A facile route to a macroporous silver network for methanol oxidation
Qunwei
Tang
,
Ziying
Tang
,
Jihuai
Wu
*,
Jianming
Lin
and
Miaoliang
Huang
Institute of Materials Physical Chemistry, Huaqiao University, Quanzhou, 362021, P. R. China. E-mail: jhwu@hqu.edu.cn; Fax: +86 595-22692229; Tel: +86 595-22693899
First published on 11th October 2011
Abstract
A macroporous silver network was facilely synthesized using eggshell membrane as template and explored for methanol oxidation reaction, showing a significantly enhanced electrocatalytic ability compared with a traditional silver mirror electrode. The scanning electron microscopy observation showed a hierarchically ordered macroporous silver network because of the Ag+-protein binding. Energy dispersive X-ray spectrum, X-ray diffraction patterns and X-ray photoelectron spectra were used to characterize the resultant macroporous Ag network. The cyclic voltammetric studies at room temperature showed an excellent electrocatalytic activity for a methanol oxidation reaction in an acidic medium. The macroporous silver network was also checked in the electro-oxidation of methanol at high temperatures. A much higher electrocatalytic activity at 250 °C than that at 50 °C made them potential candidates for high-temperature proton exchange membrane fuel cells. The research opens a new insight to design low-cost, highly active and stable catalysts for methanol oxidation. The facile synthesis, excellent properties, alterable supports and low cost allow this material to be used in high-temperature direct methanol fuel cells.
1. Introduction
Direct methanol fuel cells (DMFCs) have the potential for commercial applications in energy conversion and energy storage due to their high power density and portability, but their development has been impeded by problems with the membrane electrode assembly. As a key performance of DMFCs, the methanol oxidation reaction (MOR) of DMFCs plays an important role in controlling the properties of a fuel cell, and efficient electrocatalysts are essential for practical applications of the fuel cells.1–3Protons are produced when methanol gas flow permeates the anode catalyst layer, so, to increase the distance of methanol gas across anode catalyst layer is helpful in enhancing MOR and fuel cell performances. For this purpose, it is a prerequisite to not only increase the specific surface area of catalysts with good electrical conductivity but in addition to reduce the cost and develop a facile route to synthesis. Owing to the slow kinetics of the MOR at anode in DMFCs, generally, Pt nanoparticles are always used as catalysts. It is mandatory to support Pt nanoparticles onto a high-surface-area active carbon or carbon black to increase the surface area of the catalysts. In addition, the catalyst support also provides good electrical conductivity to the catalyst layer. However, the commonly employed carbon-supports suffer from: oxidation to surface oxides, and eventually to CO2 at the cathode during long-term operations in an oxygen-rich environment (21–100%); high operating-potentials, namely 0.6 V versus the standard hydrogen/methanol electrode and above; elevated temperatures (>60 °C); low pH; and significant levels of water in both vapour and liquid phases.4–8 Therefore, the development of cheap and stable electrocatalysts is also crucial for the large-scale commercial application of DMFCs.Template synthesis is an effective way to control product morphology, and thus many functional materials have been successfully templated with controllable morphologies.9–14 In the current work, a facile one-step route has been applied for the synthesis of a macroporous Ag network for methanol oxidation using an eggshell membrane (ESM) template. ESM consists of interwoven shell membrane fibers which have been used as a template for perovskite ceramics,9TiO2 nanotubes,10 metal nanoclusters,11 flexible conducting polymers supported Pt/Ag catalysts,12 and tris(8-hydroxyquinoline)aluminum nanowires.13 It is known that avian eggshells are formed by layered organization of calcified shell and organic ESMs containing collagen types I, V, and X, and glycosaminoglycans.15 The Ag+ can be adsorbed onto the surface of the ESM by means of the interaction with the amines, amides, and carboxylic surface functional groups,16 providing a possibility to form the macroporous Ag network.
