Reusable and efficient CoCl2 ·6H2O/cationic 2,2’-bipyridyl system-catalyzed S-arylation of aryl halides with thiols in water under air†
Ming-Tzu
Lan
,
Wei-Yi
Wu
,
Shao-Hsien
Huang
,
Kai-Luen
Luo
and
Fu-Yu
Tsai
*
Institute of Organic and Polymeric Materials, National Taipei University of Technology, 1, Sec. 3, Chung-Hsiao E. Rd., Taipei 106, Taiwan. E-mail: fuyutsai@ntut.edu.tw; Fax: +886 2 2731 7174
First published on 25th October 2011
Abstract
A CoCl2·6H2O/cationic 2,2’-bipyridyl system was proven to be an efficient and reusable catalyst for the coupling of aryl halides with thiols in water under aerobic conditions, leading to the formation of thioethers. Aryl iodides and bromides could couple with various thiols giving the corresponding thioethers using only a 3 mol% catalyst loading in the presence of 1 equiv KOH and 1.5 equiv Zn at 100 °C. For aryl chlorides, reaction at 140 °C and prolongation of the reaction time was performed. After reaction, the residual aqueous solution could be reused several times without any additional treatment and regeneration, making this cobalt-catalyzed S-arylation reaction greener.
Introduction
The importance of aryl sulfide moieties stems from their usefulness in biological, pharmaceutical, and material science applications.1 In modern organic synthesis, transition-metal-catalyzed cross-coupling of aryl halides and thiols for the formation of aryl sulfides is one of the most powerful tools.2Catalysts Cu,3Pd,4Ni,5 and Fe6 are widely employed for this coupling reaction. The use of inexpensive Co as a catalyst for C–S coupling, however, is still rare. Recently, Cheng’s group found that CoI2(dppe) can catalyze aryl–sulfur bond formation in CH3CN using Zn as the reductant.7 This method represents the missing link for the well-documented first-series transition-metal catalyzed S-arylation between Fe and Cu.The development of environmentally-benign catalytic systems, in particular using water as the reaction medium, has attracted much attention in recent years due to this green solvent being cheap, nontoxic, safe, and environmentally-benign as compared with organic solvents.8 Furthermore, the easy separation of the catalyst-containing aqueous solution from the organic products enables reuse of the catalyst. Although several transition-metals, such as Cu9 and Fe10, have been proven to be reusable catalysts for S-arylation using water as the solvent, this cross-coupling reaction catalyzed by cobalt in water under air has yet to be developed, and the design of a greener and reusable cobalt-containing catalyst for S-arylation as an alternative protocol to complement other metal-catalyzed systems is considered of high practical value.
We have recently reported the combination of a water-soluble cationic 2,2’-bipyridyl ligand 1 and FeCl3.6H2O as a reusable catalytic system for the coupling of aryl iodides and thiols.10 Herein, we report that the replacement of iron by cobalt to associate with 1 was a reusable and efficient catalyst for the C–S bond-forming reaction of aryl halides, not only aryl iodides but also bromides and chlorides, with thiols in water under aerobic conditions by an operationally-simple procedure without the use of any air- or moisture-sensitive reagents (Scheme 1).
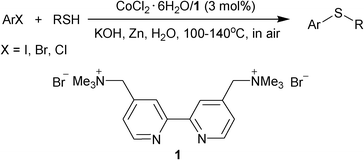 | ||
| Scheme 1 Cobalt-catalyzed S-arylation in water. | ||
Experimental
General
