DOI:
10.1039/C1RA00624J
(Paper)
RSC Adv., 2011,
1, 1574-1578
Room-temperature synthesis of Prussian blue analogue Co3[Co(CN)6]2 porous nanostructures and their CO2 storage properties
Received
22nd August 2011
, Accepted 23rd August 2011
First published on 25th October 2011
Abstract
A nanosized Prussian blue analogue Co3[Co(CN)6]2 (PBA) with novel morphology was achieved at room temperature by simple mixing K3[Co(CN)6]2 and Co(CH3COO)2·4H2O in the presence of sodium dodecylbenzenesulfonate (SDBS). The as-prepared product was characterized by X-ray powder diffraction (XRD), field emission scanning electron microscopy (FE-SEM), and transmission electron microscopy (TEM). The effects of synthetic parameters such as different surfactants on the morphology and size of the particles were investigated. The experimental results showed that the amount of SDBS employed in the reaction system played an important role in determining the size and shape of the products. More interestingly, the as-prepared product showed high CO2 adsorption at room temperature and 1 bar of pressure, which is higher than that of the bulk materials. These results prove that the gas adsorption properties of Co3[Co(CN)6]2 are highly dependent on not only its surface area but also its morphology, and the materials at the nanoscale are more favorable to adsorption applications.
Introduction
Recently, growing attention has been paid to Prussian blue analogues (PBA) for their applications in hydrogen storage,1–4 molecular magnetics,5 optics,6 and so on.7 Although great efforts have been paid to PBA, and many unique properties have been found, in these studies the main focus is the properties of bulk PBA. However, it was found that the shape and size are important factors to fine-tune the properties of materials.8–9 In this regard, PBA nanomaterials often exhibit excellent size- and shape-dependent chemical and physical properties, which cannot be observed in their bulk analogues. For example, Co3[Co(CN)6]2 irregular nanoparticles have been shown to exhibit improved CO2 storage properties over bulk Co3[Co(CN)6]2.10 Therefore, it is a strong desire to prepare the PBA with different sizes and shapes, at the nanoscale, to investigate their properties.
The Prussian blue analogues of chemical formula M3II[MIII(CN)6]2·nH2O (MII![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) MIII transition metals) are constructed from octahedral MIII(CN)63− complexes, which are bridged into a simple cubic lattice by M2+ ions. Co3[Co(CN)6]2·nH2O, is a typical Prussian blue analogue with the chemical formula M3II[MIII(CN)6]2·nH2O, and its hydrogen storage properties have been reported by Kaye et al.11 Moreover, there are several kinds of morphologies of Co3[Co(CN)6]2 with different sizes that have been reported at the nanoscale, such as Co3[Co(CN)6]2 polyhedra and nanorods by solvothermal synthesis in microemulsion system12 and nanoparticles in reverse microemulsions.13 However, the hydrothermal methods and reverse microemulsion are relatively complex processes, which is not consistent with the low cost idea. On the other hand, Zn3[Co(CN)6]2 (microspheres and micropolyhedrons),14Mn3[Co(CN)6]2 (nanocubes)15 and nanosized zeolitic imidazolate frameworks (ZIF-8)16 have been obtained by simple mixing the reactants at room temperature, but little progress has been made toward understanding and controlling the growth process of Co3[Co(CN)6]2 with a complicated morphology at room temperature in a simple way. In this paper, we report on the synthesis of Co3[Co(CN)6]2 truncated cubes with porous structure at room temperature in the presence of surfactants, which maybe follow the non-classical crystallization. By carefully controlling experimental conditions, Co3[Co(CN)6]2 nanostructures with different sizes can be efficiently obtained. As the mesocrystals can be synthesized by the aggregation of preformed small particles, a porous crystal may form, and a crystal with complex morphologies could be easily obtained. Recently, Jiang and co-workers reported the synthesis of Prussian blue mesocrystals with complicated morphologies by a hydrothermal method in the presence of hydrochloric acid and a polymer, which could be explained by the non-classical crystallization.17–18 However, developing a more facile means to fabricate PBA mesocrystals with complex nanostructures remains a challenge. Therefore, We believe that researching the nonclassical crystallization of PBA Co3[Co(CN)6]2 could complement the current research area. More interestingly, the as-prepared Co3[Co(CN)6]2 exhibit enhanced CO2 storage properties compared to that of the bulk.
