Network dimensionalities and thermal expansion properties of metal nitroprussides†
Tomoyuki
Matsuda
a,
Jungeun
Kim
b and
Yutaka
Moritomo
*a
aGraduated School of Pure and Applied Science, University of Tsukuba, Tsukuba, Ibaraki 305-8571, Japan. Fax: +81 29 853 4337; Tel: + 81 29 853 4337E-mail: moritomo@sakura.cc.tsukuba.ac.jp
bJASRI/SPring-8, 1-1-1 Kouto, Sayo-cho, Sayo-gun, Hyogo 679-5198, Japan
First published on 1st November 2011
Abstract
We investigated the thermal expansion properties of metal nitroprussides, MA[Fe(CN)5NO]·zH2O (MA = Mn, Fe, Co, Ni, Cu, Zn, and Cd), which have various network structures, i.e. three-dimensional (3D) cubic (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m for MA = Fe, Co, and Ni), distorted three-dimensional (distorted-3D) orthorhombic (Pnma for MA = Mn, Zn, and Cd), and two-dimensional (2D) orthorhombic (Amm2 for MA = Cu) structures. In the cubic system, in which the unbridged NO groups are randomly distributed, isotropic positive thermal expansion was observed. On the contrary, in the orthorhombic system, in which the unbridged NO groups are uniaxially distributed, positive (negative) thermal expansion was observed along (perpendicular to) the uniaxial direction. We interpreted the characteristic thermal responses in terms of the rotational vibrations of the [Fe(CN)5NO] units as well as their steric hindrances due to the network dimensionality.
m for MA = Fe, Co, and Ni), distorted three-dimensional (distorted-3D) orthorhombic (Pnma for MA = Mn, Zn, and Cd), and two-dimensional (2D) orthorhombic (Amm2 for MA = Cu) structures. In the cubic system, in which the unbridged NO groups are randomly distributed, isotropic positive thermal expansion was observed. On the contrary, in the orthorhombic system, in which the unbridged NO groups are uniaxially distributed, positive (negative) thermal expansion was observed along (perpendicular to) the uniaxial direction. We interpreted the characteristic thermal responses in terms of the rotational vibrations of the [Fe(CN)5NO] units as well as their steric hindrances due to the network dimensionality.
Introduction
Thermal expansion properties in network structures are attracting a considerable interest of the materials scientists due to the possibility of zero/negative thermal expansion properties.1 Recently, coordination polymer frameworks have been found to show negative thermal expansion (NTE) behavior.2–6 The NTE behavior in such framework systems is generally attributed to the flexibility of the structure, in which the low-energy transverse and/or rotational vibrations of the molecular units are thermally populated. Such a flexibility of the lattice causes interesting physical properties, such as pressure-induced structural phase transition,7 pressure-induced amorphization,8 and a large Grüneisen parameter.9 In addition, materials with NTE behavior are useful in a variety of electronics applications and as components of high-precision thermometers.Prussian blue type cyanides, AxMA[MB(CN)6]y·zH2O (A = alkali metal ion, MA = Mn, Fe, Co, Ni, Cu, Zn and Cd, and MB = Fe, Co, Pt), show characteristic thermal expansion properties.4–6 The compounds belong to face-centered cubic (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m; Z = 4) structures, in which MA and MB are bridged by the cyano groups and form a three-dimensional (3D) network structure.10 The alkali metal cations and water molecules are accommodated in the interstitial sites of the lattice. The thermal expansion coefficient (β ≡ dlna/dT) of the lattice constant a systematically decreases with an increase in a: β = +1.46 × 10−5 K−1 for Rb0.49Co[Fe(CN)6]0.80·3.8H2O (a = 9.9431(3) Å) and β = −0.88 × 10−5 K−1 for Cs1.00Cd[Fe(CN)6]1.00·0.5H2O (a = 10.7551(1) Å). The systematic change in β is well explained by the thermally-induced rotational vibrations of the [Fe(CN)6] units as well as their steric hindrances. According to this scenario, we may significantly control the thermal expansion properties by the modification of the dimensionality of the cyano-bridged network.