Chemicals were purchased from commercial suppliers and were used without further purification. The cationic 2,2’-bipyridyl ligand was prepared according to the published procedures.11 Melting points were recorded using melting point apparatus and were uncorrected. All 1H and 13C NMR spectra were recorded in CDCl3 at 25 °C on a Varian 200 or Bruker 400 NMR spectrometer. GC analysis was performed on a SHIMADZU GC-14B equipped with a fused silica capillary column. Elemental analyses were performed and high-resolution mass spectra (HRMS) were recorded at the Instrument Center Service, National Science Council of Taiwan. All known S-arylation products obtained were pure, and their spectra (1H, and 13C NMR) are given in the ESI.†Typical procedure for the cobalt-catalyzed S-arylation reaction
A 20 mL reaction tube was charged with CoCl2·6H2O (0.03 mmol), 1 (0.03 mmol), and H2O (5 mL). After stirring the solution at room temperature for 10 min, aryl halide (1.2 mmol), thiol (1.0 mmol), KOH (1.0 mmol), and Zn (1.5 mmol) were added. The reaction mixture was stirred at 100 °C under air for 24 h (aryl iodide, see Table 1 and 2) or 48 h (aryl bromide, see Table 3). In the case of aryl chloride, the reaction vessel was sealed under air by a Teflon-coated screw cap and stirred at 140 °C for 72 h (see Table 4). After cooling the reaction mixture to room temperature, the aqueous solution was extracted with hexane or EtOAc, the organic phase was dried over MgSO4, and the solvent was then removed under vacuum. Column chromatography on silica gel afforded the desired product.| Entry | [M]/1 (mol%) | Base (mmol) | Zn (mmol) | Yield (%)b |
|---|---|---|---|---|
| a Reaction conditions: 2a (1.2 mmol), 3a (1.0 mmol), [M]/1, Zn (1.5 mmol), KOH (1.0 mmol), and H2O (5 mL) at 100 °C for 24 h. b GC yields. Isolated yields are given in parentheses. c Reaction time was 12 h. d 2,2’-Bipyridyl was used as the ligand. e In the absence of ligand 1. f 99.999% purity CoCl2.6H2O was used. | ||||
| 1 | CoCl2.6H2O (3) | KOH (1) | – | 42 (34) |
| 2 | CoCl2.6H2O (3) | KOH (1) | 1.0 | 58 (47) |
| 3 | CoCl2.6H2O (3) | KOH (1) | 1.5 | 99 (94) |
| 4 | CoCl2.6H2O (3) | KOH (2) | 1.5 | 88 (79) |
| 5 | CoCl2.6H2O (2) | KOH (1) | 1.5 | 80 (75) |
| 6 | CoCl2.6H2O (4) | KOH (1) | 1.5 | 99 (93) |
| 7c | CoCl2.6H2O (3) | KOH (1) | 1.5 | 69 (60) |
| 8d | CoCl2.6H2O (3) | KOH (1) | 1.5 | 49 (43) |
| 9e | CoCl2.6H2O (3) | KOH (1) | 1.5 | 33 (27) |
| 10 | CoCl2.6H2O (3) | K2CO3 (1) | 1.5 | 90 (80) |
| 11 | CoCl2.6H2O (3) | Cs2CO3 (1) | 1.5 | 59 (52) |
| 12 | CoCl2.6H2O (3) | Na2CO3 (1) | 1.5 | 15 |
| 13 | CoCl2.6H2O (3) | K3PO4 (1) | 1.5 | 55 (45) |
| 14 | CoCl2.6H2O (3) | CsF (1) | 1.5 | 81 (74) |
| 15 | CoCl2.6H2O (3) | KF (1) | 1.5 | 71 (62) |
| 16 | Co(NO3)2.6H2O (3) | KOH (1) | 1.5 | 73 (58) |
| 17f | CoCl2.6H2O (3) | KOH (1) | 1.5 | 98 (92) |
| 18 | CuO (0.01) | KOH (1) | 1.5 | 10 |
| 19 | Cu2O (0.01) | KOH (1) | 1.5 | 9 |
| 20 | PdCl2(NH3)2 (0.01) | KOH (1) | 1.5 | 5 |
| 21 | CuCl2 (0.01) | KOH (1) | 1.5 | 0 |
| 22 | CuCl (0.01) | KOH (1) | 1.5 | 0 |
| 23 | PdCl2 (0.01) | KOH (1) | 1.5 | 0 |
| Entry | Aryl iodide (2 | Thiol (3 | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: 2 (1.2 mmol), 3 (1.0 mmol), CoCl2.6H2O/1 (3 mol%), KOH (1.0 mmol), Zn (1.5 mmol), and H2O (5 mL) at 100 °C for 24 h. b Isolated yields. | |||
| 1 |
 2a
2a
|
 3b
3b
|
 4b, 72
4b, 72 |
| 2 | 2a |
 3c
3c
|
 4c, 91
4c, 91 |
| 3 | 2a |
 3d
3d
|
 4d, 71
4d, 71 |
| 4 |
 2b
2b
|
 3a
3a
|
 4e, 99
4e, 99 |
