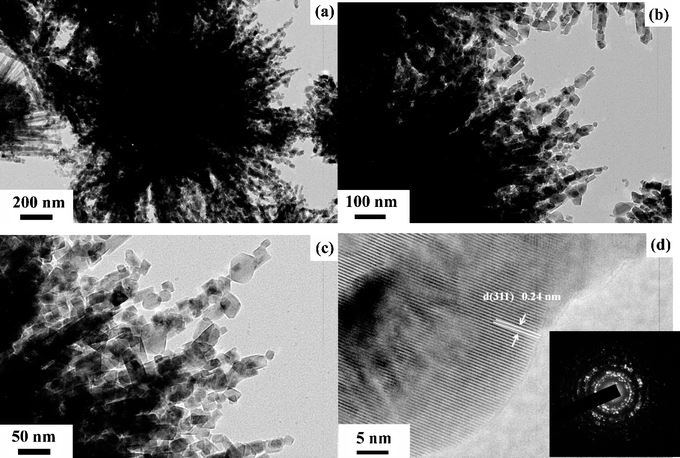 | ||
| Fig. 4 Bright-field (a, b and c) TEM, HR-lattice (d) TEM images and corresponding SAED (the inset in d) of the as-synthesized urchin-like Co3O4 microspherical superstructures. | ||
N2 adsorption–desorption measurements were used to study the specific porosity and textural properties of the urchin-like Co3O4 microspherical superstructures. Fig. 5 depicts the adsorption isotherms of the microspherical superstructures. The isotherm can be classified as type IV according to the international union of pure and applied chemistry classification (IUPAC). Of note, a distinct hysteresis loop can be observed in the large range of ca. 0.5∼1.0 P/P0, indicating that the as-prepared Co3O4 superstructures own multi-modal and hierarchical porosity, i.e., mesopores together with macropores,15,17–19 which should be mainly caused by the “V-type” porous channels among the oriented Co3O4 NWs. The average pore diameter of the superstructures is obtained ca. 28 nm from the Barrett-Joyner-Halenda (BJH) method by calculating from the adsorption branch of the isotherm. Moreover, the BET SSA and mesoporous volume of the Co3O4 microspherical superstructures are quantitatively summarized as ca. 113 m2 g−1 and 0.32 cm3 g−1, respectively.
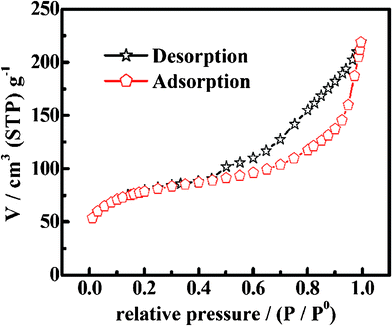 | ||
| Fig. 5 The N2 adsorption–desorption isotherm of the urchin-like Co3O4 microspherical superstructures. | ||
3.2 Electrochemical properties
Cyclic voltammetry is considered to be a suitable tool to demonstrate the capacitive behavior of any electroactive material.1 Typical CV curves of the Co3O4 microspherical superstructures under various sweeping rates, as indicated, are presented in Fig. 6. Clearly, the shape of the curves, as demonstrated in Fig. 6, indicates that the characteristics of the measured capacitance is very different from that of the pure electric double layer capacitance, for which the CV curve is close to an ideal rectangular shape. With the similarity in the shape of the CV curves in Fig. 6 to that previously reported by B.N. Popov et al. and L. Cao et al.,3,20 the discussion on electron transition and chemical reaction is referred to the corresponding citations. The subsequent increases in current with insignificant shape change in the CV curves with rapid current responses on voltage reversal still happens at each end potential with increasing sweep rate under the same potentials, suggesting good electrochemical capacitive nature of the microspherical superstructures in the 3 M KOH aqueous solution.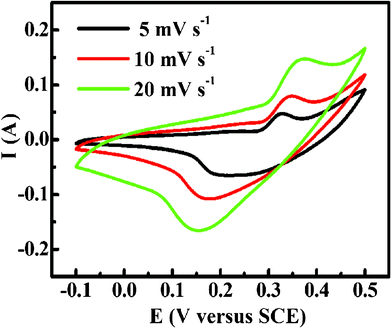 | ||
| Fig. 6 The CV curves of the as-synthesized Co3O4 microspherical superstructures at various scan rates as indicated. | ||
Fig. 7 shows the galvanostatic constant current charge–discharge curves of the urchin-like Co3O4 microspherical superstructures at a series of current densities using a potential window spanned from −0.10 to 0.45 versus SCE. A symmetric triangular shape during the charge–discharge processes is presented and resemble those of EDLCs. However, a closer look reveals that the slopes of the CP curves slightly differ from linearity as a result of the pseudo-Faradaic processes of the electrode. An important parameter, coulombic efficiency (η) of the electrode, can be evaluated from eqn (4) based on the CP plots depicted in Fig. 7