m; Z = 4) structures, in which MA and MB are bridged by the cyano groups and form a three-dimensional (3D) network structure.10 The alkali metal cations and water molecules are accommodated in the interstitial sites of the lattice. The thermal expansion coefficient (β ≡ dlna/dT) of the lattice constant a systematically decreases with an increase in a: β = +1.46 × 10−5 K−1 for Rb0.49Co[Fe(CN)6]0.80·3.8H2O (a = 9.9431(3) Å) and β = −0.88 × 10−5 K−1 for Cs1.00Cd[Fe(CN)6]1.00·0.5H2O (a = 10.7551(1) Å). The systematic change in β is well explained by the thermally-induced rotational vibrations of the [Fe(CN)6] units as well as their steric hindrances. According to this scenario, we may significantly control the thermal expansion properties by the modification of the dimensionality of the cyano-bridged network.
In this work, we systematically studied the thermal expansion properties of metal nitroprussides, MA[Fe(CN)5NO]·zH2O (MA = Mn, Fe, Co, Ni, Cu, Zn, and Cd), in which one of the CN groups are replaced by the NO group. The unbridged NO group modified the dimensionality of the cyano-bridged network structure. Based on the structural analysis, we classified the network structure into three categories, i.e., 3D cubic (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m for MA = Fe, Co, and Ni), distorted three-dimensional (distorted-3D) orthorhombic (Pnma for MA = Mn, Zn, and Cd), and two-dimensional (2D) orthorhombic (Amm2 for MA = Cu) structures. We found that the variation of the network dimensionality crucially influences the thermal expansion properties. We interpreted the dimensionality-dependence of the thermal expansion in terms of the rotational vibrations of the [Fe(CN)5NO] units as well as their steric hindrances.
m for MA = Fe, Co, and Ni), distorted three-dimensional (distorted-3D) orthorhombic (Pnma for MA = Mn, Zn, and Cd), and two-dimensional (2D) orthorhombic (Amm2 for MA = Cu) structures. We found that the variation of the network dimensionality crucially influences the thermal expansion properties. We interpreted the dimensionality-dependence of the thermal expansion in terms of the rotational vibrations of the [Fe(CN)5NO] units as well as their steric hindrances.
Experimental
Powders of MA[Fe(CN)5NO]·zH2O (MA = Fe, Co, Ni, Cu, Zn, and Cd) were prepared by reacting aqueous solutions of MnCl2·4H2O, FeCl2·4H2O, CoCl2·6H2O, NiCl2·6H2O, CuCl2·2H2O, ZnCl2, CdCl2·2.5H2O, and Na2[Fe(CN)5NO]·2H2O. The precipitated powders were filtered and washed with water and then dried in air. Chemical compositions of the prepared samples were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) for metal elements and standard microanalytical methods for C, H, and N elements. Elemental analyses showed that the formulas of the obtained compounds were Mn[Fe(CN)5NO]·2.1H2O (Mn), Fe[Fe(CN)5NO]·5.0H2O (Fe), Co[Fe(CN)5NO]·5.4H2O (Co), Ni[Fe(CN)5NO]·5.8H2O (Ni), Cu[Fe(CN)5NO]·2.2H2O (Cu), Zn[Fe(CN)5NO]·2.0H2O (Zn), Cd[Fe(CN)5NO]·2.3H2O(Cd), respectively: calculated wt% for Mn: Mn, 17.80; Fe, 18.09; C, 