| 5 | 2b |
 3b
3b
|
 4f, 84
4f, 84 |
| 6 |
 2c
2c
|
 3a
3a
|
 4g, 85
4g, 85 |
| 7 | 2c |
 3b
3b
|
 4h, 84
4h, 84 |
| 8 | 2c |
 3c
3c
|
 4i, 90
4i, 90 |
| 9 | 2c |
 3d
3d
|
 4j, 74
4j, 74 |
| 10 |
 2d
2d
|
 3a
3a
|
 4k, 99
4k, 99 |
| 11 | 2d |
 3b
3b
|
 4l, 92
4l, 92 |
| 12 |
 2e
2e
|
 3a
3a
|
 4m, 77
4m, 77 |
| 13 | 2e |
 3b
3b
|
 4n, 71
4n, 71 |
| 14 |
 2f
2f
|
 3a
3a
|
 4o, 95
4o, 95 |
| 15 | 2f |
 3b
3b
|
 4p, 89
4p, 89 |
| 16 |
 2g
2g
|
 3a
3a
|
 4q, 83
4q, 83 |
| 17 | 2g |
 3b
3b
|
 4r, 85
4r, 85 |
| 18 |
 2h
2h
|
 3a
3a
|
 4s, 81
4s, 81 |
| 19 |
 2i
2i
|
3a |
 4t, 72
4t, 72 |
| 20 | 2a |
 3e
3e
|
 4u, 44
4u, 44 |
| 21 | 2b | 3e |
 4v, 50
4v, 50 |
| 22 | 2a |
 3f
3f
|
 4w, 33
4w, 33 |
| 23 | 2b | 3f |
 4x, 51
4x, 51 |
| 24 | 2c | 3f |
 4y, 30
4y, 30 |
| Entry | Aryl bromide (5 | Thiol (3 | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: 5 (1.2 mmol), 3 (1.0 mmol), CoCl2.6H2O/1 (3 mol%), KOH (1.0 mmol), Zn (1.5 mmol), and H2O (5 mL) at 100 °C for 48 h. b Isolated yields. | |||
| 1 |
 5a
5a
|
 3a
3a
|
 4a, 74
4a, 74 |
| 2 | 5a |
 3b
3b
|
 4b, 68
4b, 68 |
| 3 | 5a |
 3c
3c
|
 4c, 86
4c, 86 |
| 4 | 5a |
 3d
3d
|
 4d, 66
4d, 66 |
| 5 |
 5b
5b
|
 3a
3a
|
 4e, 93
4e, 93 |
| 6 | 5b |
 3b
3b
|
 4f, 82
4f, 82 |
| 7 |
 5c
5c
|
 3a
3a
|
 4g, 78
4g, 78 |
| 8 | 5c |
 3b
3b
|
 4h, 73
4h, 73 |
| 9 | 5c |
 3c
3c
|
 4i, 72
4i, 72 |
| 10 | 5c |
 3d
3d
|
 4j, 64
4j, 64 |
| 11 |
 5d
5d
|
 3a
3a
|
 4k, 61
4k, 61 |
| 12 | 5d |
 3b
3b
|
 4l, 61
4l, 61 |
| 13 |
 5e
5e
|
 3a
3a
|
 4m, 74
4m, 74 |
| 14 | 5e |
 3b
3b
|
 4n, 68
4n, 68 |
| 15 |
 5f
5f
|
 3a
3a
|
 4o, 86
4o, 86 |
| 16 | 5f |
 3b
3b
|
 4p, 80
4p, 80 |
| 17 |
 5g
5g
|
 3a
3a
|
 4q, 73
4q, 73 |
| 18 | 5g | 3b |
 4r, 71
4r, 71 |
| 19 |
 5h
5h
|
 3a
3a
|
 4s, 53
4s, 53 |
| 20 |
 5i
5i
|
3a |
 4t, 48
4t, 48 |
| 21 | 5a |
 3e
3e
|
 4u, 27
4u, 27 |
| 22 | 5b | 3e |
 4v, 30
4v, 30 |
| 23 | 5a |
 3f
3f
|
 4w, 26
4w, 26 |
| 24 | 5b | 3f |
 4x, 45
4x, 45 |
| Entry | Aryl chloride (6 | Thiol (3 | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: 6 (1.2 mmol), 3 (1.0 mmol), CoCl2.6H2O/1 (3 mol%), KOH (1.0 mmol), Zn (1.5 mmol), and H2O (5 mL) at 140 °C for 72 h. b Isolated yields. | |||
| 1 |
 6a
6a
|
 3a
3a
|
 4a, 43
4a, 43 |
| 2 | 6a |
 3b
3b
|
 4b, 48
4b, 48 |
| 3 | 6a |
 3c
3c
|
 4c, 50
4c, 50 |
| 4 |
 6b
6b
|
 3a
3a
|
 4e, 56
4e, 56 |
| 5 | 6b |
 3b
3b
|
 4f, 45
4f, 45 |
| 6 |
 6c
6c
|
 3a
3a
|
 4g, 32
4g, 32 |
| 7 | 6c |
 3c
3c
|
 4i, 37
4i, 37 |
| 8 |
 6d
6d
|
 3a
3a
|
 4k, 30
4k, 30 |
| 9 | 6d |
 3b
3b
|
 4l, 33
4l, 33 |
| 10 |
 6e
6e
|
 3a
3a
|
 4m, 38
4m, 38 |
| 11 | 6e |
 3b
3b
|
 4n, 33
4n, 33 |
| 12 |
 6f
6f
|
 3a
3a
|
 4o, 42
4o, 42 |
| 13 | 6f |
 3b
3b
|
 4p, 40
4p, 40 |
1-(4-(Phenethylthio)phenyl)ethanone (4v)
Pale yellow solid. Mp: 48–50 °C. 1H NMR (CDCl3, 400 MHz) δ = 2.55 (s, 3H), 2.97 (t, J = 8.0 Hz, 2H), 3.23 (t, J = 8.0 Hz, 2H), 7.20−7.25 (m, 3H), 7.29−7.33 (m, 4H), 7.85 (d, J = 8.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ = 26.3, 33.4, 35.1, 126.5 (2C), 126.6, 128.4 (2C), 128.5 (2C), 128.7 (2C), 133.9, 139.6, 144.1, 197.0. Found: C, 74.86; H, 6.49.; S, 12.19. Calcd. for C16H16OS: C, 74.96; H, 6.29. S, 12.51.Hexylphenylsulfide (4w)
Pale yellow oil. 1H NMR (CDCl3, 400 MHz) δ = 0.88 (t, J = 6.8 Hz, 3H), 1.26−1.33 (m, 4H), 1.38−1.46 (m, 2H), 1.60−1.68 (m, 2H), 2.91 (t, J = 7.2 Hz, 2H), 7.13−7.17 (m, 1H), 7.24−7.33 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ = 14.0, 22.5, 28.5, 29.2, 31.4, 33.7, 125.6, 128.8 (2C), 128.9 (2C), 137.1. Found: C, 74.61; H, 9.48.; S, 16.39. Calcd. for C12H18S: C, 74.16; H, 9.34.; S, 16.50.1-(4-(Hexylthio)phenyl)ethanone (4x)
