Ferrous methanesulfonate as an efficient and recyclable catalyst for the tetrahydropyranylation of alcohols and phenols under solvent-free conditions
Min
Wang
*a,
Zhiguo
Song
b,
Xin
Wan
a and
Shuang
Zhao
a
aCollege of Chemistry, Chemical Engineering and Food Safety, Bohai University, Jinzhou, 121000, China. E-mail: minwangszg@yahoo.com.cn
bCenter for Science & Technology Experiment, Bohai University, Jinzhou, 121000, China
First published on 3rd November 2011
Abstract
Ferrous methanesulfonate catalyzed tetrahydropyranylation of alcohols (benzylic, primary, isomerical, tertiary, cyclic, allyl, and furyl) and phenols at room temperature under solvent-free conditions has been developed. The catalytic activity of sixteen metal methanesulfonates was compared under the same conditions, ferrous methanesulfonate proved to be the best. It can be recovered easily and reused for several times without distinct deterioration in catalytic activity. During the competitive protection of the hydroxyl groups between an alcohol and a phenol, tetrahydropyranyl ether formed exclusively with the alcohol. A possible catalytic mechanism was proposed.
Introduction
Protection of hydroxyl groups plays an essential role in multistep organic synthesis including natural products and biologically active compounds. Among the protecting groups, tetrahydropyranyl (THP) is the most useful due to its low cost and stability towards strongly basic media, Grignard reagents, acylating agents, alkyllithiums, and metal hydrides.1 Tetrahydropyranylation has been performed with a variety of reagents and catalysts including LiOTf,2 polyaniline salt,3 PdCl2(CH3CN)2,4 Fe(ClO4)3,5 Bu4NBr3,6 VO(OAc)2,7 CAN,8 Fe(HSO4)3,9 H14[NaP5W30O110],10 Bi(NO3)3·5H2O,11 NbCl5,12 Pyridinium chloride,13 Moβ,14 activated carbon supported H2SO4,15 2,4,6-trichloro[1,3,5]triazine,16 and Dowex 50WX4-10017etc. Most of these proved to be efficient for this reaction. However, some of them have several drawbacks, such as elevated temperature, long reaction time, harmful organic solvent, expensive catalysts, and high catalyst to substrate ratio. Thus, there is still demand for the introduction of mild and efficient methods for this transformation.Recently, metal methanesulfonates (M(CH3SO3)x·aH2O) have attracted attention because of their distinct advantages such as low toxicity, low cost and relative insensitivity to air and moisture. In the course of our continuous investigations, we observed that they could act as mild and water-tolerant Lewis acid catalysts for the esterification,18 the Biginelli,19 and the Mannich reactions.20 We also found ferrous methanesulfonate (Fe(CH3SO3)2·4H2O, abbreviated as Fe(MS)2) could be used as a heterogeneous and recyclable catalyst for the protection of carbonyl groups.21 In further extension to our work, we wish to disclose a new and efficient protocol for the protection of hydroxyl groups in the presence of catalytic amounts of M(CH3SO3)x·aH2O under mild conditions (Scheme 1).
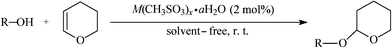 | ||
| Scheme 1 Tetrahydropyranylation of alcohol and phenol catalyzed by metal methanesulfonates. | ||
Experimental
Instruments and chemicals
Infrared spectra were recorded on Spectrum GX series Fourier Transform instrument of PerkinElmer. 1H NMR spectra were recorded on a Bruker ARX-300 spectrometer in CDCl3 using TMS as an internal standard. GC analysis was carried out on a PerkinElmer Auto System XL Gas Chromatograph. Elemental analyses were carried out on EA 2400II elemental analyzer (PerkinElmer).M(CH3SO3)x·aH2O were synthesized according to the literature.22 They were characterized by IR and TG. All the other chemicals (AR grade) were commercially available and used without further purification.