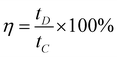 | (4) |
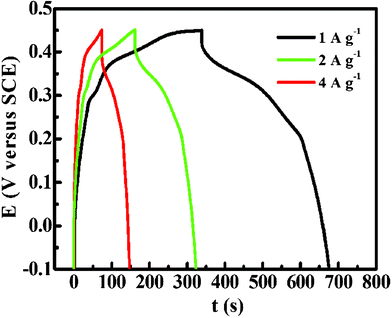 | ||
| Fig. 7 CP plots of the as-synthesized urchin-like Co3O4 microspherical hierarchical superstructures at different current densities as indicated. | ||
In addition, the SCs of the Co3O4 microspherical superstructures were calculated from the CP curves shown in Fig. 7 based on the eqn (5)
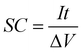 | (5) |
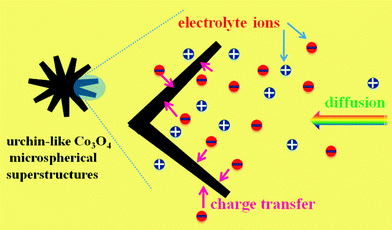 | ||
| Fig. 8 Schematic of the optimized ion diffusion path in urchin-like Co3O4 microspherical hierarchical superstructures. | ||
The electrochemical process mentioned above can be further verified by the following EIS measurement. Fig. 9 demonstrates the Nyquist plot of the EIS tests in 3 M KOH solution with a frequency loop from 105 Hz to 0.01 Hz using a perturbation amplitude of 5 mV at 0.3 V versus SCE. The intersection of the plots at the x-axis represents the solution resistance (Rs),25 which includes the following three terms: the resistance of the KOH aqueous solution, the intrinsic resistance of the electroactive materials themselves and the contact resistance at the interface between electroactive materials and the current collector. As seen from the inset, Rs can be found to be only ca. 0.53 Ω, which reveals the good electronic conductivity of the as-synthesized Co3O4 microspherical superstructures. And at the high-medium frequency region, a semicircle can be found and its diameter stands for the charge transfer resistance (Rct) in the electrochemical process, which is approximated to ca. 0.14 Ω judging from the slope of the curve at low-frequency region (the inset in Fig. 9). Such small value means a low charge transfer resistance during the redox reaction for energy storage. At the low frequency region, another x-intersection is equal to the Rs + 1/3Rdiffusion,26 where Rdiffusion shows the ion diffusion resistance. The ion diffusion resistance was calculated as only ca. 0.36 Ω according to the plot shown in the inset in Fig. 9, which means that the electrolyte ions can be quickly transported from the bulk solution to the surfaces of Co3O4 NWs and NPs. In general, the rate of an electrode process depends on diffusion as well as charge transfer. The EIS measurement presents that urchin-like Co3O4 microspherical superstructures own a reduced ion diffusion path and charge transfer resistance. Therefore, the as-synthesized urchin-like Co3O4 microspherical superstructures can obtain a large SC and electrochemical utilization even at high charge–discharge rates. In addition, in the low frequency region, the slope of the impedance plot almost tends to a vertical asymptote, indicating the good electrochemical capacitance of the urchin-like hierarchical superstructures in the KOH aqueous solution.