Pale yellow solid. Mp 69−71 °C. 1H NMR (CDCl3, 400 MHz) δ = 0.91 (t, J = 6.8 Hz, 3H), 1.29−1.36 (m, 4H), 1.43−1.50 (m, 2H), 1.67−1.74 (m, 2H), 2.56 (s, 3H), 2.99 (t, J = 7.6 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H), 7.85 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 100 MHz): δ 13.7, 22.3, 26.1, 28.3, 28.5, 31.1, 31.7, 126.0 (2C), 128.4 (2C), 133.5, 137.6, 196.6. HRMS calcd for C14H20OS, 236.1236; found, 236.1235.Typical procedure for the reuse of the catalytic aqueous solution
The reaction was conducted following the procedure described previously under the reaction conditions shown in Table 5. After cooling the reaction mixture to room temperature, the unreacted Zn powder and solidified product 4e were separated from the aqueous phase by centrifugation. The solid portion was washed with EtOAc and dried over MgSO4. The pure product was isolated by column chromatography. The residual aqueous solution was then charged with 2b or 5b, 3a, KOH, and Zn for the next reaction.| Entry | Ar–X | ArSH | Yield (%)b in each cycle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | |||
| a Reaction conditions: aryl halide (1.2 mmol), thiophenol (1.0 mmol), CoCl2.6H2O/1 (3 mol%), KOH (1 mmol), Zn (1.5 mmol), and H2O (5 mL) at 100 °C for 24 h (Entry 1) or 48 h (Entry 2). b Isolated yields. | |||||||||
| 1 |
 2b
2b
|
 3a
3a
|
99 | 95 | 91 | 88 | 80 | 73 | 60 |
| 2 |
 5b
5b
|
 3a
3a
|
93 | 88 | 83 | 80 | 73 | 62 | 54 |
Results and discussion
In order to optimize the reaction conditions, the coupling of iodobenzene 2a and thiophenol 3a catalyzed by analysis grade CoCl2·6H2O combined with 1 was examined; the results are summarized in Table 1. It is known that the employment of 3a as the limiting reagent can diminish the formation of the disulfide by-product.12 Therefore, the reaction gave the desired product, 4a, in a 42% GC yield when the reaction mixture, 2a (1.2 mmol) and 3a (1.0 mmol), was stirred in water (5 mL) under aerobic conditions in the presence of CoCl2·6H2O/1 (3 mol%) and 1.0 mmol KOH at 100 °C for 24 h (Entry 1). In order to improve the yield for the formation of 3a, Zn powder was employed as the reductant,7 and we found that 1.5 equiv of Zn and 1 equiv of KOH rendered the best result (Entries 2–4). Decreasing or increasing the catalyst loading did not afford better yields than the use of 3 mol% of catalyst (Entries 5 and 6). Reducing the reaction time to 12 h resulted in only a 69% GC yield (Entry 7). Employment of neutral 2,2’-bipyridyl as the ligand or the absence of 1 delivered 4a in only 49% and 33% yields, respectively (Entries 8 and 9), indicating the importance of 1 in this aqueous catalytic system. Using the various bases screened, low to high yields were obtained (Entries 10–15), and although the use of K2CO3 afforded a 90% yield, the employment of KOH as the base in Entry 3 produced optimal results. Co(NO3)2·6H2O was also used as a catalyst to test the reaction; however, only a 73% GC yield was obtained (Entry 16). Buchwald and Bolm recently reported that a FeCl3-catalyzed cross-coupling reaction may result owing to contamination by copper.13 To ensure that the reaction was catalyzed by cobalt, 99.999% purity CoCl2·6H2O was associated with 1 as the catalyst and a 98% GC yield of 4a was obtained (Entry 