Typical procedure for the tetrahydropyranylation
To a mixture of alcohol/phenol (15 mmol) and 3,4-dihydro-2H-pyran (DHP, 18 mmol) was added Fe(MS)2 (0.3 mmol) at room temperature. The mixture was stirred until completion of the reaction (monitored by GC). After reaction, the organic layer was washed twice with saturated NaHCO3 solution (10 mL), dried (Na2SO4), and evaporated to yield the almost pure product. The products were purified further by column chromatography on silica gel (ethyl acetate/n-hexane = 1/9 as the eluent). Then the pure products were identified by bp, IR, 1H NMR, and elemental analysis, which agreed favorably with those of authentic samples.23–33Results and discussion
To explore the feasibility of M(CH3SO3)x·aH2O-catalyzed tetrahydropyranylation, the reaction of benzyl alcohol with DHP was selected as a model. The reactions were carried out at room temperature under solvent-free conditions for 1.0 h. Sixteen M(CH3SO3)x·aH2O (2 mol%, based on alcohol) include rare earth metal (La, Ce(III), Pr, Nd, Yb), alkaline earth metal (Mg, Ca, Sr, Ba), transition metal (Mn, Fe(II), Co, Ni, Cu, Zn) and alkali metal (Li) methanesulfonates were screened for their ability to catalyze the protection of hydroxyl group. Fe(MS)2 proved to be the best since it resulted in the highest product yield (97%). Pr(MS)3 and Co(MS)2 provide much lower yields in 5% and 3%, respectively. No other metal salts are found to be effective. The catalytic behavior of Fe(MS)2 is significantly superior to other metal methanesulfonates catalysts. This would be ascribed to the higher Lewis acidity of Fe(MS)2. During the reaction, DHP is activated by Fe(MS)2 to facilitate the nucleophilic attack of alcohol.Next, we focused on the optimal amount of Fe(MS)2. The results showed that the lower amount (1 mol%) gave a low yield (91%), and the bigger amount (3 mol%) could not cause an obvious increase on the yield. Hence, 2 mol% of Fe(MS)2 was the appropriate amount for the reaction.
Then, the reusability of Fe(MS)2 was investigated. Fe(MS)2 is a pale green crystal catalyst, which is slightly soluble in the reactants and products of this reaction. After reaction, Fe(MS)2 could be recovered by filtration and washed with acetone, then reused for its next run. It could be recycled five times without distinct loss of activity, which provides product yields in 97%, 93%, 89%, 84%, and 80%, respectively.
Further, we carried out the reactions between DHP and various alcohols or phenols to explore the reaction scope of Fe(MS)2-catalyzed tetrahydropyranylation, the results were summarized in Table 1. As can be seen, all reactions proceeded very cleanly (checked by GC) and no polymeric by-product of DHP was observed. A broad selection of alcohols, including benzylic, primary, isomerical, tertiary, cyclic, allyl, and furyl alcohols, were converted to their corresponding THP ethers successfully. For linear chain aliphatic alcohols, the protection of short-chain alcohols proceeds faster than in case of the long-chain ones. tert-Butyl alcohol, which is an acid-sensitive alcohol, also undergoes tetrahydropyranylation smoothly and no dehydration was observed (Table 1, entry 7). Protection of phenolic hydroxyl group was also achieved, and phenol whose benzene ring substituted with a strong electron-donating group reacted quickly with excellent yield (Table 1, entries 15 and 16). However, p-chlorophenol undergoes tetrahydropyranylation with low yield, and the yield did not increase even if the reaction time was prolonged (Table 1, entry 17). In addition, chemoselective protection of alcohol in the presence of phenol was achieved (Table 1, entry 18). Benzyl alcohol converted to corresponding THP ether while phenol remained unreacted, which demonstrated that this method showed excellent selectivity in the alcohol. Note, under all conditions without the addition of Fe(MS)2, no THP ether was formed.
| Entry | Alcohol/phenol | Time (h) | Yield (%) | Bp (°C) | References for bp and spectroscopic data of products |
|---|---|---|---|---|---|
| a Melting point | |||||
| 1 | PhCH2OH | 1.0 | 97 | 104–105/4 mm | 23–26 |
| 2 | CH3OH | 6.0 | 88 | 125 | 27 |
| 3 | C2H5OH | 6.0 | 83 | 146 | 27 |
| 4 | n-C3H7OH | 6.5 | 83 | 165 | 27 |
| 5 | i-C3H7OH | 3.5 | 81 | 159–160 | 23, 28 |
| 6 | n-C4H9OH | 7.0 | 82 | 183 | 17, 27, 29 |
| 7 | t-C4H9OH | 9.0 | 62 | 169–170 | 28, 30 |
| 8 | i-C4H9OH | 5.0 | 74 | 80/30 mm | 23 |
| 9 | n-C8H17OH | 8.0 | 66 | 100/4 mm | 23, 26, 31 |
| 10 | n-C12H25OH | 9.0 | 64 | 142–144/2 mm | 23 |
| 11 | c-C6H11OH | 9.0 | 70 | 79–80/2 mm | 23–25, 32 |
| 12 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2OH CHCH2OH |
5.0 | 85 | 126 | 23–25, 27 |
| 13 | Furfuryl alcohol | 6.0 | 90 | 124/24 mm | 23, 27 |
| 14 | PhOH | 1.3 | 91 | 90–92/3.5 mm | 23–25, 27 |
| 15 | 4-CH3C6H4OH | 1.3 | 97 | 99/3 mm | 26, 33 |
| 16 | 4-CH3OC6H4OH | 1.5 | 96 | 120/1.5 mm | 23, 26, 33 |
| 17 | 4-ClC6H4OH | 1.0 | 53 | 48–49a | 25, 33 |
| 18 | PhCH2OH+PhOH | 1.5 | 92+0 | — |
In order to show the efficiency and applicability of the present method, the catalytic activity of Fe(MS)2 was compared with that of some reported catalysts in the literatures, the results are summarized in Table 2. It can be seen Fe(MS)2 was superior to the others in terms of catalyst amount, reaction temperature, reaction time and products yields.