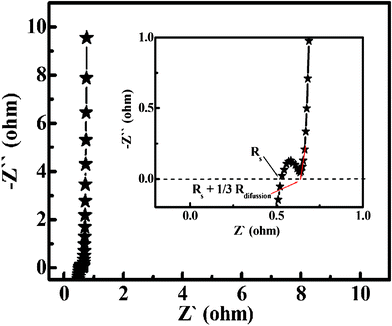 | ||
| Fig. 9 EIS plot of the as-synthesized urchin-like Co3O4 microspherical hierarchical superstructures at 0.3 V versus SCE. (The inset is an enlarged curve of the high frequency region). | ||
The cycle performance of any electrode material is one of the most important parameters for its practical applications. The cycle life for the urchin-like Co3O4 microspherical superstructures was carried out at a constant current density of 4 A g−1. As shown in Fig. 10, after continuous 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles, the SC decreases from ca. 536 to 413 F g−1. A SC degradation of ca. 23% reveals good electrochemical stability of the unique Co3O4 microspherical superstructures at large current densities.
000 charge–discharge cycles, the SC decreases from ca. 536 to 413 F g−1. A SC degradation of ca. 23% reveals good electrochemical stability of the unique Co3O4 microspherical superstructures at large current densities.
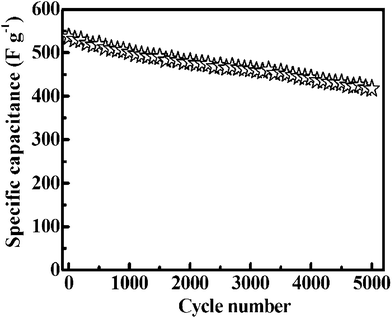 | ||
| Fig. 10 Cycling performance of the as-synthesized hierarchical Co3O4 superstructures at 4 A g−1. | ||
4. Conclusions
To recap, we propose an efficient synthetic strategy to synthesize urchin-like Co3O4 microspherical superstructures, which are assembled by one-dimension Co3O4 nanowires consisting of Co3O4 nanoparticles. Electrochemical data shows that the as-synthesized urchin-like Co3O4 microspherical superstructures can deliver a specific capacitance of 614 F g−1 at 1 A g−1, and 536 F g−1 at 4 A g−1 in 3 M KOH solution. Such unique urchin-like superstructures with good electronic conductivity facilitate the electrolyte ions and electrons to easily contact the electroactive Co3O4 nanowire/nanoparticle building blocks at high rates, ensuring that sufficient Faradaic reactions can take place at high current densities for energy storage. Furthermore, the capacitance degradation of ca. 23% after 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous charge–discharge cycles at 4 A g−1 reveals their good electrochemical stability at large current densities. Importantly, the preparation we described here is much simple and cost-effective for large-scale production of the Co3O4 microspherical superstrutures with good electrochemical performance for ECs and even for Li-ion battery applications.
000 continuous charge–discharge cycles at 4 A g−1 reveals their good electrochemical stability at large current densities. Importantly, the preparation we described here is much simple and cost-effective for large-scale production of the Co3O4 microspherical superstrutures with good electrochemical performance for ECs and even for Li-ion battery applications.
Acknowledgements
This work was financially supported by State Basic Research Program of PRC (973 Program) (no. 2007CB209703), National Natural Science Foundation of PRC (no. 20873064), Natural Science Foundation of Anhui Province (no. 10040606Q07) and 2010 Young Teachers' Foundation of Anhui University of Technology (no. QZ201003).References
- B. E. Conway, Electrochemical Supercapacitors, Scientific Fundaments and Technological Applications; Kluwer Academic/Plenum: New York, 1999 Search PubMed.
- T. Y. Wei, C. H. Chen, K. H. Chang, S. Y. Lu and C. C. Hu, Chem. Mater., 2009, 21, 3228–3233 CrossRef CAS.
- C. Lin, J. A. Ritter and B. N. Popov, J. Electrochem. Soc., 1998, 145, 4097–4103 CrossRef CAS.
- C. Z. Yuan, L. Chen, B. Gao, L. H. Su and X. G. Zhang, J. Mater. Chem., 2009, 19, 246–252 RSC.
- W. Sugimoto, H. Iwata, Y. Yasunaga, Y. Murakami and Y. Takasu, Angew. Chem., Int. Ed., 2003, 42, 4092–4096 CrossRef CAS.