19.45; N, 27.22; H, 1.37%: found; Mn, 17.55; Fe, 18.10; C, 19.48; N, 27.18; H, 1.31%, calculated wt% for Fe: Fe, 30.86; C, 16.60; N, 23.23; H, 2.79%: found; Fe, 30.43; C, 16.71; N, 23.17; H, 2.53%, calculated wt% for Co: Co, 15.84; Fe, 15.01; C, 16.14; N, 22.58; H, 2.92%: found; Co, 15.72; Fe, 15.02; C, 16.34; N, 22.34; H, 2.68%, calculated wt% for Ni: Ni, 15.17; Fe, 14.86; C, 15.98; N, 22.37; H, 3.02%: found; Ni, 15.24; Fe, 14.85; C, 16.02; N, 22.23; H, 2.86%, calculated wt% for Cu: Cu, 19.91; Fe, 17.50; C, 18.82; N, 26.34; H, 1.39%: found; Cu, 19.77; Fe, 17.78; C, 18.97; N, 26.32; H, 1.21%, calculated wt% for Zn: Zn, 20.60; Fe, 17.60; C, 18.92; N, 26.48; H, 1.27%: found; Zn, 20.08; Fe, 17.46; C, 19.01; N, 26.46; H, 0.90%, calculated wt% for Cd: Cd, 30.40; Fe, 15.10; C, 16.24; N, 22.73; H, 1.25%: found; Cd, 30.22; Fe, 15.27; C, 16.54; N, 22.62; H, 0.81%.In order to precisely determine a and the space groups of the above-synthesized metal nitroprussides, X-ray powder-diffraction patterns were measured at the synchrotron-radiation facility, SPring-8. First, powder samples were filled into a 0.3 mmϕ glass capillary. The capillary was put on a Debye–Scherrer camera at the BL02B2 beamline of SPring-8.11 The wavelength of the X-ray (λ ∼ 0.5 Å) was calibrated by the lattice constant of standard CeO2 powder. The sample temperature was controlled by the cooled nitrogen gas. The exposure time was 5 min. The lattice constants of each compound were refined by the RIETAN-FP program,12 and the 2θ range used in the Rietveld analyses was 3°–30°
Results and discussion
Materials
The metal nitroprussids are known to show several types of crystal structure.13–30 We performed Rietveld analysis of the synchrotron-radiation X-ray powder diffraction patterns of the obtained compounds and found that the compounds are classified into three types of crystal structure according to the metal ion (Table 1). We show in Fig. 1 the prototypical diffraction patterns at 100 K together with the results of Rietveld refinements. In the inset of Fig. 1, we depict schematic crystal structures.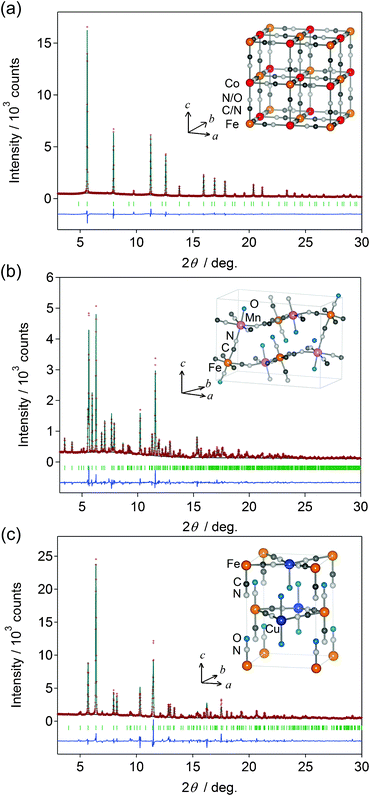 | ||
| Fig. 1 X-Ray powder diffraction patterns at 100 K and Rietveld analysis of Co (a), Mn (b), and Cu (c). Red dots, black line, and blue line are the observed plots, calculated pattern, and their difference, respectively. Green bars represent the calculated positions of the Bragg reflections. (inset) Schematic illustration of the unit cell. | ||
| Space group | Lattice constant / Å | |||
|---|---|---|---|---|
| a | b | c | ||
| Mn[Fe(CN)5NO]·2.1H2O | Pnma | 14.0371(5) | 7.5464(2) | 10.4127(3) |
| Fe[Fe(CN)5NO]·5.0H2O |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
10.3144(3) | — | — |
| Co[Fe(CN)5NO]·5.4H2O |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
10.2501(2) | — | — |
| Ni[Fe(CN)5NO]·5.8H2O |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
10.1537(2) | — | — |
| Cu[Fe(CN)5NO]·2.2H2O | Amm2 | 7.2231(4) | 6.9700(4) | 10.0850(6) |
| Zn[Fe(CN)5NO]·2.0H2O | Pnma | 13.8463(3) | 7.4099(2) | 10.3789(2) |