17). We also analyzed the remaining metal impurities in the analysis grade CoCl2.6H2O by ICP-MASS, which showed that this metal salt contained 0.8 ppm of Cu and 0.2 ppm of Pd. Then, we ran several controlled experiments to determine whether the trace amounts of impurities were able to catalyze the S-arylation or not. Therefore, 0.01 mol% of CuO, Cu2O, and PdCl2(NH3)2 were combined with 1 as the catalytic systems in water for S-arylation under the same conditions as shown in Entry 3, which gave only yields between 5% and 10%, respectively (Entries 18–20). In addition, no desired product was observed when CuCl2, CuCl, and PdCl2 were employed as the catalysts (Entries 21–23). These results clearly demonstrate that the S-arylation is indeed catalyzed by cobalt and that trace quantities of impurities do not affect this reaction in an obvious manner.After the optimal conditions were obtained, differently-substituted aryl iodides coupled with thiols were screened to determine the scope of this protocol. As shown in Table 2, the coupling of 2a with aryl thiols 3 gave the corresponding products, 4b–4d, in high yields (Entries 1–3). The cobalt/1-catalyst system proved suitable for the coupling of both electron-deficient and electron-rich aryl iodides with 3 to afford the corresponding diaryl sulfides in isolated yields between 71% and 99% (Entries 4–17). Furthermore, the results of this reaction were not affected by sterically-demanding ortho-substituted aryl iodides such as 2h and 2i; these underwent a coupling reaction with 3a leading to the formation of the corresponding products in 81% and 72% yields, respectively (Entries 18 and 19). Unfortunately, when alkyl-substituted thiols were employed, only moderate to good yields of thioethers were obtained (Entries 20–24).
With regard to aryl bromides, prolongation of the reaction time to 48 h was required to obtain a satisfactory outcome (Table 3). Hence, bromobenzene 5a could couple with a variety of aryl thiols, 3a–3d, in yields between 66% and 86% (Entries 1–4). For electron-poor aryl bromides, such as 5b, high product yields were obtained (Entries 5 and 6). In the case of electron-rich aryl bromides, isolated yields of over 60% could be obtained (Entries 7–14). 1-Bromonaphthalene 5f and meta-substituted 5g could also couple with 3a and 3b, giving the corresponding thioethers in yields between 71% and 86% (Entries 15–18). The coupling reaction of sterically-hindered 5h and 5i with 3a did occur, although the yields were lower than those of iodide analogs (Entries 19 and 20). As expected, aryl bromides coupled with alkyl-substituted thiols at a much slower rate, affording yields between 26% and 45% (Entries 21–24).
The reactivity of aryl chlorides was also studied. Reactions of a series of aryl chlorides and thiols were evaluated using a sealable tube under air at 140 °C for 72 h (Table 4). The use of chlorobenzene 6a and electron-poor 6b coupled with 3 giving yields between 43% and 56% (Entries 1–5). However, lower product yields were obtained when electron-rich 6c–6e were employed (Entries 6–11). Under identical conditions, coupling of 6f with 3 furnished 4o and 4p in moderate yields (Entries 12 and 13). Although the employment of aryl chlorides did not provide excellent yields of S-arylation products, there have been no previous reports of the use of aryl chlorides for the cross-coupling of C–S bonds in a cobalt-catalyzed reaction.