2. Experimental
2.1. Materials and reagents
Eggshell membranes were obtained from commercial eggs. AgNO3, glucose, 1-methylimidazolium, trifluoromethanesulfonic acid, H2SO4 and HCl were purchased from Sigma-Aldrich and used as received.2.2. Separation of eggshell membrane
Commercial eggs were gently broken and emptied via the blunt end. The eggshells were washed with deionized water and then the inner shell membrane and the limiting membrane were manually removed. The remaining eggshells were immersed in a 1 M HCl aqueous solution to dissolve the CaCO3 shell, leaving the organic outer shell membrane.2.3. Fabrication of macroporous Ag network
After the outer shell membrane was washed with water thoroughly, the resultant ESM was immersed in 50 mM AgNO3 aqueous solution for a week. The Ag+ adsorbed ESM was subsequently washed with deionized water, dried at 80 °C, reduced by 5 wt% glucose aqueous solution for 2 days and calcined at 450 °C for 1 h to obtain the final Ag network because of the decomposition of Ag2O at more than 300 °C. The macroporous Ag network was formed by the Ag from the decomposition of Ag2O instead of the Ag nanoparticles reduced by 5 wt% glucose. At high temperatures such as 450 °C, the eggshell will be burnt out, so, the resultant product is pure Ag network.2.4. Preparation of Ag mirror
The glass substrates were thoroughly cleaned by acetone and deionized water. After being completely dried at 60 °C, the substrates were immersed in a 50 mM AgNO3 aqueous solution. The Ag mirror with an average size of Ag nanoparticles of 50 nm was then prepared by dipping into 5 wt% glucose aqueous solution.2.5. Synthesis of 1-methylimidazolium trifluoromethanesulfonate ([MIm][Tfo])
Protic ionic liquid, [MIm][Tfo], was synthesized by stirring the mixture of 1-methylimidazolium with equivalent molar amount of trifluoromethanesulfonic acid at 100 °C. The resultant viscous oil was cooled to room temperature. After milling and sifting using a 40-mesh screen, powdered [MIm][Tfo] was obtained.2.6. Measurements of electrocatalytic activity for methanol oxidation
The electrocatalytic activities of the macroporous Ag network and Ag mirror for methanol oxidation were evaluated by two methods using a three-compartment glass cell (VersaSTAT 3): the macroporous Ag network or Ag mirror were used as working electrodes, Pt foil as a counter electrode, Ag/AgCl as a reference electrode, (i) 2 M methanol in 0.25 M H2SO4 aqueous solution and (ii) melting [MIm][Tfo] were used as electrolytes, respectively. The working temperatures for route (i) and route (ii) were 25 °C and 50–200 °C, respectively. In the case of route (ii), the curves were recorded under a dry methanol gas-bubbling atmosphere. All the electrodes were immersed in the testing [MIm][Tfo] with a tube for methanol bubbling placed close to the working electrode. Before the measurement, the electrolytes were both deoxygenated by nitrogen bubbling for 10 min. The 2nd cycles were plotted at the scan rate of 50 mV s−1.2.7. Characterizations
The samples were mounted on a metal stub, and their surfaces were coated with gold. The morphology was observed and photographed by an S-4700 Hitachi cold field emission scanning electron microscopy (FESEM). X-ray photoelectron spectra (XPS) were performed on a Sigma Probe X-ray spectroscope (Thermo VG Scientific). The X-ray diffraction (XRD) analysis of the samples were carried out using a D8 ADVANCE X-ray diffractometer of Germany Bruker Co., Cu-Kα of 0.1540 nm wavelength, running at 40 kV and 30 mA, scanning from 10 to 70° at a speed of 5° min−1.3. Results and discussion