| Cd[Fe(CN)5NO]·2.3H2O | Pnma | 14.2012(5) | 7.6830(3) | 10.5029(4) |
The cubic compounds (Fe, Co, and Ni) show 3D network structures similar to the case of the Prussian blue analogues. Here, we note that the five cyano groups of [Fe(CN)5NO] are bridged to the neighboring MA while the NO group is unbridged.27
On the contrary, the orthorhombic compounds (Mn, Cu, Zn, and Cd) have rather complicated network structures (Fig. 2). The Mn, Zn, and Cd compounds show distorted-3D network structure. Within the ab plane (Fig. 2a, left), MA atoms are surrounded by four cyanide nitrogen atoms of [Fe(CN)5NO], forming cyano-bridged 2D layers (MA—N—C—Fe). Along the c axis (Fig. 2a, right), one side of MA is coordinated to cyanide nitrogen atom of [Fe(CN)5NO], while the other side is coordinated to the oxygen atom of the water ligand. That is, the five cyano groups of [Fe(CN)5NO] are bridged to the neighboring MA, while the NO group is unbridged. Due to the steric confinements between the free NO group of [Fe(CN)5NO], and water ligand of MA, the cyano-bridged MA-Fe layers along the ab plane are significantly distorted. The Cu compound shows 2D network structure. Within the ab plane (Fig. 2a, left), Cu atoms are surrounded by four cyanide nitrogen atoms of [Fe(CN)5NO]. Along the c axis (Fig. 2a, right), however, both sides of Cu atoms are coordinated to the oxygen atoms of water ligands. That is, the four CN groups of [Fe(CN)5NO] are bridged to the neighboring Cu in the ab plane while the remaining CN group and the NO group are unbridged along the c axis. The cyano-bridged Cu-Fe layers stack alternately along the c axis, causing 2D network structure.
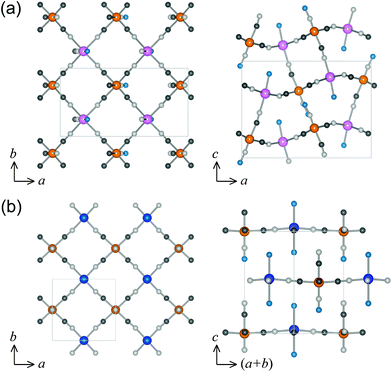 | ||
| Fig. 2 Schematic crystal structures of distorted-3D network for Mn, Zn, and Cd (a), and 2D network for Cu (b). (left) The projection in the ab plane. (right) Network structure perpendicular to the ab plane. | ||
Thermal expansion properties
We show typical examples of the magnified X-ray diffraction patterns around 422 reflection in Fe, Co, and Ni in Fig. S1 (ESI†). The 422 reflection shifts to the high-angle side with decrease in temperature, reflecting PTE. The lattice constants were refined against temperature. The reliable factor RB ranges from 2.64 to 6.28%. The temperature dependences of the normalized lattice constants are plotted in Fig. 3. We determined β by the least-squares fittings; β = 8.5 × 10−6 / K (Fe), 6.9 × 10−6 / K (Co), and 7.0 × 10−6 / K (Ni).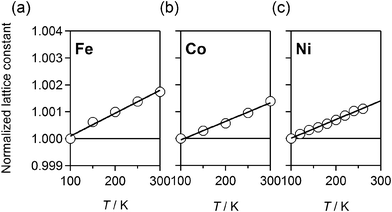 | ||
| Fig. 3 Temperature dependences of the normalized lattice constants for Fe (a), Co (b), and Ni (c). The lines are the results of the least-squares fittings. | ||