MIII transition metals) are constructed from octahedral MIII(CN)63− complexes, which are bridged into a simple cubic lattice by M2+ ions. Co3[Co(CN)6]2·nH2O, is a typical Prussian blue analogue with the chemical formula M3II[MIII(CN)6]2·nH2O, and its hydrogen storage properties have been reported by Kaye et al.11 Moreover, there are several kinds of morphologies of Co3[Co(CN)6]2 with different sizes that have been reported at the nanoscale, such as Co3[Co(CN)6]2 polyhedra and nanorods by solvothermal synthesis in microemulsion system12 and nanoparticles in reverse microemulsions.13 However, the hydrothermal methods and reverse microemulsion are relatively complex processes, which is not consistent with the low cost idea. On the other hand, Zn3[Co(CN)6]2 (microspheres and micropolyhedrons),14Mn3[Co(CN)6]2 (nanocubes)15 and nanosized zeolitic imidazolate frameworks (ZIF-8)16 have been obtained by simple mixing the reactants at room temperature, but little progress has been made toward understanding and controlling the growth process of Co3[Co(CN)6]2 with a complicated morphology at room temperature in a simple way. In this paper, we report on the synthesis of Co3[Co(CN)6]2 truncated cubes with porous structure at room temperature in the presence of surfactants, which maybe follow the non-classical crystallization. By carefully controlling experimental conditions, Co3[Co(CN)6]2 nanostructures with different sizes can be efficiently obtained. As the mesocrystals can be synthesized by the aggregation of preformed small particles, a porous crystal may form, and a crystal with complex morphologies could be easily obtained. Recently, Jiang and co-workers reported the synthesis of Prussian blue mesocrystals with complicated morphologies by a hydrothermal method in the presence of hydrochloric acid and a polymer, which could be explained by the non-classical crystallization.17–18 However, developing a more facile means to fabricate PBA mesocrystals with complex nanostructures remains a challenge. Therefore, We believe that researching the nonclassical crystallization of PBA Co3[Co(CN)6]2 could complement the current research area. More interestingly, the as-prepared Co3[Co(CN)6]2 exhibit enhanced CO2 storage properties compared to that of the bulk.
Experimental section
The typical synthetic experiments were as follows, Solution A: 0.04 mmol K3[Co(CN)6]2 and 0.3 g SDBS were dissolved in 10 mL distilled water system under agitated stirring to get an absolute transparent solution. Solution B: 0.075 mmol of Co(CH3COO)2·nH2O was dissolved in 10 mL distilled water. Solution B was added into solution A slowly and regularly using a syringe to form a red colloid solution. The whole reaction process was kept at room temperature with agitated stirring. After 10 min, the reaction was aged at room temperature without any interruption for 24 h. The resulting pink precipitation was filtered and washed several times with absolute ethanol and finally dried in an oven at 60 °C.
Characterization
All chemicals used in the synthesis of Co3[Co(CN)6]2·nH2O were obtained from commercial sources and are used without further purification. The powder X-ray diffraction (XRD) patterns were collected on a Japan Rigaku D/MAX-cAX-ray diffractometer equipped with Cu Ka radiation over the 2θ range of 10–70. Transmission electron microscopy (TEM) images were obtained on a Hitachi H-800 transmission electron microscope, using an accelerating voltage of 200 kV. Field emission scanning electron microscopy (FE-SEM) images were performed on a JEOL JSM-6700 M scanning electron microscope. Thermogravimetric analysis (TGA) was carried out using a Shimadzu-50 thermoanalyser under nitrogen gas flow at 10 °C min−1 in the temperature range 30–800 °C. Specific surface areas were computed from the results of N2 physisorption at 77 K (Micromeritics ASAP 2020) by using the BET (Brunauer–Emmet–Teller) and BJH (Barrett–Joyner–Halenda).
Results and discussion
Fig. 1 shows the X-ray diffraction pattern of the as-prepared product. All the reflections can be readily indexed as a pure face-centered cubic (fcc) phase of Co3[Co(CN)6]2·nH2O [space group: Fm3m (no. 225)] with lattice constant α = 10.2 Å, which is in good agreement with the standard values for bulk cubic Co3[Co(CN)6]2·nH2O (JCPDS No.77-1161). The sharp peaks indicate that the as-prepared product has good crystallinity. The FT-IR spectrum (Fig. 2) of the as-prepared sample exhibits a dominant peak at 2170 cm−1 attributed to the CN− stretching. The presence of crystal water can be deduced from the occurrence of a sharp ν(O–H) band due to water incorporated in a crystalline lattice (3645 cm−1) and from the occurrence of the δ(OH2) vibration (1610 cm−1).