- J. Xu, L. Gao, J. Y. Cao, W. C. Wang and Z. D. Chen, Electrochim. Acta, 2010, 56, 732–736 CrossRef CAS.
- S. L. Xiong, C. Z. Yuan, X. G. Zhang, B. J. Xi and Y. T. Qian, Chem. Eur. J., 2009, 15, 5320–5326 CrossRef CAS.
- M. B. Zheng, J. Cao, S. T. Liao, J. S. Liu, H. Q. Chen, Y. Zhao, W. J. Dai, G. B. Ji, J. M. Cao and J. Tao, J. Phys. Chem. C, 2009, 113, 3887–3894 CAS.
- Y. Y. Gao, S. L. Chen, D. X. Cao, G. L. Wang and J. L. Yin, J. Power Sources, 2010, 195, 1757–1760 CrossRef CAS.
- T. Sugimoto and E. Matijevieć, J. Inorg. Nucl. Chem., 1979, 41, 165–172 CrossRef CAS.
- S. Ardizzone, G. Spinolo and S. Trasatti, Electrochim. Acta, 1995, 40, 2683–2686 CrossRef CAS.
- J. Liu, G. Z. Cao, Z. G. Yang, D. H. Wang, D. Dubois, X. D. Zhou, G. L. Graff, L. R. Pederson and J. G. Zhang, ChemSusChem, 2008, 1, 676–697 CrossRef CAS.
- C. Z. Yuan, B. Gao, L. F. Shen, S. D. Yang, L. Hao, X. J. Lu, F. Zhang, L. J. Zhang and X. G. Zhang, Nanoscale, 2011, 3, 529–545 RSC.
- J. S. Hu, L. S. Zhong, W. G. Song and L. J. Wan, Adv. Mater., 2008, 20, 2977–2982 CrossRef CAS.
- C. Z. Yuan, X. G. Zhang, L. H. Su, B. Gao and L. F. Shen, J. Mater. Chem., 2009, 19, 5772–5777 RSC.
- W. Wei and Z. Z. Yang, Adv. Mater., 2008, 20, 2965–2969 CrossRef CAS.
- C. Z. Yuan, X. G. Zhang, L. R. Hou, L. F. Shen, D. K. Li, F. Zhang, C. G. Fan and J. M. Li, J. Mater. Chem., 2010, 20, 10809–10816 RSC.
- J. R. Matos, M. Kruk, L. P. Mercuri, M. Jaroniec, L. Zhao, T. Kamiyama, O. Terasaki, T. J. Pinnavaia and Y. Liu, J. Am. Chem. Soc., 2003, 125, 821–829 CrossRef CAS.
- C. Z. Yuan, S. L. Xiong, X. G. Zhang, L. F. Shen, F. Zhang, B. Gao and L. H. Su, Nano Res., 2009, 2, 722–732 CrossRef CAS.
- L. Cao, M. Lu and H. L. Li, J. Electrochem. Soc., 2005, 152, A871–A875 CrossRef CAS.
- M. B. Zheng, J. Cao, S. T. Liao, J. S. Liu, H. Q. Chen, Y. Zhao, W. J. Dai, G. B. Ji, J. M. Cao and J. Tao, J. Phys. Chem. C, 2009, 113, 3887–3894 CAS.
- Y. Liu, W. W. Zhao and X. G. Zhang, Electrochim. Acta, 2008, 53, 3296–3304 CrossRef CAS.
- Y. Y. Gao, S. L. Chen, D. X. Cao, G. L. Wang and J. L. Yin, J. Power Sources, 2010, 195, 1757–1760 CrossRef CAS.
- T. Y. Wei, C. H. Chen, K. H. Chang, S. Y. Lu and C. C. Hu, Chem. Mater., 2009, 21, 3228–3233 CrossRef CAS.
- C. Z. Yuan, X. G. Zhang, Q. F. Wu and B. Gao, Solid State Ionics, 2006, 177, 1237–1242 CrossRef CAS.
- K. Wang, J. Y. Huang and Z. X. Wei, J. Phys. Chem. C, 2010, 114, 8062–8067 CAS.
| This journal is © The Royal Society of Chemistry 2011 |