Next, we show in Fig. S2 (ESI†) typical examples of the magnified X-ray diffraction patterns around 020, 311, and 212 reflections in Mn, Zn, and Cd, which have distorted-3D networks. The 311 and 212 reflections shift to the high-angle side with a decrease in temperature, reflecting positive thermal expansion (PTE). On the contrary, the 020 reflection shifts to the low-angle side with a decrease in temperature, reflecting NTE along the b axis. The lattice constants are refined against temperature. The reliable factor RB ranges from 1.01 to 2.80%. To evaluate the thermal expansion of cyano-bridged ab plane, we use the geometrical average of the lattice constants (a' ≡ a1/2 × b1/2). We show the temperature dependences of a' and c values in Fig. 4. We determined β by the least-squares fittings. The β values are −9.8 × 10−7 / K (a'), and 5.35 × 10−5 / K (c) for Mn, −4.3 × 10−6 / K (a'), and 5.19 × 10−5 / K (c) for Zn, and −3.4 × 10−6 / K (a'), and 6.63 × 10−5 / K (c) for Cd.
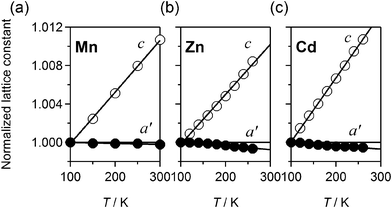 | ||
| Fig. 4 Temperature dependences of the normalized a' (≡ a1/2 × b1/2) and c for Mn (a), Zn (b), and Cd (c). The lines are the results of the least-squares fittings. | ||
We show in Fig. S3 (ESI†) a typical example of magnified X-ray diffraction patterns around 200, 020, 004, and 220 reflections in Cu, which have a 2D network structure. The 020 and 004 reflections shift to the high-angle side with decrease in temperature, reflecting PTE along the b and c axes. On the contrary, 200 and 220 reflections shift to the low-angle side with decrease in temperature, reflecting NTE along the a axis and ab plane. The lattice constants of Cu are refined against temperature, and the reliable factor RB ranges from 3.08 to 3.41%. To evaluate the thermal expansion of cyano-bridged ab plane, we use the geometrical average of the lattice constants (a' ≡ a1/2 × b1/2). We show in Fig. 5 the normalized values of a' and c against temperature. We determined β by the least-squares fitting, and the β values are −5.2 ×10−6 / K (a'), and 1.20 × 10−4 / K (c).
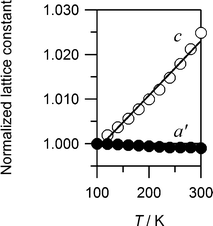 | ||
| Fig. 5 Temperature dependence of the normalized a' (≡ a1/2 × b1/2) and c for Cu. The lines are the results of the least-squares fittings. | ||
Relation between network dimensionalities and thermal expansion behavior
Here, we discuss the relation between thermal expansion behavior and dimensionality. We plot the in-plane and out-of-plane components of the thermal expansion against MA for MA[Fe(CN)5NO]·zH2O. The Fe, Co, and Ni compounds show 3D network, the Mn, Zn, and Cd compounds show a distorted-3D network, and the Cu compounds shows a 2D network.In the in-plane components (Fig. 6a), the distorted-3D and 2D systems show NTE, while the 3D system shows PTE. This dimensionality dependences of the thermal expansion is well understood by the steric hindrance effect. The origin of the NTE can be ascribed to the rotational vibrations of the [Fe(CN)5NO] units, analogous to the case of the Prussian blue analgoues.9 In the distorted-3D and 2D systems, there exist enough spaces for the [Fe(CN)5NO] units to show thermally-induced rotational vibrations (Fig. 2). Then, the rotational vibration causes the NTE as observed. In the 3D system, however, the steric hindrance suppress the rotational vibration. Then, the inherent PTE overcomes the NTE behavior.