| Entry | Catalyst (mol%) | Temperature | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | LiOTf (60) | Reflux | 2.5 | 96 | 2 |
| 2 | Polyaniline sulfate salt (14) | 50 °C | 8.0 | 59 | 3 |
| 3 | PdCl2(CH3CN)2 (10) | R.T. | 1.0 | 72 | 4 |
| 4 | Fe(ClO4)3 (3) | R.T. | 1.5 | 98 | 5 |
| 5 | n-Bu4N+Br3−(TBATB) (2.5) | R.T. | 1.0 | 85 | 6 |
| 6 | VO(OAc)2 (27) | R.T. | 1.0 | 95 | 7 |
| 7 | H14[NaP5W30O110] (0.1) | Reflux | 2.0 | 90 | 10 |
| 8 | NbCl5 (10) | R.T. | 2.5 | 90 | 12 |
| 9 | Ru(acac)3 (2) | R.T. | 16.0 | 80 | 24 |
| 10 | Ionic liquid/PPh3.HBr (10) | R.T. | 4.0 | 96 | 34 |
| 11 | ZrCl4 (5) | Reflux | 3.0 | 92 | 35 |
| 12 | La(NO3)3·6H2O (10) | R.T. | 2.5 | 93 | 36 |
| 13 | Fe(MS)2 (2) | R.T. | 1.0 | 97 | — |
Reddy et al. proposed a tentative mechanism for the formation of THP ethers.14 Although the definite mechanism of the reaction is not clear, the Lewis acid property of the catalyst directed us to accept the presented mechanism in Scheme 2, as a plausible one. First, DHP is activated by Fe(MS)2 to afford 1. Nucleophilic attack of alcohol to 1 gives 2, which upon proton transfer produces final product 3 and simultaneously releases the catalyst for the next run.
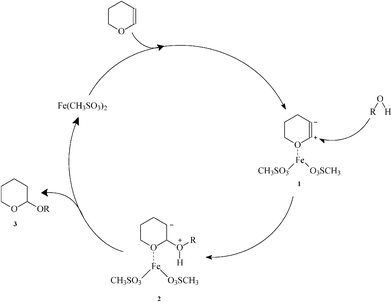 | ||
| Scheme 2 Proposed catalytic mechanism. | ||
Conclusions
In conclusion, a new efficient and chemoselective method has been developed for the tetrahydropyranylation of a wide range of alcohols and phenols in the presence of Fe(MS)2. The highly catalytic nature, observed chemoselectivity, reusability of catalyst, and the fact that Fe(MS)2 is non-toxic, easy to handle and insensitive to air and moisture make this procedure especially attractive.Acknowledgements
This research work was financially supported by the Liaoning commission of Science and Technology (No. 20091001) and Liaoning commission of Education (No. 2009A033).References
- T. W. Greene and P. G. M. Wuts, Protective groups in organic synthesis, 2nd ed., Wiley, New York, 1991, 346 Search PubMed.
- B. Karimi and J. Maleki, Tetrahedron Lett., 2002, 43, 5353 CrossRef CAS.
- S. Palaniappan, M. S. Ram and C. A. Amarnath, Green Chem., 2002, 4, 369 RSC.
- Y. G. Wang, X. X. Wu and Z. Y. Jiang, Tetrahedron Lett., 2004, 45, 2973 CrossRef CAS.
- M. M. Heravi, F. K. Behbahani, H. A. Oskooie and R. H. Shoar, Tetrahedron Lett., 2005, 46, 2543 CrossRef CAS.
- S. Naik, R. Gopinath and B. K. Patel, Tetrahedron Lett., 2001, 42, 7679 CrossRef CAS.