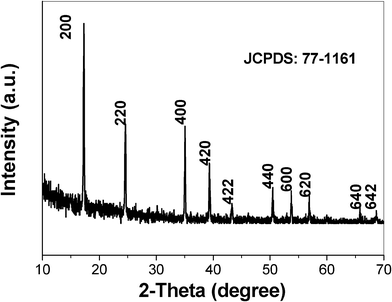 |
| | Fig. 1 The XRD pattern of the product obtained in the presence of 0.3 g SDBS. | |
 |
| | Fig. 2 The FT-IR spectrum of the product was obtained in the presence of 0.3 g SDBS. | |
Fig. 3 illustrates the SEM and TEM images of the Co3[Co(CN)6]2·nH2O. As to what can be observed from Fig. 3a, the nanoparticles turn out to be large with relative uniformity in size and shape. The inset in Fig. 3a indicates that the nanoparticles are non-agglomerated. As shown in Fig. 3b, when the product is air-dried on the TEM grid after centrifuging, the uniform size and shape of the nanoparticles resulted in excellent dispersity. Two different morphologies of truncated nanocubes and spheroids could be observed as shown by the arrows and inset in Fig. 3b. Based on a statistical evaluation of at least 20 nanoparticles, a mean particle diameter was deduced to be around 170 nm. A series of comparison experiments have been carried out to investigate the influence of the conditions on the morphology and size of the products. Large numbers of them indicated that the morphology and size of the product had a strong dependence on the reaction conditions. When the amount of SDBS increases to 0.6 g, the morphology and size of the products changed as shown in Fig. 4. Low magnification SEM images clearly demonstrate the formation of uniform nanoparticles with an average size of 500 nm (Fig. 4a and 4b). The crystal seems to be pseudo truncated cubes. The relatively flat square faces and the curved faces represent the six square faces and eight truncated faces of a truncated cube, respectively. The inset in Fig. 4b shows the typical TEM image of an individual particle, which clear indicates the truncated nanocube shape. The perfect nanocubes, which attach to the surfaces of the big particles, are also formed as shown by the red arrows in Fig. 4a and 4b. Moreover, the white boxes in Fig. 4b reveal that the truncated faces of the nanoparticles are stratiform with the porous structure, which could be further confirmed by the high magnification SEM images of a typical individual (Fig. 4c and 4d). This result indicates that the curved part of the crystals may be assembled by the smaller nanoparticles. The pH value of the solution was found to be 9, using pH meter, which may be attributed to the hydrolysis of the CH3COOH− ions. It is reported that the Prussian blue microcrystals have been prepared by selective etching in a weak acidity system.19 Therefore, we consider that the weak alkali conditions also contributes to the rough curved faces by selective etching. Selective area electron diffraction (SAED) shows ordered spots like a single crystal (the inset in Fig. 4d), which is another important feature of the mesocrystal.20 In fact, the morphologies shown in Fig. 4c and 4d are the same, which is quite similar to the morphology of mesocrystals of inorganic superstructures,21 and the distinct morphologies can be identified because of different directions of observation. Therefore, we deduce that the two kinds of shapes observed in Fig. 3b are also identical. When the amount of SDBS increases to 1 g, an irregular microcrystal forms (Fig. 5a). Such an irregular crystal is formed by attachment of small cubes. Highly magnified SEM images show that the cubes attach tightly, even embed into the surface. Fig. 6 showed the XRD patterns of the as-prepared samples formed under 0.6 g and 1 g SDBS, which are also in good agreement with cubic Co3[Co(CN)6]2·nH2O. Changing the SDBS to PVP did not result in the formation of the complex morphology, and non-uniform nanoparticles with a wide size distribution were obtained in the presence of 0.6 g PVP with other experimental parameters kept constant (Fig. 5b). Therefore, the amount of SDBS has played an important role in controlling the morphology of the nanoparticles during the reaction. On the other hand, the traditional synthesis methodology of Prussian blue and its analogues are based on a direct precipitation reaction of the Mm+ cations and the [M'(CN)6]n− anions.22 It is well-known that Prussian blue analogue Co3[Co(CN)6]2·nH2O has a very small solubility-product constant, indicating that upon direct mixing, Co2+ and [Co(CN)6]3− immediately react to form Co3[Co(CN)6]2·nH2O. For this kind of rapid reaction, control over the morphology and size of the products is difficult.23 It can be reasonably inferred that to control the morphology and size of the materials, especially for those with small Ksp values, a slow reaction process is very useful. In our experiment, dropwise addition with a syringe, low concentrations of reactants and the SDBS were employed to provide suitable conditions for a slow reaction process, and therefore form products with relative uniform size and shape. Most importantly, compared with the previous synthesis methods of Co3[Co(CN)6]2·nH2O nanostructure,12,13 the preparation of Co3[Co(CN)6]2·nH2O truncated nanocubes in our case is the most simple as it does not require high temperatures and complex processes.