![(a) In-plane, and (b) out-of-plane components of the thermal expansion against MA for MA[Fe(CN)5NO]·zH2O. The Fe, Co, and Ni compounds with 3D network (closed squares), the Mn, Zn, and Cd compounds with distorted- 3D network (closed diamonds), and the Cu compound with 2D network (closed circles).](/image/article/2011/RA/c1ra00547b/c1ra00547b-f6.gif) | ||
| Fig. 6 (a) In-plane, and (b) out-of-plane components of the thermal expansion against MA for MA[Fe(CN)5NO]·zH2O. The Fe, Co, and Ni compounds with 3D network (closed squares), the Mn, Zn, and Cd compounds with distorted- 3D network (closed diamonds), and the Cu compound with 2D network (closed circles). | ||
In the out-of-plane components (Fig. 6b), the magnitude of PTE increases with a decrease in the dimensionality. This is because the chemical bonds between the cyano-bridged layers become weaker as the dimensionality decreases. Actually, the number N of the cyano-bridge per one [Fe(CN)5NO] between the layers decreases from 1.67 (= 2 × (5/6)) for the 3D system, 1 for the distorted-3D system, and 0 for the 2D system. The reduction of the cyano-bridge weakens the inter-layer interaction, and enhances the PTE behavior.
Conclusions
In conclusion, we demonstrated that the anisotropic thermal expansion properties of the metal nitroprussides significantly depend on the dimensionality of the network structure. The dimensionality dependence of the thermal expansion is well explained by the rotational vibrations of [Fe(CN)5NO] units as well as their steric hindrances. Here, we emphasize that the metal nitroprussides are a unique system, in which the dimensionality of the cyano-bridged transition metal network can be controlled by chemical substitution of the MA site.Acknowledgements
This work was supported by a Grant-In-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (No. 21244052). The synchrotron-radiation X-ray powder diffraction experiments were performed at the SPring-8 BL02B2 beamline with approval of the Japan Synchrotron Radiation Research Institute (JASRI). Elementary analysis was performed at Chemical Analysis Division, Research Facility Center for Science and Engineering, University of Tsukuba.References
- T. A. Mary, J. S. O. Evans, T. Vogt and A. W. Sleight, Science, 1996, 272, 90 CAS.
- A. L. Goodwin, M. Calleja, M. J. Conterio, M. T. Dove, J. S. O. Evans, D. A. Keen, L. Peters and M. G. Tucker, Science, 2008, 319, 794 CrossRef CAS.
- Y. Wu, A. Kobayashi, G. J. Halder, V. K. Peterson, K. W. Chapman, N. Lock, P. D. Southon and C. J. Kepert, Angew. Chem., Int. Ed., 2008, 47, 8929 CrossRef CAS.
- S. Margadonna, K. Prassides and A. N. Fitch, J. Am. Chem. Soc., 2004, 126, 15390 CrossRef CAS.
- K. W. Chapman, P. J. Chupas and C. J. Kepert, J. Am. Chem. Soc., 2006, 128, 7009 CrossRef CAS.
- T. Matsuda, J.E. Kim, K. Ohoyama and Y. Moritomo, Phys. Rev. B, 2009, 79, 172302 CrossRef.
- J. S. O. Evans, Z. Hu, J. D. Jorgensen, D. N. Argyriou, S. Short and A. W. Sleight, Science, 1997, 275, 61 CrossRef CAS.
- C. A. Perottoni and J. A. H. da Jornada, Science, 1998, 280, 886 CrossRef CAS.