Fig. 1a and 1b show the top-view SEM images of the natural ESM template used in this work. It reveals that the ESM is a macroporous network composed of interwoven and coalescing shell membrane fibers ranging in diameter from 0.5 to 1 μm. The presence of macropores with pore sizes of 1–3 μm is evident. Due to the flexibility of ESM, the resultant Ag+ adsorbed ESM network is flexible and can be bent many times with no breakage. SEM images of the resultant hierarchically macroporous Ag network obtained from in situreduction of the Ag+ are shown in Fig. 1c and 1d. The as-obtained macroporous Ag network is composed of interwoven and coalescing Ag fibers ranging in diameter mainly from 0.8 to 1.2 μm, slightly bigger than the diameters of the initial ESM fibers. From the cross-sectional SEM photograph, one can see that the thickness of the Ag network is around 5 μm (Fig. 1e). The formed Ag network can be confirmed by EDX analysis (Fig. 1f). The detected C element comes from the incomplete calcined ESM.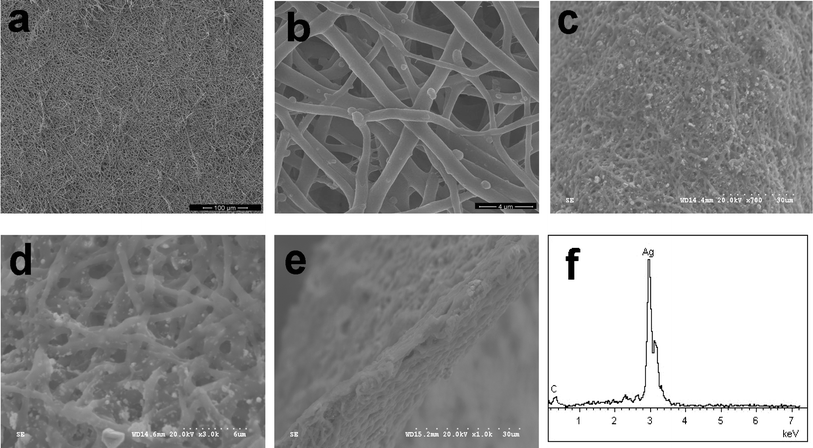 | ||
| Fig. 1 Top-view SEM photographs of (a) & (b) ESM and (c) & (d) macroporous Ag network at low and high magnifications, respectively. Images (e) & (f) are the cross-sectional SEM photograph and EDX spectrum of the resultant Ag network, respectively. | ||
The crystal phase of the macroporous Ag network can be confirmed by XRD analysis (Fig. 2a). As a comparison, the XRD pattern of a traditional Ag mirror composing of Ag nanoparticles with an average size of 50 nm is also provided. Peaks at 2θ = 38.3, 44.6 and 64.7° appear in the XRD patterns of macroporous Ag network and Ag mirror, which can be assigned to the diffraction of (111), (220) and (200) planes of the fcc silver, confirming the metal essence of silver clusters. Fig. 2b shows the typical XPS survey spectra of the Ag network and Ag nanoparticles. The detection of Ag4p, Ag4d, Ag4s, Ag3d, and Ag3p shows that the Ag network is comprised of metallic Ag which is similar to Ag nanoparticles. The presence of C1s is the result of residual ESM, which is consistent with the results of EDX analysis.
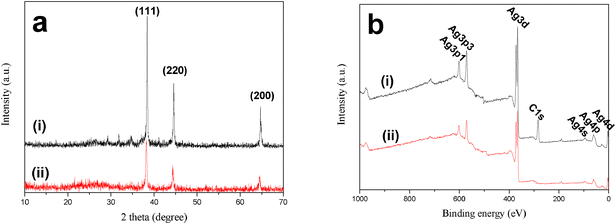 | ||
| Fig. 2 (a) XRD patterns and (b) XPS spectra of the (i) macroporous Ag network and (ii) traditional Ag mirror. | ||
To examine the electrocatalytic activity of the macroporous Ag network for methanol oxidation, we have studied the MORs using two different routes: (i) in 2 M methanol and 0.25 M H2SO4 solution at room temperature and (ii) in melting [MIm][Tfo] under methanol gas bubbling conditions with temperature range of 50–250 °C. Fig. 3a shows the CV curves of the macroporous Ag network and Ag mirror for the electro-oxidation of 2 M methanol in 0.25 M H2SO4 solution. The peak current density at ca. 0.25 V corresponding to the methanol oxidation in macroporous Ag network is 2.67 times higher than that in Ag mirror, revealing a significant enhancement of electrocatalytic activity. Owing to the potential use in high-temperature DMFCs, the oxidation ability of methanol by the resultant catalyst is performed in melting [MIm][Tfo] in the temperature range of 50–250 °C. When methanol gas is bubbled into [MIm][Tfo] around the working electrodes, the oxidative voltage corresponding to the MOR stands up at ca. 0.31 V in the macroporous Ag network and Ag mirror (Fig. 3b and 3c). The weak oxidation peak intensities at low temperatures (below 120 °C) indicate low proton production and poor fuel cell performances, while the current density significantly increases when the temperature is higher than 120 °C. The catalytic activity of the macroporous Ag network at 250 °C is 3.27 times larger than that at 50 °C. However, the catalytic activity at 250 °C is only 2.37 times higher than that at 50 °C for Ag mirror (Fig. 3d), meaning much less Ag amount and low cost of the catalyst for high temperature proton exchange membrane fuel cells (PEMFCs). The significant improvement in the catalyst performance derives from the highly catalytic kinetics at high temperatures.17–19 It is well-known that the development of PEMFCs operated above 120 °C in dry conditions is a significant milestone for their practical applications. Therefore, the resultant macroporous Ag network provides great advantages for the use in fuel cells under anhydrous and high-temperature conditions due to the high surface area and good catalytic activity.