![SEM and TEM images of the as-prepared Co3[Co(CN)6]2·nH2O nanoparticles obtained in the presence of 0.3 g SDBS.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f3.gif) |
| | Fig. 3
SEM and TEM images of the as-prepared Co3[Co(CN)6]2·nH2O nanoparticles obtained in the presence of 0.3 g SDBS. | |
![SEM images of the as-prepared Co3[Co(CN)6]2·nH2O nanoparticles obtained in the presence of 0.6 g SDBS at different magnifications. The inset is the typical TEM image of a particle.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f4.gif) |
| | Fig. 4
SEM images of the as-prepared Co3[Co(CN)6]2·nH2O nanoparticles obtained in the presence of 0.6 g SDBS at different magnifications. The inset is the typical TEM image of a particle. | |
![(a) The SEM image of Co3[Co(CN)6]2·nH2O obtained with 1 g SDBS; (b) The SEM image of Co3[Co(CN)6]2·nH2O obtained with 0.6 g PVP with the other experimental parameters kept constant.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f5.gif) |
| | Fig. 5 (a) The SEM image of Co3[Co(CN)6]2·nH2O obtained with 1 g SDBS; (b) The SEM image of Co3[Co(CN)6]2·nH2O obtained with 0.6 g PVP with the other experimental parameters kept constant. | |
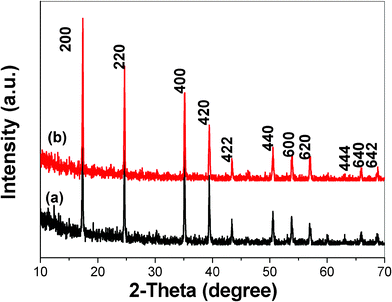 |
| | Fig. 6 The XRD patterns of the products obtained in the presence of 0.6 g and 1 g SDBS: (a) 0.6 g; (b) 1 g. | |
Microporosity of the as-prepared products was demonstrated by BET sorption isotherm measurements performed at 77 K using liquid nitrogen. Before measurements, the samples were heated to 200 °C for 10 h under vacuum to completely dehydrate the samples, according to the results of thermogravimetric analysis (Fig. 7). The adsorption–desorption results were shown in Fig. 8 and 9. The typical Type I isotherms are observed for both products. The Brunauer–Emmett–Teller (BET) specific surface area of Co3[Co(CN)6]2·nH2O obtained under 0.3 g SDBS was calculated to be 450.0626 m2 g−1 with a micropore volume of 0.196 cm³ g−1, which is lower than that of bulk Co3[Co(CN)6]2·nH2O (720 m2 g−1), and it therefore appears that some residual species are indeed present in the pores and the possibility of porous Co3[Co(CN)6]2·nH2O as a reservoir for various gases. Moreover, a narrow porous volume distribution of about 2–4 nm in the pore size has been indicated, which could prove that the nanoparticles that formed the product is a porous structure. The BET specific surface area of Co3[Co(CN)6]2·nH2O obtained under 0.6 g SDBS were calculated to be 719.3642 m2 g−1 with a micropore volume of 0.31 cm³ g−1, which is in close agreement with the value of bulk Co3[Co(CN)6]2·nH2O and its irregular nanoparticles.10 Two narrow porous volume distributions of 2–3.5 nm and 9–11 nm were observed from the inset in Fig. 9, which is large enough to accommodate all the gases and other substances, such as heavy metal ions, drug and so on.