- R. Mittal, S. L. Chaplot, H. Schober and T. A. Mary, Phys. Rev. Lett., 2001, 86, 4692 CrossRef CAS.
- A. Ludi and H. U. Güdel, Struct. Bonding, 1973, 14, 1 CrossRef CAS.
- E. Nishibori, M. Takata, K. Kato, M. Sakata, Y. Kubota, S. Aoyagi, Y. Kuroiwa, M. Yamakata and N. Ikeda, Nucl. Instrum. Methods Phys. Res., Sect. A, 2001, 467–468, 1045 CrossRef CAS.
- F. Izumi and K. Momma, Solid State Phenom., 2007, 130, 15 CrossRef CAS.
- L. Reguera, J. Balmaseda, C. P. Krap and E. Reguera, J. Phys. Chem. C, 2008, 112, 10490 CAS.
- J. B. Ayers and W. H. Waggoner, J. Inorg. Nucl. Chem., 1969, 31, 2045 CrossRef CAS.
- D. B. Brown, Inorg. Chem., 1975, 14, 2582 CrossRef CAS.
- L. A. Gentil, E. J. Baran and P. J. Aymonino, Inorg. Chim. Acta, 1976, 20, 251 CrossRef CAS.
- J. C. Long, J. L. Thomas and J. C. Lombardi, J. Inorg. Nucl. Chem., 1978, 40, 1627 CrossRef CAS.
- D. F. Mullica, E. L. Sappenfield, D. B. Tippin and D. H. Leschnitzer, Inorg. Chim. Acta, 1989, 164, 99 CrossRef CAS.
- D. F. Mullica, D. B. Tippin and E. L. Sappenfield, Inorg. Chim. Acta, 1990, 174, 129 CrossRef CAS.
- D. F. Mullica, D. B. Tippin and E. L. Sappenfield, J. Coord. Chem., 1992, 25, 175 CrossRef CAS.
- E. Reguera, J. F. Bertrán, J. Miranda and A. Dago, Hyperfine Interact., 1993, 77, 1 CrossRef CAS.
- E. Reguera, A. Dago, A. Gómez and J. F. Bertrán, Polyhedron, 1996, 15, 3139 CrossRef CAS.
- Z. Z. Gu, O. Sato, T. Iyoda, K. Hashimoto and A. Fujishima, Chem. Mater., 1997, 9, 1092 CrossRef CAS.
- A. Benavente, J. A de Morán, O. E. Piro, E. E. Castellano and P. J. Aymonino, J. Chem. Crystallogr., 1997, 27, 343 CrossRef CAS.
- N. Machida, S. Ohkoshi, Z. J. Zhong and K. Hashimoto, Chem. Lett., 1999, 907 CrossRef CAS.
- A. Gómez, E. Reguera and L. M. D. Cranswick, Polyhedron, 2001, 20, 165 CrossRef.
- J. Balmaseda, E. Reguera, A. Gomez, J. Roque, C. Vazquez and M. Autie, J. Phys. Chem. B, 2003, 107, 11360 CrossRef CAS.
- M. Zentková, M. Mihalik, I. Tóth, Z. Mitróová, A. Zentko, M. Sendek, J. Kováč, M. Lukáčová, M. Maryško and M. Miglierini, J. Magn. Magn. Mater., 2004, 272–276, E753 CrossRef.
- J. T. Culp, C. Matranga, M. Smith, E. W. Bittner and B. Bockrath, J. Phys. Chem. B, 2006, 110, 8325 CrossRef CAS.
- E. Torres-Garcia, J. Balmaseda, L. F. del Castillo and E. Reguera, J. Therm. Anal. Calorim., 2006, 86, 371 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Temperature dependences of magnified X-ray powder diffraction patterns. See DOI: 10.1039/c1ra00547b/ |
| This journal is © The Royal Society of Chemistry 2011 |