The reusability of the aqueous catalytic system is very important from the practical and economic viewpoints. In order to clarify whether the aqueous solution after reaction still possessed activity for a further catalytic cycle or not, a successive addition experiment was performed. After completion of the coupling reaction of 2b and 3a (Table 2, Entry 4), a fresh portion of 2b, 3a, KOH, Zn, and water was charged directly to the unseparated reaction mixture and the second cycle was executed under conditions identical to those of the first run; this procedure was repeated until the completion of the fifth cycle. We found that the overall isolated yield of 4e was 480%, corresponding to an average of a 96% yield for each cycle. This result clearly reveals that the aqueous solution after reaction is still active for further S-arylation and its loss of activity is slight.
The reaction outcome of the successive addition experiment prompted us to study the reusability of this CoCl2·6H2O/1catalytic system for S-arylation. We chose 2b and 5b as the representative aryl halides to couple with 3a under the conditions shown in Tables 2 and 3 for S-arylation. As shown in Table 5, cross-coupling of 2b and 3a with 3 mol% catalyst loading in the presence of Zn and KOH led to the formation of 4e in a 99% yield in 24 h. After completion of the first cycle and cooling of the reaction mixture to room temperature, unreacted Zn powder and solidified 4e were separated from the aqueous phase by centrifugation. The solid portion was washed with EtOAc, dried over MgSO4, and purified by column chromatography. The remaining aqueous solution was then recharged with 2b, 3a, Zn, and KOH for a further cycle. It was found that the residual aqueous solution could be reused at least six times with only a slight decrease in activity (Entry 1). In comparison with the results of the successive addition experiment, the slow gradual decrease of product yield in each reuse run may be mainly due to a loss of catalyst concentration upon successive separation of the aqueous solution from the solid in each cycle. In the case of 5b, the reuse runs also proceeded well for each cycle (Entry 2). It is noteworthy that there is no need to separate the catalyst from the reaction medium and reactivate prior to reuse, indicating that the use of this catalytic system may meet the goals of green chemistry.
In comparison with other reported catalyst-reusable first-series transition-metal-catalyzed S-arylation reactions, such as Cu9 and Fe10, using water as the reaction medium, this cobalt-catalyzed reaction can be performed under air with a much lower catalyst loading, although the addition of Zn as a reductant is required. With regard to aryl halides, only aryl iodides are suitable for Fe-catalyzed reaction. Although both aryl iodide and bromide reactions can be catalyzed by a Cu catalyst, in this work not only iodides and bromides but also chlorides were able to react. The present protocol provides a useful alternative utilizing a less expensive transition-metal as a reusable catalyst for S-arylation.
Conclusion
In conclusion, we have developed a reusable and efficient water-soluble CoCl2·6H2O/cationic 2,2’-bipyridyl catalytic system for the cross-coupling of aryl halides and thiols. This method employs cationic 2,2’-bipyridyl as the ligand to bring the less expensive CoCl2·6H2O into the aqueous phase; therefore, the reaction can be performed in water under air, rendering the experimental and reuse procedures very simple. In addition, the aqueous catalytic system can be separated from the organic products by simple centrifugation and reused at least six times with only a slight decrease in activity, indicating that it may be an alternative protocol to complement other metal-catalyzed systems and has the potential for use in practical applications. Further studies of the use of this catalytic system in other organic reactions are now under investigation by our group.Acknowledgements
This research was financially supported by the National Science Council of Taiwan (NSC98-2113-M-027-001-MY3). We thank Prof. Chung-Yuan Mou (National Taiwan University) for performing ICP-MASS to determine the amount of impurity in the cobalt salt.References
- (a) D. J. Procter, J. Chem. Soc., Perkin Trans. 1, 2001, 335 RSC; (b) G. Liu, J. R. Huth, E. T. Olejniczak, R. Mendoza, P. DeVries, S. Leitza, E. B. Reilly, G. F. Okasinski, S. W. Fesik and T. W. Von Geldern, J. Med. Chem., 2001, 44, 1202 CrossRef CAS; (c) J. Hassan, M. Sevignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS; (d) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (e) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS; (f) G. De Martino, M. C. Edler, G. La Regina, A. Coluccia, M. C. Barbera, D. Barrow, R. I. Nicholson, G. Chiosis, A. Brancale, E. Hamel, M. Artico and R. Silvestri, J. Med. Chem., 2006, 49, 947 CrossRef CAS; (g) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS; (h) I.-P. Beletskaya and V. P. Ananikov, Eur. J. Org. Chem., 2007, 3431 CrossRef CAS; (i) A. Gangjee, Y. Zeng, T. Talreja, J. J. McGuire, R. L. Kisliuk and S. F. Queener, J. Med. Chem., 2007, 50, 3046 CrossRef CAS.
- T. Kondo and T. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS.