![Nitrogen adsorption–desorption isotherms and pore size distribution (the inset) of Co3[Co(CN)6]2·nH2O in the presence of 0.3 g SDBS.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f8.gif) |
| | Fig. 8
Nitrogen adsorption–desorption isotherms and pore size distribution (the inset) of Co3[Co(CN)6]2·nH2O in the presence of 0.3 g SDBS. | |
![Nitrogen adsorption–desorption isotherms and pore size distribution (the inset) of Co3[Co(CN)6]2·nH2O in the presence of 0.6 g SDBS.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f9.gif) |
| | Fig. 9
Nitrogen adsorption–desorption isotherms and pore size distribution (the inset) of Co3[Co(CN)6]2·nH2O in the presence of 0.6 g SDBS. | |
Considering that the Co3[Co(CN)6]2·nH2O nanoparticles, which possess porous nanostructures and high surface area, may apparently contribute to CO2 storage properties, we attempted to measure the gas sorption properties of Co3[Co(CN)6]2·nH2O nanoparticles at room temperature. The samples obtained in the presence of 0.3 g and 0.6 g SDBS were denoted as S1 and S2, respectively. S1 and S2 were activated at 200 °C for 10 h to remove the adsorbed water molecules from the nanopores of the structure. Fig. 10 shows the adsorption isotherms for S1 and S2, where the calculated CO2 wt% at room temperature and 1 bar pressure were 8% and 8.7%, respectively, which are higher than that of Co3[Co(CN)6]2 irregular cubic-shaped nanocrystals (7.8%).10 This result shows that S1 and S2 are suitable for gas adsorption. Although the surface area of Co3[Co(CN)6]2 irregular cubic-shaped nanocrystals (744 m2 g−1) is much higher than that of S1 (450 m2 g−1),10 better gas adsorption performance is shown for S1. Moreover, the surface area of S2 is in agreement with the data of Co3[Co(CN)6]2 irregular cubic-shaped nanocrystals and the bulk material (720 m2 g−1) but the much better gas adsorption performance is shown for S2. These results prove that the gas adsorption properties of Co3[Co(CN)6]2 are highly dependent on not only its surface area but also its morphology, and materials at the nanoscale are more favorable to adsorption applications. On the other hand, we deduce that the narrow porous volume distributions of S1 and S2 are also responsible for their high adsorption properties. Of course, the detailed relationship between the microstructure and adsorption–desorption properties is complex, which requires further studies. Furthermore, the adsorption quantity raised with increasing pressure, indicating a stronger adsorption ability of our product at high pressures.
 |
| | Fig. 10
CO2 uptake of S1 (black) and S2 (red) | |
Conclusion
In conclusion, for the first time we demonstrate a facile method for preparing Co3[Co(CN)6]2·nH2O uniform truncated nanocubes with two different sizes (170 nm and 500 nm) at room temperature. The results demonstrate that it is possible to control the morphology and structure of Co3[Co(CN)6]2·nH2O by adjusting the process parameters, such as surfactant and amount of surfactant. Moreover, the as-prepared Co3[Co(CN)6]2·nH2O uniform truncated nanocubes displayed higher CO2 adsorption at room temperature and 1 bar pressure, compared to that of the bulk Mn3[Co(CN)6]2·nH2O under the same conditions. Studies pertaining to the gas adsorption of the Co3[Co(CN)6]2·nH2O nanoparticles at high pressure are in progress.
Acknowledgements
This work was supported by the National Natural Science Foundation (NSFC, 21071137).
References
- M. R. Hartman, V. K. Peterson and Y. Liu, Chem. Mater., 2006, 18, 3221 CrossRef CAS.
- J. Jiménez-Gallegos, J. Rodríguez-Hernández, H. Yee-Madeira and E. Reguera, J. Phys. Chem. C, 2010, 114, 5043 Search PubMed.
- R. K. Motkuri, P. K. Thallapally, B. P. McGraila and S. B. Ghorishi, CrystEngComm, 2010, 12, 4003 RSC.
- W. Chapman Karena, D. Southon Peter, L. Weeks Colin and J. Kepert. Cameron, Chem. Commun., 2005, 3322–3324 Search PubMed.