![Cyclic voltammograms (CVs) of the macroporous Ag network and Ag mirror recorded in (a) 2 M methanol and 0.25 M H2SO4 solution. CV curves of the (b) macroporous Ag network and (c) Ag mirror in melting [MIm][Tfo] under methanol gas bubbling conditions. (d) Plots of current density as a function of working temperature. The curves were scanned from −0.8 to 1.0 V and back to −0.8 V at a scan rate of 50 mV s−1.](/image/article/2011/RA/c1ra00712b/c1ra00712b-f3.gif) | ||
| Fig. 3 Cyclic voltammograms (CVs) of the macroporous Ag network and Ag mirror recorded in (a) 2 M methanol and 0.25 M H2SO4 solution. CV curves of the (b) macroporous Ag network and (c) Ag mirror in melting [MIm][Tfo] under methanol gas bubbling conditions. (d) Plots of current density as a function of working temperature. The curves were scanned from −0.8 to 1.0 V and back to −0.8 V at a scan rate of 50 mV s−1. | ||
4. Conclusions
In summary, we have described a facile route to prepare a hierarchically ordered macroporous Ag network for MOR. The catalysts were characterized by SEM, EDX, XRD and XPS techniques. Their porous structures provide a large surface area and distance for the electro-oxidation of methanol, showing a much higher electrocatalytic activity than that of a bare Ag mirror. The electrocatalytic performances of the catalysts at 250 °C are around 3.27 times larger than that at 50 °C, indicating good potential use in high-temperature DMFCs. A macroporous Ag network catalyst is very important for practical applications. Besides the fabrication of an Ag network, many networks of other noble metals, transition metals and rare metals and their oxides can also be designed as long as they can form nitrates and chlorides. The free metal ions can be adsorbed by the active groups of ESM because of the metal–protein bonding. The research opens a new insight to design low-cost, highly active and stable catalysts for methanol oxidation. The facile synthesis, excellent properties, alterable supports and low cost allow this material to be used in high-temperature DMFCs and other fields.Acknowledgements
The authors gratefully acknowledge the financial support of the National High Technology Research and Development Program of China (No. 2009AA03Z217), the National Natural Science Foundation of China (No. 90922028).References
- R. Bashyam and P. Zelenay, Nature, 2006, 443, 63–66 CrossRef CAS.
- H. A.Gasteiger and N. M. Markovic, Science, 2009, 324, 48–49 CrossRef.
- D. R. Rolison, Science, 2003, 299, 1698–1701 CrossRef CAS.
- F. T. Wagner, S. G. Yan, P. T. Yu, Handbook of Fuel Cells-Fundamentals, Technology and Applications. John Wiley & Sons Press, 2010 Search PubMed.
- S. Y. Huang, P. Ganesan and B. N. Popov, Appl. Catal., B, 2011, 102, 71–77 CrossRef CAS.
- S. Kraemer, K. Wikander, G. Lindbergh, A. Lundblad and A. E. C. Palmqvist, J. Power Sources, 2008, 180, 185–190 CrossRef.
- V. Parry, G. Berthome, J. C. Joud, O. Lemaire and A. A. Franco, J. Power Sources, 2011, 196, 2530–2538 CrossRef CAS.
- K. Miyazaki, H. Shirakata, T. Abe, N. Yoshizawa and Z. Ogumi, Fuel Cells, 2010, 10, 960–965 CrossRef CAS.
- D. Dong, Y. Wu, X. Zhang, J. Yao, Y. Huang, D. Li, C. Z. Li and H. Wang, J. Mater. Chem., 2011, 21, 1028–1032 RSC.
- D. Yang, L. Qi and J. Ma, Adv. Mater., 2002, 14, 1543–1546 CrossRef CAS.
- C. Shao, B. Yuan, H. Wang, Q. Zhou, Y. Li, Y. Guan and Z. X. Deng, J. Mater. Chem., 2011, 21, 2863–2866 RSC.
- Q. W. Tang, J. H. Wu, Z. Y. Tang, Y. Li, J. M. Lin and M. L. Huang, J. Mater. Chem., 2011, 21, 13354–13364 RSC.
- T. Lee, S. C. Chang and J. F. Peng, Thin Solid Films, 2010, 518, 5488–5493 CrossRef CAS.
- Q. W. Tang, J. H. Wu, Y. Li, J. M. Lin, Z. Y. Tang and M. L. Huang, J. Mater. Chem., 2011, 21, 12932–12934 Search PubMed.
- J. E. Dennis, D. A. Carrino, K. Yamashita and A. I. Caplan, Matrix Biol., 2000, 19, 683–692 CrossRef CAS.
- M. Goto and K. Suyama, Appl. Biochem. Biotechnol., 2000, 84-86, 1021–1038 CrossRef CAS.
- S. Y. Lee, A. Ogawa, M. Kanno, H. Nakamoto, T. Yasuda and M. Watanabe, J. Am. Chem. Soc., 2010, 132, 9764–9773 CrossRef CAS.
- J. Zhang, Z. Xie, J. Zhang, Y. Tang, C. Song, T. Navessin, Z. Shi, D. Song, H. Wang, D. P. Wilkinson, Z. S. Liu and S. Holdcroft, J. Power Sources, 2006, 160, 872–891 CrossRef CAS.
- B. Lin, S. Cheng, L. Qiu, F. Yan, S. Shang and J. Lu, Chem. Mater., 2010, 22, 1807–1813 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