- (a) P. S. Herradura, K. A. Pendola and R. K. Guy, Org. Lett., 2000, 2, 2019 CrossRef CAS; (b) C. Palomo, M. Oiarbide, R. Lopez and E. Gomez-Bengoa, Tetrahedron Lett., 2000, 41, 1283 CrossRef CAS; (c) C. G. Bates, R. K. Gujadhur and D. Venkataraman, Org. Lett., 2002, 4, 2803 CrossRef CAS; (d) F. Y. Kwong and S. L. Buchwald, Org. Lett., 2002, 4, 3517 CrossRef CAS; (e) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2002, 4, 4309 CrossRef CAS; (f) Y.-J. Wu and H. He, Synlett, 2003, 1789 CrossRef CAS; (g) C. G. Bates, P. Saejueng, M. Q. Doherty and D. Venkataraman, Org. Lett., 2004, 6, 5005 CrossRef CAS; (h) W. Deng, Y. Zou, Y.-F. Wang, L. Liu and Q.-X. Guo, Synlett, 2004, 1254 CAS; (i) Y.-J. Chen and H.-H. Chen, Org. Lett., 2006, 8, 5609 CrossRef CAS; (j) D. Zhu, L. Xu, F. Wu and B. Wan, Tetrahedron Lett., 2006, 47, 5781 CrossRef CAS; (k) L. Rout, T. K. Sen and T. Punniyamurthy, Angew. Chem., Int. Ed., 2007, 46, 5583 CrossRef CAS; (l) X. Lu and W. Bao, J. Org. Chem., 2007, 72, 3863 CrossRef; (m) H. Zhang, W. Cao and D. Ma, Synth. Commun., 2007, 37, 25 CrossRef CAS; (n) L. Rout, P. Saha, S. Jammi and T. Punniyamurthy, Eur. J. Org. Chem., 2008, 640 CrossRef CAS; (o) H.-J. Xu, X.-Y. Zhao, J. Deng, Y. Fu and Y.-S. Feng, Tetrahedron Lett., 2009, 50, 434 CrossRef CAS; (p) M. T. Herrero, R. SanMartin and E. Domínguez, Tetrahedron, 2009, 65, 1500 CrossRef CAS; (q) S. Bhadra, B. Sreedhar and B. C. Ranu, Adv. Synth. Catal., 2009, 351, 2369 CrossRef CAS; (r) C. Gonzalez-Arellano, R. Luque and D. J. Macquarrie, Chem. Commun., 2009, 1410 RSC; (s) C.-K. Chen, Y.-W. Chen, C.-H. Lin, H.-P. Lin and C.-F. Lee, Chem. Commun., 2010, 46, 282 RSC; (t) Y.-S. Feng, Y.-Y. Li, L. Tang, W. Wu and H.-J. Xu, Tetrahedron Lett., 2010, 51, 2489 CrossRef CAS; (u) Y.-B. Huang, C.-T. Yang, J. Yi, X.-J. Deng, Y. Fu and L. Liu, J. Org. Chem., 2011, 76, 800 CrossRef CAS; (v) D. J. C. Prasad and G. Sekar, Org. Lett., 2011, 13, 1008 CrossRef CAS; (w) D. J. C. Prasad and G. Sekar, Synthesis, 2010, 79 CAS; (x) S. Kovács and Z. Novák, Org. Biomol. Chem., 2011, 9, 711 RSC.
- (a) G. Y. Li, Angew. Chem., Int. Ed., 2001, 40, 1513 CrossRef CAS; (b) U. Schopfer and A. Schlapbach, Tetrahedron, 2001, 57, 3069 CrossRef CAS; (c) M. Murata and S. L. Buchwald, Tetrahedron, 2004, 60, 7397 CrossRef CAS; (d) T. Itoh and T. Mase, Org. Lett., 2004, 6, 4587 CrossRef CAS; (e) C. Mispelaere-Canivet, J.-F. Spindler, S. Perrio and P. Beslin, Tetrahedron, 2005, 61, 5253 CrossRef CAS; (f) M. A. Fernández-Rodríguez, Q. Shen and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 2180 CrossRef; (g) J. Y. Lee and H. L. Phil, J. Org. Chem., 2008, 73, 7413 CrossRef CAS; (h) Z. Jiang, J. She and X. Lin, Adv. Synth. Catal., 2009, 351, 2558 CrossRef CAS; (i) C. C. Eichman and J. P. Stambuli, J. Org. Chem., 2009, 74, 4005 CrossRef CAS; (j) C. S. Bryan, J. A. Braunger and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 7064 CrossRef CAS; (k) C.-F. Fu, Y.-H. Liu, S.-M. Peng and S.-T. Liu, Tetrahedron, 2010, 66, 2119 CrossRef CAS; (l) M. A. Fernández-Rodríguez and J. F. Hartwig, Chem.–Eur. J., 2010, 16, 2355 CrossRef.