- H. Tokoro and S. Ohkoshi, Dalton Trans., 2011, 40, 6825, 10.1039/c0dt01829e.
- S. Ferlay, T. Mallah, R. Quahes, P. Veillet and M. Verdaguer, Nature, 1995, 378, 701 CrossRef CAS.
- J. G. Moore, E. J. Lochner, C. Ramsey, N. S. Dalal and A. E. Stiegman, Angew. Chem., Int. Ed., 2003, 42, 2741 CrossRef CAS.
- J. H. Yang, H. S. Wang, L. H. Lu, W. D. Shi and H. J. Zhang, Cryst. Growth Des., 2006, 6, 2438 CAS.
- J. F. Zhai, Y. M. Zhai, L. Wang and S. J. Dong, Inorg. Chem., 2008, 47, 7071 CrossRef CAS.
- C. A. Fernandez, S. K. Nune, R. K. Motkuri, P. K. Thallapally, C. M. Wang, J. Liu, G. J. Exarhos and B. Peter, Langmuir, 2010, 26, 18591 CrossRef CAS.
- S. S. Kaye and J. R. Long, J. Am. Chem. Soc., 2005, 127, 6506 CrossRef CAS.
- M. H. Cao, X. L. Wu, X. Y. He and C. W. Hu, Chem. Commun., 2005, 2241 RSC.
- H. M. Buchold Daniel and Feldmann. Claus, Chem. Mater., 2007, 19, 3302–3380 CrossRef.
- D. J. Du, M. H. Cao, X. Y. He, Y. Y. Liu and C. W. Hu, Langmuir, 2009, 25, 7057 CrossRef CAS.
- L. Hu, P. Zhang, Q. W. Chen, N. Yan and J. Y. Mei, Dalton Trans., 2011, 40, 5557 RSC.
-
(a) S. K. Nune, P. K. Thallapally, A. Dohnalkova, C. M. Wang, J. Liu and G. J. Exarhos, Chem. Commun., 2010, 46, 4878 RSC;
(b) J. Cravillon, R. Nayuk, S. Springer, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2011, 23, 2130 CrossRef CAS.
- M. Hu, J. S. Jiang, R. P. Jia and Y. Zeng, CrystEngComm, 2009, 11, 2257–2259 RSC.
- M. Hu, J. S. Jiang, R. P. Jia and Y. Zeng, CrystEngComm, 2010, 12, 2679–2683 RSC.
- M. Hu, J. S. Jiang and Y. Zeng, Chem. Commun., 2010, 46, 1133–1135 RSC.
- H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576–5591 CrossRef.
- H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576 CrossRef.
- X. L. Wu, M. H. Cao, C. W. Hu and X. Y. He, Cryst. Growth Des., 2006, 6, 26 CAS.
- T. Uemura and S. Kitagawa, J. Am. Chem. Soc., 2003, 125, 7814 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) MIII transition metals) are constructed from octahedral MIII(CN)63− complexes, which are bridged into a simple cubic lattice by M2+ ions. Co3[Co(CN)6]2·nH2O, is a typical Prussian blue analogue with the chemical formula M3II[MIII(CN)6]2·nH2O, and its hydrogen storage properties have been reported by Kaye et al.11 Moreover, there are several kinds of morphologies of Co3[Co(CN)6]2 with different sizes that have been reported at the nanoscale, such as Co3[Co(CN)6]2 polyhedra and nanorods by solvothermal synthesis in microemulsion system12 and nanoparticles in reverse microemulsions.13 However, the hydrothermal methods and reverse microemulsion are relatively complex processes, which is not consistent with the low cost idea. On the other hand, Zn3[Co(CN)6]2 (microspheres and micropolyhedrons),14Mn3[Co(CN)6]2 (nanocubes)15 and nanosized zeolitic imidazolate frameworks (ZIF-8)16 have been obtained by simple mixing the reactants at room temperature, but little progress has been made toward understanding and controlling the growth process of Co3[Co(CN)6]2 with a complicated morphology at room temperature in a simple way. In this paper, we report on the synthesis of Co3[Co(CN)6]2 truncated cubes with porous structure at room temperature in the presence of surfactants, which maybe follow the non-classical crystallization. By carefully controlling experimental conditions, Co3[Co(CN)6]2 nanostructures with different sizes can be efficiently obtained. As the mesocrystals can be synthesized by the aggregation of preformed small particles, a porous crystal may form, and a crystal with complex morphologies could be easily obtained. Recently, Jiang and co-workers reported the synthesis of Prussian blue mesocrystals with complicated morphologies by a hydrothermal method in the presence of hydrochloric acid and a polymer, which could be explained by the non-classical crystallization.17–18 However, developing a more facile means to fabricate PBA mesocrystals with complex nanostructures remains a challenge. Therefore, We believe that researching the nonclassical crystallization of PBA Co3[Co(CN)6]2 could complement the current research area. More interestingly, the as-prepared Co3[Co(CN)6]2 exhibit enhanced CO2 storage properties compared to that of the bulk.