- B. M. Choudary, V. Neeraja and M. L. Kantam, J. Mol. Catal. A: Chem., 2001, 175, 169 CrossRef CAS.
- K. Pachamuthu and Y. D. Vankar, J. Org. Chem., 2001, 66, 7511 CrossRef CAS.
- F. Shirini, M. A. Zolfigol and A. R. Abri, Chin. Chem. Lett., 2007, 18, 803 CrossRef CAS.
- M. M. Heravi, M. Haghighi, F. Derikvand and F. F. Bamoharram, Synth. Commun., 2006, 36, 3103 CrossRef CAS.
- A. T. Khan, S. Ghosh and L. H. Choudhury, Eur. J. Org. Chem., 2005, 4891 CrossRef CAS.
- K. Nagaiah, B. V. S. Reddy, D. Sreenu and A. V. Narsaiah, Arkivoc, 2005, 192 CAS.
- A. R. Hajipour, M. Kargosha and A. E. Ruoho, Synth. Commun., 2009, 39, 1084 CrossRef CAS.
- N. Narender, K. S. K. Reddy, M. A. Kumar, C. N. Rohitha and S. J. Kulkarni, Catal. Lett., 2010, 134, 175 CrossRef CAS.
- J. H. Yang, X. Zhang and W. Y. Liu, Chin. Chem. Lett., 2008, 19, 893 CrossRef CAS.
- B. Akhlaghinia and E. Roohi, Turk. J. Chem., 2007, 31, 83 CAS.
- P. S. Poon, A. K. Banerjee, L. Bedoya, M. S. Laya, E. V. Cabrera and K. M. Albornoz, Synth. Commun., 2009, 39, 3369 CrossRef CAS.
- M. Wang, Z. C. Wang, Z. L. Sun and H. Jiang, React. Kinet. Catal. Lett., 2005, 84, 223 CrossRef CAS.
- M. Wang, Z. G. Song, H. Gong and H. Jiang, Prep. Biochem. Biotechnol., 2008, 38, 105 CrossRef CAS.
- M. Wang, Z. G. Song and H. Jiang, Org. Prep. Proced. Int., 2009, 41, 315 CrossRef CAS.
- M. Wang, G. F. Tian, Z. G. Song and H. Jiang, Chin. Chem. Lett., 2009, 20, 1034 CrossRef CAS.
- M. Wang, Z. G. Song, H. Jiang and H. Gong, J. Therm. Anal. Calorim., 2009, 98, 801 CrossRef CAS.
- S. Hoyer and P. Laszlo, Synthesis, 1986, 655 CrossRef CAS.
- R. Varala and S. R. Adapa, Can. J. Chem., 2006, 84, 1174 CrossRef CAS.
- P. P. Sun and Z. X. Hu, Chin. J. Chem., 2004, 22, 1341 CrossRef CAS.
- A. Bongini, G. Cardillo, M. Orena and S. Sandri, Synthesis, 1979, 618 CrossRef CAS.
- G. F. Woods and D. N. Kramer, J. Am. Chem. Soc., 1947, 69, 2246 CrossRef CAS.
- G. O. Pierson and O. A. Runquist, J. Org. Chem., 1968, 33, 2572 CrossRef CAS.
- P. S. Poon, A. K. Banerjee, L. Bedoya, M. S. Laya, E. V. Cabrera and K. M. Albornoz, Synth. Commun., 2009, 39, 3369 CrossRef CAS.
- J. R. Stephens, P. L. Butler, C. H. Clow, M. C. Oswald, R. C. Smith and R. S. Mohan, Eur. J. Org. Chem., 2003, 3827 CrossRef CAS.
- A. Semwal and S. K. Nayak, Synthesis, 2005, 71 CAS.
- S. Chandrasekhar, M. Takhi, Y. R. Reddy, S. Mohapatra, C. R. Rao and K. V. Reddy, Tetrahedron, 1997, 53, 14997 CrossRef CAS.
- T. H. Fife and L. K. Jao, J. Am. Chem. Soc., 1968, 90, 4081 CrossRef CAS.
- L. C. Branco and C. A. M. Afonso, Tetrahedron, 2001, 57, 4405 CrossRef CAS.
- N. Rezai, F. A. Meybodi and P. Salehi, Synth. Commun., 2000, 30, 1799 CrossRef CAS.
- T. S. Reddy, K. Ravinder, N. Suryakiran, M. Narasimhulu, K. C. Mahesh and Y. Venkateswarlu, Tetrahedron Lett., 2006, 47, 2341 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