- (a) H. J. Cristau, B. Chabaud, A. Chêne and H. Christol, Synthesis, 1981, 892 CrossRef CAS; (b) K. Takagi, Chem. Lett., 1987, 2221 CrossRef CAS; (c) V. Percec, J.-Y. Bae and D. H. Hill, J. Org. Chem., 1995, 60, 6895 CrossRef CAS; (d) C. Millois and P. Diaz, Org. Lett., 2000, 2, 1705 CrossRef CAS; (e) S. Jammi, P. Barua, L. Rout, P. Saha and T. Punniyamurthy, Tetrahedron Lett., 2008, 49, 1484 CrossRef CAS; (f) J. Zhang, C. M. Medley, J. A. Krause and H. Guan, Organometallics, 2010, 29, 6393 CrossRef CAS.
- (a) A. Correa, M. Carril and C. Bolm, Angew. Chem., Int. Ed., 2008, 47, 2880 CrossRef CAS; (b) J.-R. Wu, C.-H. Lin and C.-F. Lee, Chem. Commun., 2009, 4450 RSC; (c) V. K. Akkilagunta, V. P. Reddy and K. R. Rao, Synlett, 2010, 1260 CAS.
- Y.-C. Wong, T. T. Jayanth and C.-H. Cheng, Org. Lett., 2006, 8, 5613 CrossRef CAS.
- (a) For reviews see: C. J. Li, Chem. Rev., 1993, 93, p. 2023 Search PubMed; (b) G. Papadogianakis and R. A. Sheldon, New. J. Chem., 1996, 20, 175 CAS; (c) B. Cornils, J. Mol. Catal. A: Chem., 1999, 143, 1 CrossRef CAS; (d) J. P. Genet and M. Savignac, J. Organomet. Chem., 1999, 576, 305 CrossRef; (e) W. A. Herrmann (Ed.), Synthesis Methods in Organometallic and Inorganic Chemistry, Enke: Stuttgart, 2000 Search PubMed; (f) B. Cornils and W. A. Herrmann (ed.), Aqueous-Phase Organometallic Catalysis, Wiley-VCH: Weinhein, Germany, 2004 Search PubMed; (g) C.-J. Li and T.-H. Chan, Organic Reactions in Aqueous Media, John Wiley & Sons, New York, 1997 Search PubMed; (h) P. A. Grieco (Ed.), Organic Synthesis in Water, Blackie Academic and Professional, London, 1998 Search PubMed; (i) N. E. Leadbeater, Chem. Commun., 2005, 2881 RSC; (j) C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS; (k) C. J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC; (l) I. T. Horváth and P. T. Anastas, Chem. Rev., 2007, 107, 2167 CrossRef; (m) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS; (n) S. Liu and J. Xiao, J. Mol. Catal. A: Chem., 2007, 270, 1 CrossRef CAS; (o) C.-J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197 CrossRef CAS; (p) I. T. Horváth, Green Chem., 2008, 10, 1024 RSC; (q) K. H. Shaughnessy, Chem. Rev., 2009, 109, 643 CrossRef CAS; (r) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS.
- M. Carril, R. SanMartin, E. Domínguez and I. Tellitu, Chem.–Eur. J., 2007, 13, 5100 CrossRef CAS.
- W.-Y. Wu, J.-C. Wang and F.-Y. Tsai, Green Chem., 2009, 11, 326 RSC.
- (a) W.-Y. Wu, S.-N. Chen and F.-Y. Tsai, Tetrahedron Lett., 2006, 47, 9267 CrossRef CAS; (b) S.-N. Chen, W.-Y. Wu and F.-Y. Tsai, Tetrahedron, 2008, 64, 8164 CrossRef CAS.
- (a) H. M. Meshram and R. Kache, Synth. Commun., 1997, 27, 2403 CrossRef CAS; (b) N. Iranpoor and B. Zeynizadeh, Synthesis, 1999, 49 CrossRef CAS.
- (a) S. L. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586 CrossRef CAS; (b) P.-F. Larsson, A. Correa, M. Carril, P.-O. Norrby and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5691 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Spectroscopic characterization data of S-arylation products, and copies of 1H and 13C NMR of compound 4v, 4w, and 4x. See DOI: 10.1039/c1ra00406a/ |
| This journal is © The Royal Society of Chemistry 2011 |