MIII transition metals) are constructed from octahedral MIII(CN)63− complexes, which are bridged into a simple cubic lattice by M2+ ions. Co3[Co(CN)6]2·nH2O, is a typical Prussian blue analogue with the chemical formula M3II[MIII(CN)6]2·nH2O, and its hydrogen storage properties have been reported by Kaye et al.11 Moreover, there are several kinds of morphologies of Co3[Co(CN)6]2 with different sizes that have been reported at the nanoscale, such as Co3[Co(CN)6]2 polyhedra and nanorods by solvothermal synthesis in microemulsion system12 and nanoparticles in reverse microemulsions.13 However, the hydrothermal methods and reverse microemulsion are relatively complex processes, which is not consistent with the low cost idea. On the other hand, Zn3[Co(CN)6]2 (microspheres and micropolyhedrons),14Mn3[Co(CN)6]2 (nanocubes)15 and nanosized zeolitic imidazolate frameworks (ZIF-8)16 have been obtained by simple mixing the reactants at room temperature, but little progress has been made toward understanding and controlling the growth process of Co3[Co(CN)6]2 with a complicated morphology at room temperature in a simple way. In this paper, we report on the synthesis of Co3[Co(CN)6]2 truncated cubes with porous structure at room temperature in the presence of surfactants, which maybe follow the non-classical crystallization. By carefully controlling experimental conditions, Co3[Co(CN)6]2 nanostructures with different sizes can be efficiently obtained. As the mesocrystals can be synthesized by the aggregation of preformed small particles, a porous crystal may form, and a crystal with complex morphologies could be easily obtained. Recently, Jiang and co-workers reported the synthesis of Prussian blue mesocrystals with complicated morphologies by a hydrothermal method in the presence of hydrochloric acid and a polymer, which could be explained by the non-classical crystallization.17–18 However, developing a more facile means to fabricate PBA mesocrystals with complex nanostructures remains a challenge. Therefore, We believe that researching the nonclassical crystallization of PBA Co3[Co(CN)6]2 could complement the current research area. More interestingly, the as-prepared Co3[Co(CN)6]2 exhibit enhanced CO2 storage properties compared to that of the bulk.

![SEM and TEM images of the as-prepared Co3[Co(CN)6]2·nH2O nanoparticles obtained in the presence of 0.3 g SDBS.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f3.gif)
![SEM images of the as-prepared Co3[Co(CN)6]2·nH2O nanoparticles obtained in the presence of 0.6 g SDBS at different magnifications. The inset is the typical TEM image of a particle.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f4.gif)
![(a) The SEM image of Co3[Co(CN)6]2·nH2O obtained with 1 g SDBS; (b) The SEM image of Co3[Co(CN)6]2·nH2O obtained with 0.6 g PVP with the other experimental parameters kept constant.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f5.gif)

![(a) Thermogravimetry (TG) of the as-prepared uniform Co3[Co(CN)6]2·nH2O nanoparticles under 0.3 g SDBS; (b) Thermogravimetry (TG) of the as-prepared uniform Co3[Co(CN)6]2·nH2O nanoparticles under 0.6 g SDBS.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f7.gif)
![Nitrogen adsorption–desorption isotherms and pore size distribution (the inset) of Co3[Co(CN)6]2·nH2O in the presence of 0.3 g SDBS.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f8.gif)
![Nitrogen adsorption–desorption isotherms and pore size distribution (the inset) of Co3[Co(CN)6]2·nH2O in the presence of 0.6 g SDBS.](/image/article/2011/RA/c1ra00624j/c1ra00624j-f9.gif)

